Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

120 3 Magnetic Exchange in Oxides
this case, the electrons can be more stable by filling states as spin pairs according to
the Pauli principle, and J<0. Experimental results that support these predictions
are shown in Fig.1.12 for a variety of direct exchange coupled compounds [13].
In oxides and other ionic compounds, the magnetic ions are not usually nearest
neighbors. Therefore, any direct exchange effects should be weak. If the collective-
electron formalism that is successful for metals is applied to oxides, r
a2
and r
b1
are
seen as too large to create a negative J , thereby predicting that a weak ferromagnetic
spin alignment might be expected. However, in most instances, this conclusion is not
supported by experiment. The cation spin ordering is most often antiferromagnetic.
Following the early suggestion of Kramers [14]thatindirect exchange must occur,
the general concept sketched in Fig. 3.1d was adopted. Indirect or superexchange is
the coupling of cations spins through the medium of nearest-neighbor anion ligands.
Ferromagnetism can also be produced by direct exchange .J > 0/ between next-
nearest neighbors, however,shown as 3d
xy
orbitals overlapping across the diagonals
of a two-dimensional model, for example, a cube face.
In addition to the mechanism where spins of like cations with half-filled orbitals
correlate through common ligands by virtual charge transfer of two electrons in
a periodic structure to produce antiferromagnetic order analogous to itinerant fer-
romagnetism in metals, real charge transfer between dissimilar ions can induce
ferromagnetism by the sharing of a single electron. Because the spins are initially
local to their parent ions, these short range transfers are commonly described as
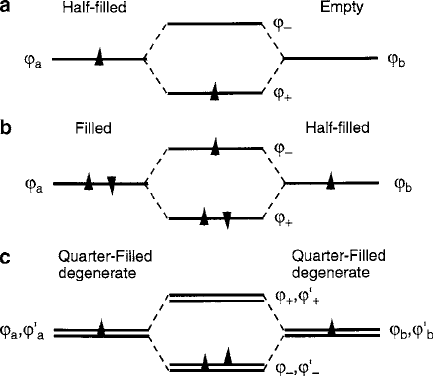
delocalized or semicovalent. Figure 3.9 illustrates two delocalization conditions:
(a) half-filled to empty and (b) filled to half-filled orbital transfers [15]. A spe-
Fig. 3.9 Molecular-orbital diagrams of ferromagnetic superexchange: (a) delocalization from
half-filled to empty, (b) delocalization from filled to half-filled, and (c) Hund’s rule ferromag-
netism in quarter-filled degenerate orthogonal metal–ligand states

3.1 Interionic Magnetic Exchange 121
cial case of ferromagnetic delocalization exchange
3
occurs between neighboring
cations of the same atomic element occupying identical lattice sites, but with dif-
ferent valence charges. When this mechanism operates in an antiferromagnetic or
ferrimagnetic system in which opposing magnetic sublattices are present, the trans-
fers are intrasublattice (homonuclear, H
2
C
for the active orbital), and the resulting
energy stabilization is called double exchange [16–19].
A somewhat related situation can occur in crystal fields (cubic, trigonal, or tetrag-
onal) that allow orbital degeneracy to survive unquenched. In Fig. 3.9c, a single
electron occupying a doublet, for example the t
2g
spin-orbit stabilized case of d
2
or
d
7
in a c=a < 1 tetragonal site, presents a degenerate antibonding state that allows
Hund’s rule to be sustained and provides metallic ferromagnetic superexchange.
A fourth type of indirect exchange occurs where the spins couple to each other
through polarization of the “charge clouds” of their mutual environment rather than
by a formally defined covalent bond. This mechanism, called RKKY after its col-
lective authors Ruderman-Kittel [20], -Kasuya [21], and -Yosida [22] and described
in most standard texts on magnetism, is generally associated with the rare-earth
or lanthanide series where the 4f
n
states are denied direct exchange interactions
with like-orbital states of neighboring cations or with crystal fields of anions by
the shielding from filled outer shells. Unpaired electron spins of the partially filled
4f
n
inner shell interact indirectly with the immediate ligand field by polarizing
the charges of their filled outer 5s
2
and 5p
6
shells that provide shielding from the
crystal fields of the ligands, as evidenced by the weaker superexchange effects of
rare-earth ions when occupying the c sublattice of magnetic garnets.
