Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

130 3 Magnetic Exchange in Oxides
of the molecular (or superexchange) field in spontaneous magnetism that was intro-
duced in Sect. 1.3.1. Recalling (1.40), we express
H
eff
D H C H
ex
D H C N
W
M; (3.26)
where N
W
D
z
n
2J
g
2
m
2
B
from (1.68) for the ferromagnetic case. For the two sublat-
tices i and j ,
N
ij
D
z
ij
n
j
2J
ij
g
i
g
j
m
2
B
;
N
ji
D
z
ji
n
i
2J
ji
g
j
g
i
m
2
B
; (3.27)
where n
i
and n
j
are the respective densities of spins of the i and j sublattices and z
ij
and z
ji
are the respective numbers of exchange-coupled neighbors.
From (3.15) we also see that the molecular field coefficient can be expressed in
terms of the covalent stabilization energy
N
ij
D
1
g
i
g
j
m
2
B
S
i
S
j
z
ij
n
j
X
n
b
2
n
U
n
: (3.28)
Since the molecular field in (3.26) from the j sublattice H
.j /
ex
D N
ij
M
j
acts on a
magnetic moment m
i
D g
i
m
B
S
i
in the i sublattice, the antiferromagnetic stabiliza-
tion energy for the i sublattice is expressed as
E
.i/
m
Dm
i
H
.j /
ex
Dg
i
m
B
S
i
N
ij
M
j
: (3.29)
Therefore, we can substitute for M
j
D n
j
g
j
m
B
S
j
and for N
ij
from (3.28) to obtain
m
i
H
.j /
ex
D z
ij
X
n
b
2
n
U
n
: (3.30)
As a result, the Brillouin-Weiss function parameter a
i
with the applied field H D 0
can be expressed as
a
i
D
m
i
H
.j /
ex
kT
D
g
i
m
B
S
i
N
ij
M
j
kT
D
z
ij
kT
X
n
b
2
n
U
n
: (3.31)
Note that there is no direct indication that m
i
H
.j /
ex
is of magnetic origin. In generic
systems where orbital angular momentum and spin-orbit coupling effects are ab-
sent, i.e., for S -state ions such as Fe
3C
or some crystal-field quenched systems of
the d
n
electron groups, the Pauli principle and Hund’s rule are responsible for the
superexchange stabilization. The influence of orbital angular momentum will be ex-
amined in Sect. 5.1.

3.2 Antiferromagnetism 131
3.2.2 Molecular Field Theory of Antiferromagnetism
To appreciate the importance of the molecular field concept in applying the theory
of superexchange to magnetic oxides, we must first review N´eel’s extension of the
Brillouin-Weiss theory of ferromagnetism to the case of opposing sublattices that are
characteristic of antiferromagnetism and its more complex cousin ferrimagnetism
[30]. For these situations, the spin values represent the number of orbital states of
the two ions that are jointly participating in the superexchangecovalent stabilization.
Although many instances of antiferromagnetism involve multiple sublattices, we
limit this discussion to the simplest case of two sublattices comprising nearest-
neighbor sites i and j occupied by ions with alternating spin directions. This is
the case of a lattice in which the magnetic ions occupy the corners of a simple cubic
structure, typical of a cubic perovskite to be examined in Sect. 3.3.2.Therearealso
situations where the magnetic sublattices are formed between next-nearest neigh-
bors, and these will be discussed later. In the ideal situation at T D 0 K, the spin
directions are assumed to be exactly parallel or antiparallel. Recalling the notions
of the molecular field introduced in Sect. 1.3., we express the magnitudes of the
resultant effective magnetic fields at the individual sites as
H
i
D H C N
ii
M
i
C N
ij
M
j
;
H
j
D H C N
jj
M
j
C N
ji
M
i
; (3.32)
where H is the applied magnetic field, N
ii
D N
jj
,andN
ij
D N
ji
are the corre-
sponding intra and intersublattice molecular field coefficients for the two sublattices
of magnetizations M
i
and M
j
. Because the interaction between sublattices is anti-
ferromagnetic, the N
ij
coefficient is negative, while N
ii
and N
jj
could be positive or
negative depending on the nature of the particular superexchange discussed in the
previous section.
