Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

150 3 Magnetic Exchange in Oxides
28. J.B. Goodenough, Magnetism and the Chemical Bond, (Wiley Interscience, New York, 1963),
Chapter 3, Table XII; also J.B. Goodenough, Phys. Rev. 117, 1442 (1960)
29. N.S. Rogado, J. Li, A.W. Sleight, and M.A. Subramanium, Adv. Mater. (Weinheim, Ger.) 17,
2225 (2005)
30. L. N´eel, Ann. Phys. (Paris) 17, 64 (1932)
31. A.H. Morrish, The Physical Principles of Magnetism, (John Wiley, New York, 1965), Chapter 8
32. A.B. Lidiard, Rept. Prog. Phys. 17, 201 (1954)
33. A.H. Morrish, The Physical Principles of Magnetism, (John Wiley, New York, 1965), p. 457
34. C.G. Shull, W.A. Strausser, and E.O. Wollan, Phys. Rev. 83, 333 (1951)
35. R.K. Nesbet, Phys. Rev. 122, 1497 (1961)
36. J.B. Goodenough and J.M. Longo, Crystallographic and Magnetic Properties of Perovskite and
Perovskite-Related Compounds, Landolt-Bornstein, Volume 4a (Springer-Verlag, New York,
1970) pp. 126–314
37. G.H Jonker and J.H. Van Santen, Physica XVI, 337 (1950)
38. J.B. Goodenough, A. Wold, N. Menyuk, and R.J. Arnott, Phys. Rev. 124, 373 (1961)
39. J.B. Goodenough and J.M. Longo, Crystallographic and Magnetic Properties of Perovskite and
Perovskite-Related Compounds, Landolt-Bornstein, Volume 4a (Springer-Verlag, New York,
1970), Fig. 39
40. J.H. Van Santen and G.H Jonker, Physica XVI, 599 (1950)

Chapter 4
Ferrimagnetism
In the previous chapters, the origins of spontaneous magnetism for parallel
(ferromagnetism) and antiparallel spin alignments (antiferromagnetism) have been
reviewed. In their pristine forms, the former occurs through direct exchange in
metals and alloys, and the latter in nonmetallic ionic compounds comprising oxy-
gen or other elements from the right-hand side of the Periodic table as the anion
lattice. Utilitarian applications of ferromagnets are self-evident to even the most
casual observer of physical phenomena, but the situation is much less so in the case
of antiferromagnetism. For the most part, antiferromagnetism has been a portal to
fundamental research in materials, particularly involving the diagnostic methods of
neutron and more recently, muon diffraction and scattering.
There are, however, select groups of transition-metal oxides that combine the
magnetic properties of ferromagnetic metals with the electrically insulating char-
acteristics of the antiferromagnetic compounds described in the previous section.
These magnetic insulators are termed ferrimagnets, and the phenomenon that char-
acterizes their magnetic properties is called ferrimagnetism. Ferrimagnetic oxides
have also served as rich sources of knowledge about the fundamental physics of ma-
terials, but unlike the antiferromagnetic oxides, they continue to add to their already
widespread uses in modern electronics technology. For these reasons, the proper-
ties of ferrites, as they are commonly designated, will be treated generously for the
remainder of this book.
4.1 Ferrimagnetic Order
In the previous chapter, the concept of multiple magnetic sublattices was intro-
duced to explain the phenomenology of antiferromagnetism. From this starting
point, we may define a ferrimagnet as an antiferromagnet with unbalanced mag-
netic sublattices due to either differing populations of similar spins or sublattices
with ions of different spin values altogether. For this to occur, it is apparent that
some kind of crystallographic selection must be involved to distinguish the sub-
lattices. In the common oxide systems where ferrimagnetism occurs, the sublattices
are defined by cation sites of different oxygen coordinations: octahedral, tetrahedral,
G.F. Dionne, Magnetic Oxides, DOI 10.1007/978-1-4419-0054-8 4,
c
Springer Science+Business Media, LLC 2009
151
152 4 Ferrimagnetism
and dodecahedral, as described in Sect. 2.2.2. In a crystal lattice, these sites form
interspersed but ordered arrays that enable each to be occupied by entirely different
ionic species, or ions of the same atomic species but of different valence, or various
combinations of both. In the simplest case, a two-sublattice molecular field theory
can be applied, but now requiring three coefficients instead of the two for the an-
tiferromagnet – one each for the ions in the same sublattice (intrasublattice), and a
third (intersublattice) linking ions between the sublattices.