3.1.4 Ferromagnetism by Spin Transfer
Although less important from a purely magnetic standpoint, the case of real transfer
by delocalization is of interest because of the electrical conductivity implications
[3]. In the analysis of the magnetism created by a single mobile spin, it must be
recognized from the outset that there can be no spin-dependent stabilization asso-
ciated solely with the transfer between the two orbital states. However, if the two
cations each have a net spin or reside in a cluster of lattice spins that would provide
an exchange field to dictate the orientation of the transferring spin, the net spins
of the cations involved in the transfer would likely be ferromagnetically aligned.
Otherwise the transfer electron would undergo a spin reversal to obey Hund’s first
3
As a primer to the terminology used in the discussion of magnetic exchange in insulators,
we first define two-electron exchange as virtual (spins do not actually switch nuclei) and
therefore covalent, with correlated spins to create antiferromagnetic order by correlation ex-
change, or simply superexchange. One-electron semicovalent exchange is described as real
(since the spin can actually switch nucleus) with delocalized spins to create ferromagnetic order
exchange.
122 3 Magnetic Exchange in Oxides
rule, violating the S D 0 requirement. If the spin retains its orientation in oppo-
sition to the polarization dictates of Hund’s rule, its energy of the e
2
=r
ij
repulsion
represents a destabilization that would offset any antiparallel alignments that are fa-
vored by unpaired spins occupying other orbital states on the same ion. The result
of an antiparallel alignment between the cations would be the discouragement of the
spin transfers through the creation of a spin trap or magnetic activation energy that
would result in a loss of the energy that would otherwise be gained by the sharing
of the spin.
As a consequence, in cases where only one of the orbital states is half-filled,
the Pauli principle is no longer the main concern because only one electron can be
involved in the coupling, and the two-electron exchange effects do not apply. Be-
cause the second orbital would be either empty or filled with no net spin in either
case, the e
2
=r
ij
repulsive term is not involved in the charge transfer. For practical
purposes, it can be convenient to view these two orbital combinations as analogous,
with electron transfer into the empty orbital and “hole” transfer into the filled or-
bital. Except for the extremes of d
0
, d
10
or certain low-spin configurations that are
diamagnetic because of low-spin S D0 configurations, Hund’s rule is followed for
ions with S D0 to prevent the loss of transfer energy that would otherwise occur
through the formation of excited states within the 3d-electron shell.
The various superexchange interactions between magnetic cations involve an-
ion mediation and can be described in greater detail. Two basic situations can be
expressed in chemical ionic notation as follows:
1. Two-electron covalent exchange (superexchange) occurs between half-filled or-
bitals depicted in Fig. 3.10a, where an electron from cation M
a
is undergoes a
virtual transfer to cation M
0
b
, requiring an excitation energy U according to
M
a
nC
C M
0
b
nC
! M
.nC1/C
a
C M
0
b
.n1/C
C U
followed by a corresponding return transfer of the electron from M
0
b
back to
M
a
to complete the exchange. Here U is for the general case of dissimilar ions
of the same valence charge. The term correlation is frequently used to describe
the antiparallel spin alignment that occurs cooperatively on opposite sides of the
cation–anion covalent bonds through virtual transfer mentioned qualitatively in
Sect. 3.1.1 and confirmed by the analytical formalism [5–10].