At thermal equilibrium, the individual sublattice magnetizations can be expressed
by
M
i
D n
i
gm
B
S
i
B
S
i
.a
i
/; (3.33)
where n
i
is the volume density of spins S
i
, B
Si
is the Brillouin-Weiss function and
a
i
D
g
i
m
B
S
i
H
i
kT
as defined previously from (3.31)and(3.32). As suggested by the
introductory analysis of ferromagnetism in Sect. 1.3, a value of the threshold (N´eel)
temperature for spontaneous antiferromagnetic alignment will emerge from a solu-
tion of (3.33). To determine the behavior at or above the N´eel temperature, we use
the approximation for B
Si
.a
i
/ ! Œ.S
i
C 1/ =3S
i
a
i
near the limit where a
i
<< 1
from (1.37) to express the individual sublattice magnetizations as
M
i
D
n
i
g
2
i
m
2
B
S
i
.S
i
C 1/
3kT
H C N
ii
M
i
C N
ij
M
j
;
M
j
D
n
j
g
2
j
m
2
B
S
j
S
j
C 1
3kT
H C N
ij
M
i
C N
jj
M
j
; (3.34)
132 3 Magnetic Exchange in Oxides
where the directions of H , M
i
,andM
j
can be treated as parallel in the paramagnetic
regime above T
N
. If we assume that each sublattice contains an equal division of
the total population n, which comprises the same ionic species with spin S and
spectroscopic factor g, S
i
D S
j
D S, g
i
D g
j
D g,andn
i
D n
j
D n=2,the
resultant magnetization becomes
M D M
i
C M
j
D
ng
2
m
2
B
S.SC 1/
3kT
H C
N
ii
C N
ij
M
(3.35)
from which the susceptibility D M=H can be written in terms of the paramagnetic
N´eel temperature
N
D
C
T C
N
; (3.36)
where C D
ng
2
m
2
B
S.SC1/
3k
and
N
D
C
2
N
ij
C N
ii
. Note that N
ij
is assumed to
be negative and of greater magnitude than N
ii
. Therefore,
N
is positive and con-
sistent with (1.43). A graphical representation of the different situations of (3.36)is
presented in Fig.1.7. In situations where N
ii
becomes equal to or greater than N
ij
,a
reordering of the sublattice spin alignments is expected to take place.
To obtain an expression for the actual N´eel temperature T
N
,(3.36) can be used
in the limit of H ! 0,
M
i
D
C
2T
N
ii
M
i
C N
ij
M
j
;
M
j
D
C
2T
N
ij
M
i
C N
ii
M
j
: (3.37)
For M
i
and M
j
each to be nonzero, the determinant of the coefficients of M
i
and M
j
must be zero. Therefore, the solution for T D T
N
becomes
T
N
D
C
2
N
ij
N
ii
: (3.38)
At this point, it is possible to compare the paramagnetic temperature with the N´eel
temperature by taking the ratio of (3.37)and(3.38):
N
T
N
D
N
ij
C N
ii
N
ij
N
ii
: (3.39)
There are limitations to the applicability of (3.39). In most cases N
ii
and N
ij
are
both negative, and the ratio
N
=T
N
>0.WhereN
ii
is negligible compared with
N
ij
, T
N
N
; where these two coefficients are comparable in size, instability in the
ordering will occur; if N
ii
were to dominate, the static spin ordering would assume
a different pattern.
To complete the picture of the susceptibility as a function of temperature for
a single-crystal antiferromagnet, we must examine the condition of the spin sys-
tems below the N´eel temperature. Here the two sublattices tend to be antiparallel
3.2 Antiferromagnetism 133
because of the superexchange stabilization, but are still influenced by an applied H .
Because of the existence of crystalline anisotropy, the direction of the applied field
relative to the preferred direction of the spins must be taken into account. The impor-
tance of magnetocrystalline anisotropy will be realized in our discussions of ferrite
in Chap. 4. For this exercise, the anisotropy is considered to be uniaxial and our dis-
cussions will be limited to the cases of H parallel and perpendicular to the easy axis.