4.1.1 Generic Ferrimagnetic Systems
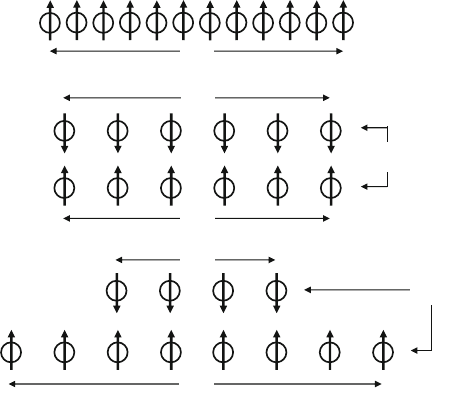
In Fig. 4.1, a one-dimensional sketch is offered to clarify the difference between
a ferromagnet, an antiferromagnet, and a ferrimagnet. In practical terms, a ferri-
magnet behaves magnetically as a ferromagnet and can be analyzed in terms of
the Brillouin–Weiss theory from Sect. 1.3.2. It must be recognized, however, that
all of the bonding linkages produce principally superexchange and therefore favor
antiparallel spin alignments. In an ideal ferrite with collinear moments, the final
ordering of the magnetic moments in the sublattices is therefore the result of com-
peting exchange fields that are generally dominated by the antiparallel influence
from intersublattice coupling. The resultant magnetic moment then becomes the
arithmetic difference of the two opposing sublattice moments.
FERROMAGNETISM
N
ii
> 0
ANTIFERROMAGNETISM
|
N
ij
| > |N
ii
,N
jj
|
N
ij
< 0
FERRIMAGNETISM
|N
ij
| > |N
ii
,N
jj
|
N
ij
< 0
N
ii
N
jj
N
ij
N
jj
N
ii
N
ii
N
ij
Fig. 4.1 One-dimensional exchange models of spontaneous spin alignment: (a) for a single mag-
netic lattice ferromagnetism: N
ii
>0;(b) for two equal sublattices with N
ij
as the dominant
coefficient, antiferromagnetism: N
ij
<0, N
ii
< or >0,(c) for two unbalanced sublattices with N
ij
dominant, ferrimagnetism: N
ij
<0, N
ii
, N
jj
< or >0
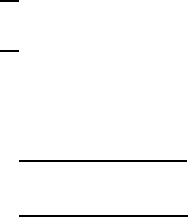
4.1 Ferrimagnetic Order 153
Although there are exotic chemical compounds in which ferrimagnetic sublattice
spin arrangements exist, our discussion of ferrimagnetism will be limited to three
families of transition-metal oxides that have become the foundation of this branch
of magnetism. The first of these systems to be recognized is the spinels designated
by the generic formula AŒB
2
O
4
,whereA is the tetrahedral site with O
4
coor-
dination and B is the octahedral site with O
6
coordination. The brackets around
B serve to indicate the octahedral sites in the actual chemical formulae. Later the
magnetic garnets with the generic formula
f
c
3
g
Œa
3
.d
3
/ O
12
were synthesized and
have become equally important particularly in microwave and optical applications.
The bracket/site designations are
f
c
g
for dodecahedral with O
12
coordination, [d]
for tetrahedral, and (a) for octahedral. It is important to note that unlike the spinels
in which the octahedral sites dominate the tetrahedral sites by a ratio of 2:1, in the
garnets the tetrahedral sites dominate by a ratio of 3:2.
A third family of ferrimagnetic compounds is the magnetoplumbites, named
after the naturally occurring of PbFe
19
O
12
(lead ferrite). These compounds are
commonly referred to as hexagonal ferrites or hexaferrites because of their sixfold
symmetrical uniaxial crystallographic structures. In many respects, hexaferrites re-
semble spinels because they feature the same ratio of octahedral to tetrahedral sites,
but also include one trigonal bipyramid .O
5
/ site (see Fig. 2.7) that contains an iron
ion, and one large site to house the usually divalent Pb, Ba, or Sr. These compounds
are important for permanent magnet applications and will be described in more de-
tail along with the spinels and garnets in Sect. 4.3.