2. One-electron semicovalent exchange occurs between dissimilar ions M and
M
0
(with d -shell occupancy differing by only one electron spin) through real
spin delocalization between half-filled ! empty or filled ! half-filled orbital
combinations:
M
nC
a
C M
0
b
.nC1/
C!M
.nC1/C
a
C M
0
b
nC
C U ˙ U
ex
;
where U
ex
is the adjustment for internal exchange energy of spin stabiliza-
tion (or destabilization if a spin flip is required) to comply with Hund’s rule
where unpaired spins occupy other states of the 3d shell, shown schematically in
Fig. 3.10b. If the spin transfers within a ferromagnetic configuration, the M
b
state
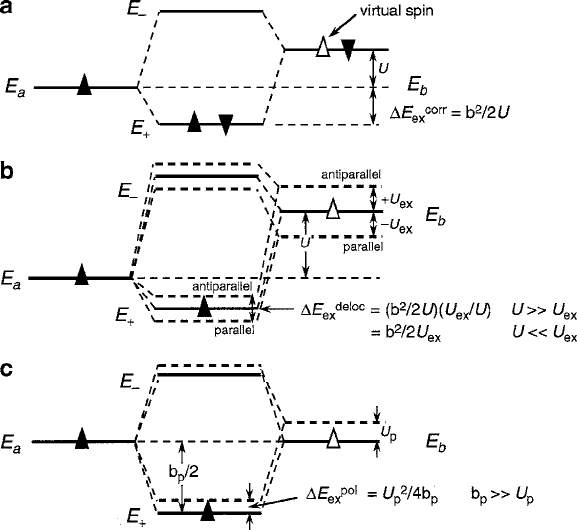
3.1 Interionic Magnetic Exchange 123
Fig. 3.10 Molecular-orbital diagrams indicating the origin of the exchange stabilization with
Aufbau spin order preferences indicated for: (a) of two-electron correlation antiferromagnetism,
(b) one-electron delocalization ferromagnetism (general case), and (c) one-electron delocalization
ferromagnetism, special case of mixed-valence charge transfer
becomes more stabilized by U
ex
, which will tend to offset U .However,ifthespin
transfers within an antiparallel configuration, the M
b
state becomes destabilized
by a similar energy.
General semicovalent exchange (Case 2) where the ions have different electron
configurations results from the delocalization of a single electron as described by
(2.46). Note that in its pure molecular form it is basically the H
C
2
molecule ion,
i.e., a one-electron case, but with U
ex
added. This situation can occur with ions
of different atomic elements, e.g., Mn
3C
d
4
and Cr
3C
d
3
in octahedral ligand
coordinations. The energy reduction from the sharing of an electron between a half-
filled/empty hybrid state is also given by (3.16), but with half of the energy because
of one electron instead of two involved in the exchange. Since there is no a pri-
ori requirement for spin polarization, i.e., no electron repulsion within the hybrid
wavefunction, the amount of energy contributed by the transfer of the single spin
will be influenced by the established alignment of the other spins occupying the or-
bital states involved. However, if a spin flip is necessary to complete the transfer in
compliance with Hund’s rule, the ionic transfer activation energy U
MM
0
would be
increased instead of reduced by U
ex
, thereby resulting in a lowering of the transfer
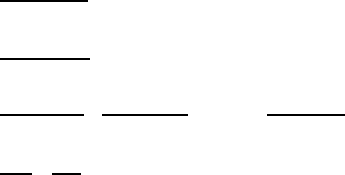
124 3 Magnetic Exchange in Oxides
probability and ferromagnetic exchange stabilization, as diagrammed in Fig. 3.10c.
The magnitudes of the respective delocalization exchange energies are given by
ˇ
ˇ
E
deloc
ex
ˇ
ˇ
b
2
.U U
ex
/
.ferromagnetic system/
ˇ
ˇ
E
deloc
ex
ˇ
ˇ
b
2
.U C U
ex
/
.antiferromagnetic system/
ˇ
ˇ
E
deloc
ex
ˇ
ˇ
b
2
.U U
ex
/
b
2
.U C U
ex
/
D 2b
2
U
ex
U
2
U
2
ex
(3.20)
2b
2
U
U
ex
U
for U
2
ex
<< U
2
in agreement with Goodenough’s VB result [23]. As discussed in connection with
real spin transfer, E
deloc
ex
represents an exchange trap energy for a mobile spin, e.g.,
a polaron with activation energy U
ex
¤ 0. Typical values of the relevant parameters
are b 2 eV, U 15 eV, and U
ex
2 eV (Slater integral), which permit the
U
2
ex
=U
2
<< 1 requirement to be satisfied. If the initial energy of M
b
is restored in
the process by an accommodation of the spin orientation, U
ex
then represents the
height of a barrier between two identical traps to be surmounted before a lossless
transfer is complete. With only one electron involved in the stabilization energy, the
MO orbital model can be used without loss of rigor.
In a special situation of Case 2 the combined energy of the initial and final
states of mixed-valence M
a
and M
b
are unchanged in an adiabatic transfer event,
with the ions essentially exchanging positions in the lattice. In this situation, the
effect is called “double exchange” and occurs according to M
nC
a
C M
.nC1/C
b
$
M
.nC1/C
a
M
nC
b
that is basically ferromagnetic as diagrammed in Fig.3.10c. How-
ever, the spin transfer still requires a mobility that is limited by an activation energy.