Because of the complexity of the analytical procedure, only the results will be pre-
sented in this text. For details of the derivation, the reader is advised to consult texts
such as Morrish [31]. The relations for the parallel and perpendicular susceptibility
are stated as
.T/ D
ng
2
m
2
B
S
2
B
0
.a
0
/
kT
1
2
N
ij
C N
ii
ng
2
m
2
B
S
2
B
0
.a
0
/
;
?
.T / D
1
N
ij
C N
ii
; (3.40)
where B
0
.a
0
/ is the first derivative of the Brillouin-Weiss function and
a
0
D
gm
B
S
kT
N
ij
N
ii
M
0
; (3.41)
where M
0
D M
i
DM
j
at H D 0. If it is assumed that N
ii
D 0,(3.40) can
be plotted as function of T=T
N
with S as a variable parameter, without assigning a
specific value to N
ij
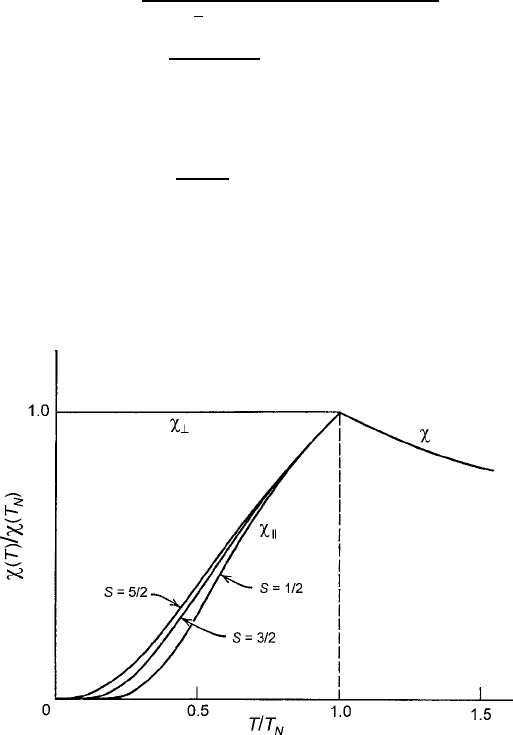
, as shown by Lidiard’s .T/calculated graphs in Fig. 3.13 [32].
In the 1= vs. T format where N
ii
¤ 0, the curves appear as sketched in Fig. 3.14.
Fig. 3.13 Susceptibility of an antiferromagnet computed as a function of temperature in units
reduced by the Neel temperature T
N
. Image is based on computations of Lidiard [32]
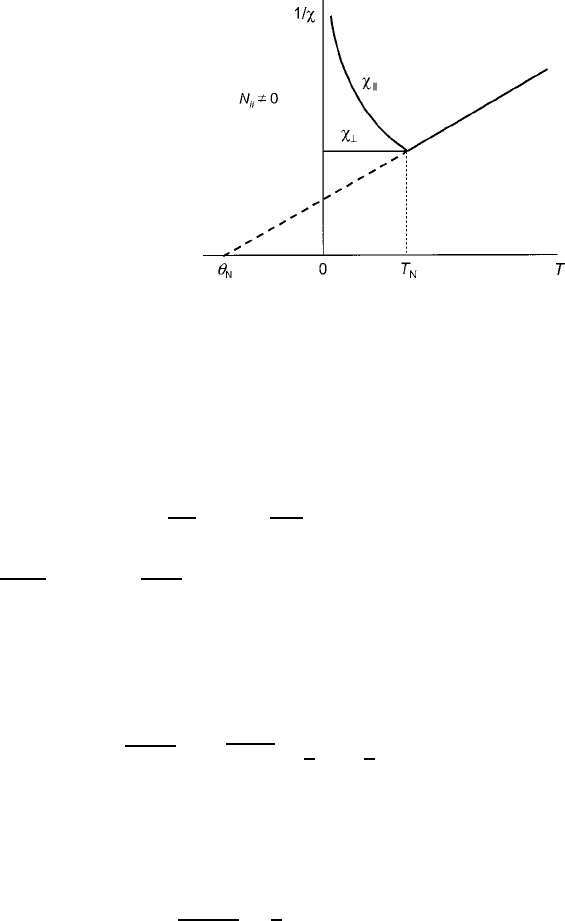
134 3 Magnetic Exchange in Oxides
Fig. 3.14 Inverse
antiferromagnetic
susceptibility modeled from
the Curie law as a function of
temperature, indicating the
asymptotic Neel temperature
N
discussed in Sect. 1.4
For polycrystalline or powdered materials consisting of randomly oriented
particles, this discussion can be extended by means of a simple argument. In
general, the applied field will form an angle with the easy axis of a particular
crystallite. Therefore, each single crystal grain will contribute a parallel and a per-
pendicular component to the magnetization, and thereby to the overall susceptibility
which can be expressed as
sc
D
M
k
H
cos C
M
?