4.1.2 Molecular Field Theory of Ferrimagnetism
Recalling the exposition of the molecular field model of antiferromagnetism from
Sect. 3.2.2, we can now apply this formalism in the manner of N´eel to the case of
a ferrimagnet [1]. For the individual sublattices of a two-site system, (3.35) with
N
ii
¤ N
jj
can be used to express the individual sublattice magnetizations as
M
i
D
C
i
T
H C N
ii
M
i
C N
ij
M
j
; (4.1)
M
j
D
C
j
T
H C N
ji
M
i
C N
jj
M
j
;
where
C
i
D
n
i
g
i
2
m
B
2
S
i
.S
i
C 1/
3k
; (4.2)
C
j
D
n
j
g
j
2
m
B
2
S
j
S
j
C 1
3k
:
154 4 Ferrimagnetism
As in the discussion of antiferromagnetism, a Curie temperature relation can be
extracted from solutions of (4.1) in the paramagnetic region above T
C
andinthe
magnetically ordered region below T
C
. Some of the details of the mathematical ma-
nipulations will be left to the readers, who can also consult standard text books, e.g.,
Morrish [2]. The analytical results, however, can serve as helpful approximations as
well as providers of physical insight into the stability of the ferromagnetic state.
In the paramagnetic region, (4.1) can be solved simultaneously to produce in-
dividual relations for M
i
and M
j
as a function of H . A susceptibility can then be
defined as D
M
i
C M
j
=H , which then leads to a rather complicated expression
for 1= vs. T that takes the form of a hyperbolic function displayed graphically in
Fig. 4.2 for which the asymptote as T !1is given by
1
D
T
C
i
C C
j
1
0
; (4.3)
where
1
0
D
1
C
i
C C
j
2
C
2
i
N
ii
C C
2
j
N
jj
C 2C
i
C
j
N
ij
: (4.4)
The asymptotic or paramagnetic Curie temperature is the intercept with the T axis,
given by
C
D
C
i
C C
j
0
: (4.5)
By the convention adopted so far in this text, all of the molecular field coefficients
are treated as negative quantities, so that
0
and therefore
C
will be negative. Equa-
tion (4.3) can be expressed as the Curie-Weiss law
D
C
i
C C
j
T C
C
: (4.6)
Fig. 4.2 Inverse susceptibility of a ferrimagnet above the Curie temperature, including the asymp-
tote of the hyperbola. Magnetization curve is added to illustrate the behavior at T<T
C
4.1 Ferrimagnetic Order 155
The above analytical method can be used to determine values of N
ii
, N
jj
,andN
ij
in
various situations that are explained in Sect. 4.2.
In the magnetically ordered region, an expression for the true Curie temperature
T
C
can be derived from (4.1) by allowing H D 0 and solving the determinant of the
coefficients of M
i
and M
j
to yield
T
C
D
1
2
C
i
N
ii
C C
j
N
jj
C
1
2
q
C
i
N
ii
C
j
N
jj
2
C 4C
i
C
j
N
ij
2
: (4.7)
In spinel and garnet ferrites, it will be shown that C
i
N
ii
C
j
N
jj
and since N
ij
is
usually the dominant coefficient, 4C
i
C
j
N
ij
2
>>
C
i
N
ii
C
j
N
jj
2
, allowing (4.7)to
be simplified to
T
C
1
2
C
i
N
ii
C C
j
N
jj
C
q
C
i
C
j
N
ij
2
: (4.8)
A first-order approximation that is often used with generally disappointing results is
T
C
q
CC
j
N
ij
2
. Because N
ii
and N
jj
are negative, N
ij
estimates arrived at by this
approximation can be substantially larger than the true values.