In real situations, the spin transfer is between mixed-valence ions of the same atomic
element, e.g., Mn
3C
d
4
and Mn
4C
d
3
or Fe
2C
d
6
and Fe
3C
d
5
.Themain
interest in these combinations is the promotion of ferromagnetism through spin
transfer (or vice-versa) by means of polaron carriers with mobility that is activated
by thermal hopping of electrons across energy barriers of height (or traps of depth)
generically defined as U
p
D U
0
C U
ex
. In special cases where exchange transfer
is strong and the trap depth is small, these effects can also be realized by covalent
tunneling through the bonding ground states. Here U
0
represents the trap energy
of a small polaron that results from adjustments of the local electrostatic (elastic)
environment [23].
For analytical purposes it is appropriate to introduce a polaron transfer integral
b
p
.Db
ab
/ integral. For the case of b
p
>> U
p
, which is of interest for polarized spin
transport, the magnitudes of the contributions can then be deduced from the one-
electron molecular orbital solutions for double exchange from (2.46), noting that the
energies E
a
E
b
D E
F
/2 in this limit. Here the extent of the exchange stabilization
that occurs because of the mutual ownership of the electron spin is determined by
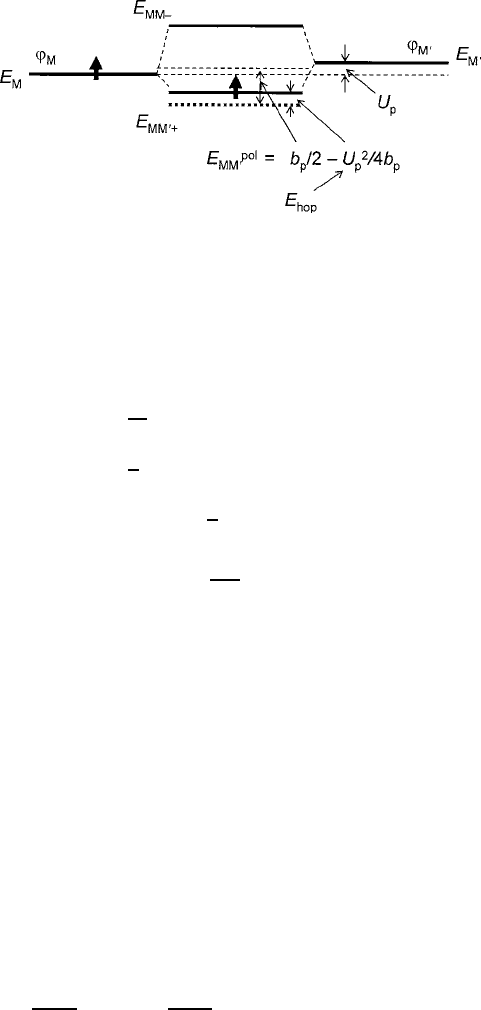
3.1 Interionic Magnetic Exchange 125
Fig. 3.11 Model of polaronic spin transfer indicating the origin of the E
hop
activation energy
the degree of sharing. When U
p
appears, both the sharing and stabilization decrease
accordingly. For a pair of identical orbital states, the analysis in Sect. 3.1.1 can be
applied to obtain the following relations
E
pol
ex
b
p
2
.for U
p
D 0/;
E
pol
ex
1
2
b
2
p
C U
2
p
1=2
for U
p
>0
;
E
hop
D E
pol
ex
1
2
b
2
p
CU
2
p
1=2
b
p
(3.21)
U
2
p
4b
p
for b
p
>> U
p
;
where E
hop
is the effective polaron trap energy after U
p
is reduced by the transfer
integral b
p
in proportion to the ratio U
p
=b
p
. The concept of polaronic electron spin
transfer is diagrammed schematically in Fig. 3.11; for a filled to half-filled hole
transfer, the mechanism is equivalent. This topic will be examined in greater detail
in Chap. 8.