H
sin : (3.42)
Since
k
D
M
k
H cos
and
?
D
M
?
H sin
,(3.42) becomes
sc
D
k
cos
2
C
?
sin
2
; (3.43)
and after averaging over all particle orientations, the susceptibility of a polycrys-
talline specimen is
p
D
k
cos
2
C
?
sin
2
D
1
3
k
C
2
3
?
: (3.44)
One additional estimate for the susceptibility should be added to this discussion.
The ratio of the polycrystalline susceptibility at T D 0 to its value at T D T
N
can
be obtained from (3.44) by setting
k
D 0 at T D 0,and
k
D
?
(a constant
value) at T D T
N
to yield
p
.0/
p
.T
N
/
D
2
3
: (3.45)
This polycrystal susceptibility can be estimated from a simple interpolation sug-
gested by (3.44) between the
k
and
?
curves in Fig. 3.14.
Application of these theoretical results to specific materials systems has been
expounded in a number of standard texts and comprehensive reviews. For our
purposes, some generic examples will be given to illustrate the relation between
covalent bonding, superexchange, and the magnetic sublattice arrangement.
3.2 Antiferromagnetism 135
3.2.3 Antiferromagnetic Spin Configurations
In nonmetallic compounds, antiferromagnetism reveals itself in a vast number of
chemical structures that include, besides the oxides, sulfides, selenides, tellurides,
and of course, halides of both simple and more complex combinations of cations in
various crystal structures both natural and synthetic. To explore this ocean of pos-
sibilities in any depth would defeat the purpose of this text, but it is, nonetheless,
important that the reader gain an appreciation of the background and the more basic
facets of this most common type of magnetic interaction. As explained in Sect. 3.1,
there are two overriding factors that determine the nature of the spin alignment
in most situations: (1) direct overlap e
g
p¢ bonding is expected to dominate the
exchange between octahedral sites and (2) the sign of the exchange constant J would
be negative in situations where the cations have similar valence charges and elec-
tronic configurations.
For particular crystal lattices, the sites that make up the opposing sublattice, des-
ignated as j , are not always the crystallographic nearest neighbors. The determining
factor in each case is the disposition of the anion (oxygen) relative to the cation
neighbor, because it is the anion that provides the chemical bond which establishes
the stabilization energy. There are three common magnetic structures that need to