The most important application of the N´eel molecular field theory of ferrimag-
netism is the computation of the spontaneous magnetization characteristic of a given
chemical composition as a function of temperature (thermomagnetization). In the
case the resultant magnetization of the opposing sublattices is given by
M D
ˇ
ˇ
M
i
M
j
ˇ
ˇ
: (4.9)
The procedure once again involves the Brillouin–Weiss function, which is applied
to each sublattice according to
M
i
.T / D M
i
.0/B
S
i
.T / ; (4.10)
M
i
.T / D M
i
.0/B
S
i
.T / ;
where
B
a
i
.T / D
m
i
H
.i/
ex
kT
D
g
i
m
B
S
i
kT
N
ii
M
i
C N
ij
M
j
; (4.11)
B
a
j
.T / D
m
j
H
.j /
ex
kT
D
g
j
m
B
S
j
kT
N
ji
M
i
C N
jj
M
j
;
and M
i
.0/ D n
i
g
i
m
B
S
i
and M
j
.0/ D n
j
g
j
m
B
S
j
. Solution of (4.11) cannot be
done in closed form, but can be accomplished by self-consistent iteration proce-
dures involving multiple sublattices simultaneously. Computer programs for this
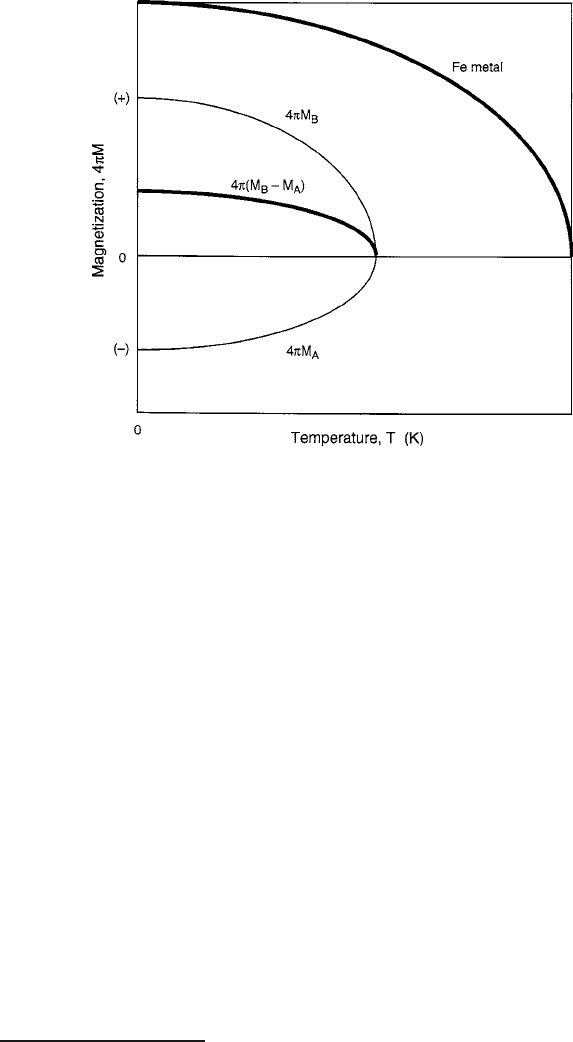
156 4 Ferrimagnetism
Fig. 4.3 Thermomagnetic characteristics of a ferromagnetic metal and a two-sublattice ferrite.
Note greater magnetization expected from the itinerant magnetism metal, for which no oxygen
occupies lattice sites and there are no opposing sublattices
purpose are listed in MIT Lincoln Laboratory Technical Reports ([3] (garnet), [4]
(RE garnet), [5] (spinel)).
1
A sketch of a typical thermomagnetization computation
isgiveninFig.4.3. The usefulness of the molecular field model has proven to be
enormous over the past four decades, particularly the refined versions of it that have
made possible the explanation and prediction of thermomagnetismbehavior of com-
pounds in which the sublattice moments are diluted with diamagnetic substitutions
for the purpose of tailoring magnetic properties to specific applications.
In most of these situations, dilution of the magnetic sublattices has been accom-
panied by departures from ideal magnetic spin alignments, commonly referred to as
“spin canting.” These reductions in the effective magnetic moments occur beyond
the normal disruptions of the magnetic ordering arising from the thermal random-
ization accounted for in the application of the Brillouin–Weiss function. Before
the more general theory of thermomagnetization is discussed, some background on
canting effects must be reviewed.
1
These published documents can be readily obtained from the U.S. National Technical Information
Service (NTIS).