3.1.5 Goodenough–Kanamori Rules
On the basis of the exchange energies of individual molecular-orbital states, rela-
tions for the total ionic exchange constants can be deduced by applying (3.14)to
combine the contributions from every occupied state. To this end, we define a resul-
tant exchange constant between ions i and j :
J
ij
D
1
2S
i
S
j
E
ex
D
1
2S
i
S
j
X
E
virtual
ex
X
E
deloc
ex
; (3.22)
126 3 Magnetic Exchange in Oxides
where E
virtual
ex
and E
deloc
ex
are for each individual orbital state and are positive
quantities by definition. In most cases, the ferromagnetic influence of the orbital
states linked by the single-electron transfers competes with the antiferromagnetism
of any lower energy half-filled orbitals. In octahedral sites, where t
2g
orbitals form
weaker bonds and e
g
stronger ¢ bonds, the resultant magnetic alignment will de-
pend on the particular ion combination. A more typical example is that of a d
4
–d
3
coupling between ions of the same atomic element. Here the ferromagnetic stabi-
lization of the single electron in the e
g
–p¢ state is offset by the antiferromagnetic
energies of the six t
2g
–p electrons. The resultant spin alignments of these particu-
lar ions can assume parallel or antiparallel configurations depending on a variety of
factors [3].
For any superexchange combination M
1
–O–M
2
, estimates of the J
12
constant
can be made if values can be assigned to the b
n
integral and excitation energy U
n
for each participating orbital state. In analytical terms, we express (3.15)as
J
12
D
1
2S
1
S
2
X
n
b
2
n
U
n
: (3.23)
This relation applies to any combination of 3d
n
ions. To make calculations, it must
first be realized that three of the five d orbitals are t
2g
–p and the other two are
e
g
–p¢. The distinction is important because the overlap integral
is significantly
less than
¢
. Estimates of these contributions that are made in Appendix 3A suggest
that b
2
=U
Wb
2
¢
=U
¢
0:1.
With this ratio one can construct models for individual ion combinations to pre-
dict the likely net superexchange interaction. As an example, the relation of (3.23)
can be applied to the d
3
–d
4
case
t
3
2g
e
0
g
to t
3
2g
e
1
g
occupying adjoining octahedral
sites:
J
virtual
12
1
2S
1
S
2
3b
2
U
.antiferromagnetic/; (3.24)
or and for the single e
g
spin
J
deloc
12
C
1
2S
1
S
2
b
2
¢
U
.ferromagnetic/; (3.25)
which yields J
12
.antiferro/=J
12
.ferro/ –0:33 if the above ratio of b
2
=U
W
b
2
¢
=U
¢
is used.
4
Since the spin system can stabilize with either alignment, the
greater stabilization energy will determine which one will dominate, i.e., which one
will provide the larger spin-ordering temperature. The result for the above example
suggests a condition of ferromagnetism dominated by the delocalization exchange
from only one of the e
g
–p¢ bonds.
4
Note that if only one electron is stabilized in a semicovalent transfer, it could be argued that the
E
virtual
ex
=E
deloc
ex
is double at 0.2, which would result in J
antiferro
12
=J
ferro
12
0:66.
3.1 Interionic Magnetic Exchange 127
By pursuing this reasoning to evaluate the probable spin alignments of various
combinations favored by particular cation–anion–cation linkages, a catalog of su-
perexchange interactions among members of the 3d
n
series can be created in-
cluding relative strengths of the expected J
12
constants. Such endeavors were
undertaken independently by Goodenough and Kanamori with results that have be-
come such valuable guidelines for the chemical design of magnetic compounds that
the Goodenough–Kanamori rules have been added to the lexicon. In Table 3.2,a
compilation of superexchange interactions based on Kanamori’s review [24]isre-
produced with some additions. The listing is restricted to some of the more standard
combinations, as examined by Anderson [6], Goodenough [25, 26], Anderson and
Hasegawa [18], and Slater [27]. Those with the same ionic valence are probably
more credible than the others because of more consistent values anticipated for b
and U .