be distinguished: simple cubic (perovskites), body-centered cubic (rutile), and face-
centered cubic (one-metal oxides). The first two are conventional in the sense that
the j sublattice consists of nearest neighbor cations and the N´eel temperature ex-
pressedby(3.38),
T
N
D
C
2
.N
ij
N
ii
/ for rutile and perovskite
C
2
N
ij
for
ˇ
ˇ
N
ij
ˇ
ˇ
>>
j
N
ii
j
: (3.46)
The general relations between J ,
P
b
2
n
=U
n
and T
N
for this antiferromagnet are
J D
1
2S
2
X
n
b
2
n
U
n
Dq
3kT
N
2zS.SC 1/
; (3.47)
from which we can express
T
N
D
z
3k
X
n
b
2
n
U
n
S C 1
S
.q D 2; simple cube/;
T
N
D
z
6k
X
n
b
2
n
U
n
S C 1
S
.q D 4; face-centered cube/; (3.48)
T
N
D
2z
3k
X
n
b
2
n
U
n
S C 1
S
.q D 1/ :
136 3 Magnetic Exchange in Oxides
t
2g
-t
2g
σ
PEROVSKITE
2p
t
2g
FACE-CENTERED CUBE
e
g
-2p
σ
-e
g
ab
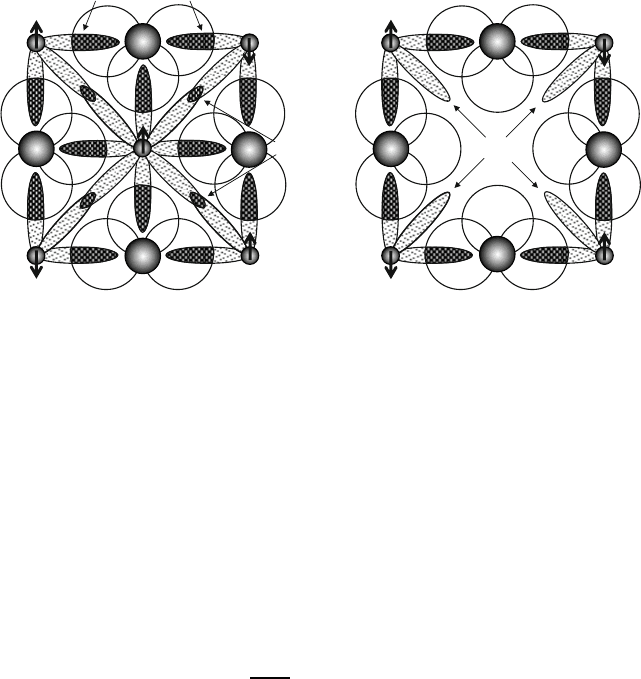
Fig. 3.15 Comparison of bonding linkages across cube faces for (a) a face-centered cube and (b)
a perovskite. The difference is contribution of direct t
2g
t
2g
bonds in (a)
Note that q D 2 for antiferromagnetism and originates from the definition of (3.38);
it is also double that of the ferromagnetic case (q D 1). A more interesting situation
is the face-centered cube shown in Fig. 3.15a, for which q D 4. In this case, the op-
posing magnetic sublattice comprises next-nearest neighbors for reasons that can be
seen from the diagram of the face. Along the unit cell edges 180
ı
M–O–M linkages
between cations occupying octahedral corner sites provide the strongest superex-
change from e
g
–p¢ bonds, and these next-nearest-neighbor interactions dominate
over the diagonal interactions between the nearest-neighbor corner to face-center
sites. If the intersublattice coefficient is greater than that of the intrasublattice coef-
ficient in this structure, i.e., jN
ij
j > .3=4/ jN
ii
j, and from [33]
T
N
D
CN
ij
4
.one-metal oxide/; (3.49)
and we see that only N
ij
can be involved and the factor of 2 in the denominator of
(3.38) is increased to 4. This occurs because of the sublattice configuration of lay-
ered <111> planes shown in Fig. 3.16 for this structure must have the 12 nearest
neighbors occurring six in-plane of the same spin alignment and six in the adjacent
plane of opposite spin alignment, thereby producing a cancellation of the N
ii
contri-
butions. However, coupling to nearest neighbors can still influence the value of N
ij
through direct linkages in tandem. The relations corresponding to (3.38) apply here
also, but with q D 4.