4.1 Ferrimagnetic Order 157
4.1.3 Magnetic Frustration and Spin Canting
In ferrimagnetism, the molecular fields comprise contributions from magnetically
opposing sublattices. As a consequence, there exists the possibility that breakdown
of the long-range magnetic order by local cancellation (or even reversal through
overcompensation) of the magnetic moments can occur through variations in spa-
tial ordering of these individual moments from inhomogeneous site distributions.
Another cause for cancellation is magnetic dilution, i.e., the replacement of the
magnetic ions by diamagnetic S D 0 substitutes (which could include actual lat-
tice vacancies). For a site in the i sublattice that is missing even one of its nearest
neighbor spins, there is a finite probability that the ion does not participate in the
exchange stabilization. Such an occurrence is called magnetic frustration, which
effectively renders the ion paramagnetic at the site in question. The resulting spin
canting is a departure from collinearity of the spin directions independent of the
thermal lattice vibrational disruptions that accompany rising temperatures. The gen-
eral subject of spin canting has been examined from various approaches and will be
reviewed in the approximate chronological order in which they were reported.
The first examination of noncollinearity of spins in magnetically ordered systems
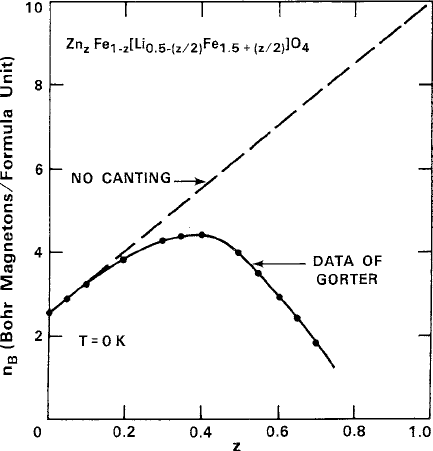
was reported by Yafet and Kittel (Y-K) [6] as an attempt to explain the magnetic be-
havior of spinel ferrites with A sites diluted by zinc. In the example of Fig. 4.4
Fig. 4.4 Magnetic moment of lithium zinc spinel ferrite at T ! 0 K, showing the canting effects
from Zn
2C
dilution of the minority tetrahedral A sublattice. Note departure from the linear N´eel
model and peak in data at z 0:4. Data are from Gorter [7]. Figure reprinted from G.F. Dionne,
J. Appl. Phys. 45, 3621 (1974) with permission.
c
1974 by the American Institute of Physics
158 4 Ferrimagnetism
for a lithium ferrite host [7], i.e., Fe
1z
Zn
z
Li
0:5z=2
Fe
1:5Cz=2
O
4
, the saturation
moment expressed in Bohr magnetons per formula unit n
B
is shown to fall below
the collinear N´eel model and reach a peak as the zinc content z ! 0:4. The ba-
sic notion of the Y-K model was that the i–i and j –j interactions under the right
conditions could form antiferromagnetic spin alignments within the their own sub-
lattices, thereby breaking up the main i –j antiferromagnetic ordering to produce
four sublattices and causing radical changes in the net magnetization. Although the
Y-K model failed to fit the data of the ferrite anomalies, it called attention to the ex-
istence of canted spins that was later confirmed in the spinel CuCr
2
O
4
by neutron
diffraction [8]. Another important result of this concept was the reasoning by de
Gennes that the canting of a sublattice would be principally the result of dilution of
the opposing sublattice [9].
There have been several attempts to devise a theory of canting that could place the
concept on a more quantitative basis. The seminal work was carried out by Gilleo
in an analysis of magnetic moment departures from the N´eel model for the mag-
netic garnets. In his most successful model, he assumed that Fe
3C
ions linked to
no more than one nearest neighbor nonmagnetic ions would not contribute to the
spontaneous magnetization [10]. This condition can be more stringent than that of
frustration, which can occur by means of a cancellation of exchange fields and there-
fore requires less dilution, but the results of the model nonetheless provided some
degree of satisfaction. In this model, the net magnetic moment per molecule in the
garnet system is
n
B
.k
d
;k
a
/ D n
Bd
.k
d
;k
a
/ n
Ba
.k
d
;k
a
/; (4.12)
where n
Bd
D 15 .1 k
d
/Œ1 E
d
.k
a
/ and n
Ba
D 10 .1 k
a
/Œ1 E
a
.k
d
/.