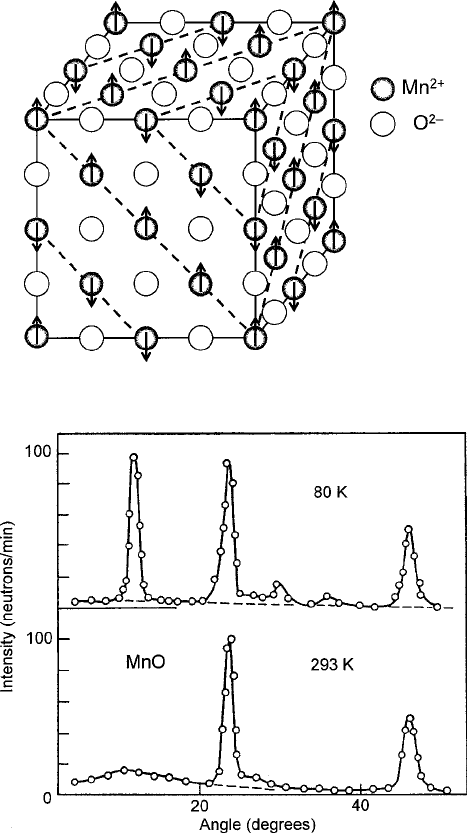
Experimental evidence of the spin configurations was obtained by the powerful
tool of neutron diffraction, which can sort out planes of common spin orientations
in a manner similar to that of X-ray diffraction with ordinary crystal lattices. An
example of these data above and below the N´eel temperature for MnO is shown in
Fig. 3.17 [34]. Note the radical difference in the patterns for the two temperature
regimes.
3.2 Antiferromagnetism 137
Fig. 3.16 Antiferromagnetic structure of a diatomic metal oxide of cubic symmetry
Fig. 3.17 Neutron-diffraction pattern from MnO, which is antiferromagnetic below the Neel tem-
perature at 120 K. The upper trace was from the antiferromagnetic state; the lower one is for
paramagnetism at room temperature. Image is adapted from Lax and Button presentation ([13],
Fig. 3.22) of data by Shull et al. [34]
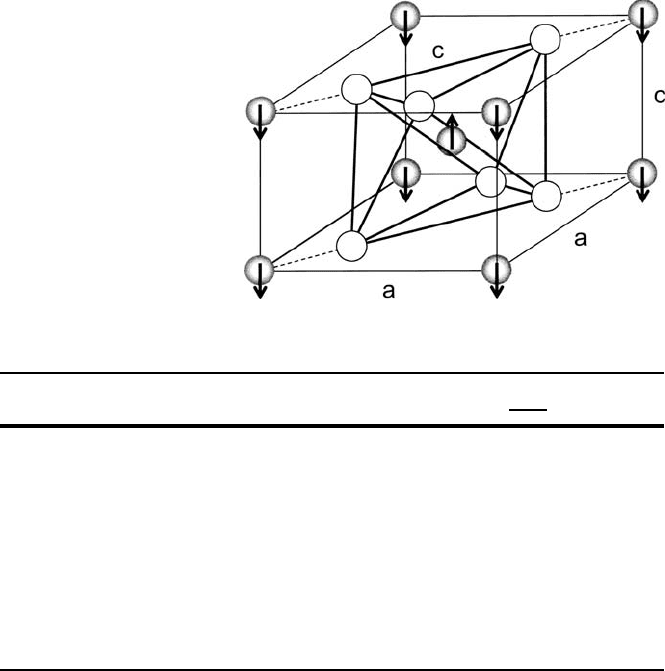
Figure3.18 shows another common antiferromagnetic structure rutile, which is
a tetragonal with magnetic cations centering octahedrally coordinated ligands with
axes directed along twofold lattice symmetry axes. The local antiferromagnetic 180
ı
cation–anion–cationarrangements produce the particular spin ordering shown in the
figure. In this case the N´eel temperature T
N
D.C =2/
N
ij
N
ii
follows (3.46).
138 3 Magnetic Exchange in Oxides
Fig. 3.18 Basic cell of rutile
indicating the octahedrally
coordinated ligands
surrounding the internal
cation with axes along a
lattice axes of twofold
symmetry
Table 3.4 Parameter data from selected antiferromagnetic compounds
Crystal
structure
C
mole
Compound T
N
.
K
/
N
.
K
/
N
=
T
N
p
.
0
/
p
.
T
N
/
(cgs units)
MnO fcc 122 610 5.0 0.69 4.40
FeO fcc 198 570 2.9 0.77 6.24
CoO fcc 293 280 0.96 3.0
NiO fcc 520 – – 0.67 –
CuO monoclin. 453 ––––
MnS fcc 165 528 3.2 0.82 4.30
MnF
2
rutile 72 113 1.6 0.75 4.08
FeF
2
rutile 79 117 1.5 0.72 3.9
CoF
2
rutile 38 53 1.4 – 3.3
NiF
2
rutile 73 116 1.6 – 1.5
MnO
a
2
rutile 84 – – 0.93 –
References may be found in the review article by Nagamiya et al. [19]
a
Mn is in the 4C state, which means that only the t
2g
orbitals are involved in the superexchange
Because of the relationship between spin alignments and bonding, simple metal
oxides (M
2C
O
2
) can form antiferromagnetic compounds of the face-centered cube
(MO) or rutile (MO
2
) types. Perovskites with magnetic structures that approximate
simple cubic usually feature M
3C
cations that have inactive e
g
–p¢ bonds. When
these 2C ions are from the upper half of the series, the N´eel temperatures are sig-
nificant because of the availability of strong e
g
–p¢ bonds. A comparison of the
properties of these ions in the two crystal structures is informative. As listed in
Table 3.4, T
N
and
N
values [19] are greater for the face-centered cubic structure.
Part of the difference can be attributed to the 180
ı
M–O–M bond angles, which
provide the largest overlap integral and greatest stabilization energy. For rutile, the
angle is less than 180
ı
, which is more typical of ferrimagnetic oxides to be examined
in Chap. 4

3.3 Antiferromagnetic Oxides 139
3.3 Antiferromagnetic Oxides
Chemical compounds with antiferromagnetic spin alignments are the most common
of materials that exhibit magnetic properties. Even magnetically undiluted mate-
rials that are paramagnetic at room temperature usually reveal a N´eel transition
if the temperature is lowered far enough. Although the present discussion is re-
stricted to oxides, halides and other compounds that incorporate ions of the 3d
n
series will be included wherever they can illustrate important features. The crys-
tallographic systems that have been studied extensively both as vehicles for basic
science investigations and for practical applications are the chemically simple one-
metal compounds already introduced in the previous section and the more complex
perovskites.
3.3.1 One-Metal Oxides
In the previous section, magnetocaloric properties of some of the divalent 3d
n
metal
oxides were used as examples of the thermal effects that occur at the antiferro-
magnetic order–disorder transition. With the exception of CuO, which features a
noncubic structure influenced by the Jahn-Teller distortions of the normally octahe-
dral sites, each of them is of the face-centered cubic structure. The relation between
the electron configurations and the N´eel temperatures are summarized in Table 3.5.
Exchange stabilization energies z
P
b
2
n
=U
n
are deduced from the T
N
values with
the aid of (3.48).
The ions from the lower half of the series are typically trivalent, which pre-
cludes their occurrence in the M
2C
O
2
face-centered cubic oxides. As a result,
lower symmetry molecular structures are formed without 180 ˚ bonds and strong
antiferromagnetism does not appear. Since the ions from n D 1, 2, or 3 configu-
rations have only t
2g
electrons, bonding is achieved by means of t
2g
orbitals with
oxygen that can be stronger than the t
2g
–p bonds of typical formations when 180
ı
angles are available. In this case, the overlaps are a combination of ¢ and .As
in all chemical compounds, the directionality of the t
2g
orbital lobes in relation to
their 2p lobe bonding partners of the oxygen ligands is the determining influence in
establishing the particular stereochemistry of the molecular structure.
Table 3.5 Superexchange data of the 3d
n
ions in one-metal oxides
Ion Config. ST
N
.
K
/
J
.
meV
/
z
P
b
2
n
=U
n
.
meV
/
E
hop
.
meV
/
Mn
2C
O t
3
2g
e
2
g
5/2 122 1:2 90 100
Fe
2C
O t
3
2g
e
2
g
2 198 2:8 136 –
Co
2C
O t
5
2g
e
2
g
3/2 293 6:8 182 300
Ni
2C
O t
6
2g
e
3
g
1 520 22:4 270 600
Cu
2C
O
a
t
6
2g
e
3
g
1/2 453 52:0 156 600
a
CuO
is of monoclinic structure probably because of the Jahn-Teller nature of the Cu
2C
ioninan
octahederal site