(Note that each Fe
3C
ion carries a magnetic moment of 5m
B
.) The parameters
E
a
.k
d
/ D 6k
d
5
5k
d
6
and E
d
.k
a
/ D 4k
a
3
3k
a
4
are the respective sublattice
canting probabilities from opposite sublattice dilution as determined from random
probability theory. From these relations both the magnetic moment at T D 0 K
and the Curie temperature can be estimated. In this attempt to fit measurement
data, only the intersublattice interactions were taken into account. Nonetheless,
the results as applied to the magnetic garnet compositions with a-site dilution
by Sc
3C
in the forms of
f
Y
3
g
ŒFe
2x
Si
x
.Fe
3
/ O
12
or by Zr
4C
with charge com-
pensating c-site Ca
2C
in the form of
f
Y
3x
Ca
x
g
ŒFe
2x
Zr
x
.Fe
3
/ O
12
have given
reasonable qualitative agreement with experiment, as shown in Fig. 4.5.Ford -site
dilution by Ge
4C
or Si
4C
with charge compensating c-site Ca
2C
in the form of
f
Y
3x
Ca
x
g
ŒFe
2
.Fe
3x
Ge
x
/ O
12
or
f
Y
3x
Ca
x
g
ŒFe
2
.Fe
3x
Si
x
/ O
12
, the model
has also given similar agreement with experiment, as shown in Fig. 4.6.
By the same procedure, Gilleo also deduced a model for the ferrimagnetic spinel
system AB
2
O
4
, with
n
B
.k
B
;k
A
/ D n
BB
.k
B
;k
A
/ n
BA
.k
B
;k
A
/; (4.13)
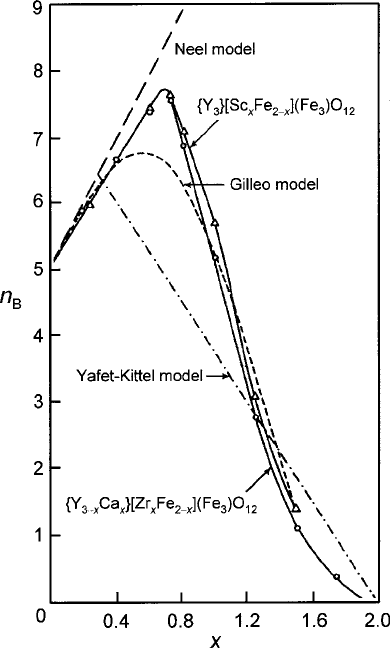
4.1 Ferrimagnetic Order 159
Fig. 4.5 Initial canting effects on magnetic moment of a-sublattice diluted yttrium-iron garnet at
T 0 K. Data of fY
3x
Ca
x
g
Œ
Fe
2x
Si
x
.
Fe
3
/
O
12
and fY
3x
Ca
x
g
Œ
Fe
2x
Zr
x
.
Fe
3
/
O
12
are from
Geller [15]. Figure reprinted from G.F. Dionne, J. Appl. Phys. 41, 4874 (1970) with permission.
c
1970 by the American Institute of Physics
where n
BB
D n
BB
0
.1 k
B
/Œ1 E
B
.k
A
/, n
BB
D n
BB
0
.1 k
A
/Œ1 E
A
.k
B
/,
and E
A
.k
B
/ D 12k
B
11
11k
B
12
, E
B
.k
A
/ D 6k
A
5
5k
A
6
. In this case the
respective undiluted Bohr magnetons per molecule are left as variables n
BB
0
and
n
BA
0
because of the greater likelihood of varying ionic spin values, i.e., other than
S D 5=2, in the spinel system.
In fashioning a physical description of spin canting in the magnetic garnets,
Geller realized the importance of the intrasublattice exchange fields and proceeded
to picture the evolution of the antiferromagnetic ground state from its initial ferri-
magnetism by examining the influence of the intrasublattice a–a and d –d exchange
fields on the stronger intersublattice a–d interaction [11]. The reasoning proceeds as
follows: When dilution of the a sublattice, for example, is made, the initial effect is a
net increase of n
B
which would proceed initially as a linear function of k
a
;however,