Table 3.2 Summary of spin alignment estimates of d
n
ion 180
ı
superexchange interactions in
octahedral sites (after Kanamori [24])
d
n
–d
n
0
Probable
combinations Cations Bond mechanism Spin alignment result
d
3
–d
3
Mn
4C
–Mn
4C
¢, -bonds
Cr
3C
–Cr
3C
A, G, A–H, S AF AF
d
8
–d
8
Ni
2C
–Ni
2C
¢-bonds
A, G, A–H, S AF AF
d
5
–d
5
Mn
2C
–Mn
2C
¢-bonds
Fe
3C
–Fe
3C
A, G, A–H, S AF AF
-bonds AF(weak)
G, A–H, S Uncertain
-bonds (Weak)
A
d
8
–d
3
Ni
2C
–V
2C
¢, -bonds
Ni
2C
–Cr
3C
A, G, A–H, S F F
d
5
–d
3
Fe
3C
–Cr
3C
¢-bonds
Mn
2C
–V
2C
A, G, A–H, S F F
Fe
3C
–V
2C
-bonds AF(weak)
Mn
2C
–Cr
3C
G, A–H Uncertain
-bonds (Weak)
A, S
d
4
–d
4
Mn
3C
–Mn
3C
Bond angle
Mn
3C
–Fe
4C
Dependent
d
6
–d
6
Fe
2C
–Fe
2C
¢-bonds
(e.g., FeO) A, G, A–H, S AF AF
Co
3C
–Co
3C
-bonds Uncertain
(e.g., Co
2
O
a
3
(Weak)
d
7
–d
7
Co
2C
–Co
2C
¢-bonds
(e.g., CoO) A, G, A–H, S AF AF
-bonds Bond-angle dependent
A Anderson mechanism; G Goodenough mechanism; A–H Anderson-Hasegawa mechanism;
S Slater mechanism
a
Not 180
ı
bond angles
128 3 Magnetic Exchange in Oxides
Table 3.3 Spin ordering of d
n
ions in octahedral oxygen cites, high-spin states,
180
ı
bonds (after Goodenough [28])
A more comprehensive study of cation combinations including those involving
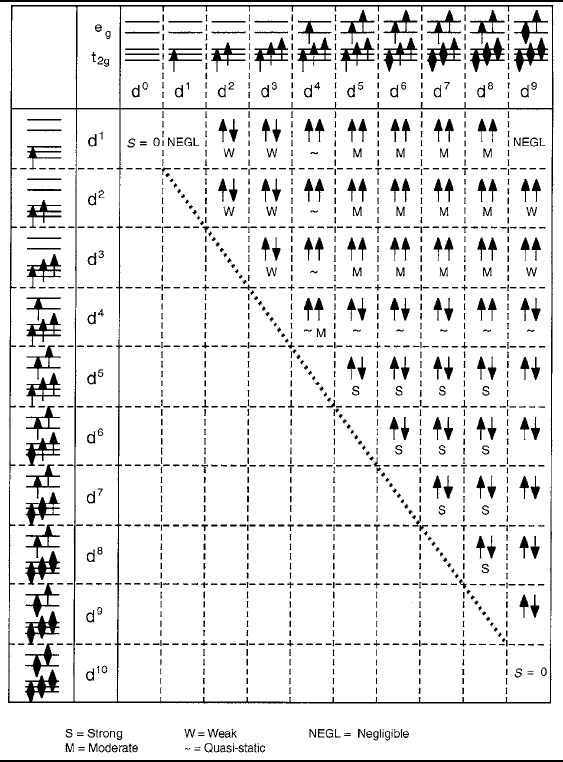
low-spin configurations is given by Goodenough [28]. In Table 3.3 results for 180
ı
bonds between cations in octahedral sites are presented. It is important, however, to
recognize that the spin ordering temperatures could be meaningful only where a ho-
mogeneous magnetic lattice is created. In most of these situations, particularly those
involving combinations far from the diagonal or of widely differing cation valences,
the actual materials structures may not be thermodynamically stable and may be
fashioned only through artificial procedures such as molecular beam epitaxial layer
growth. To estimate corresponding N´eel or Curie temperatures, the proper relations
between the spin values and the number of exchange coupled nearest neighbors must
3.2 Antiferromagnetism 129
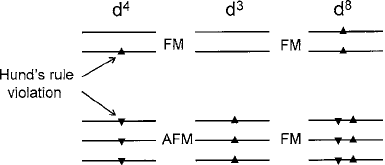
Fig. 3.12 Model of the
Goodenough–Kanamori rules
of spin alignment for d
4
, d
3
,
and d
8
configurations in
octahedral sites. E
g
shell spin
reversal in d
4
is shown
intentionally to illustrate a
Hund’s rule violation
be selected for each pair of cations. An example of the application of these rules is
offered by the Aufbau models of superexchange couplings between the aforemen-
tioned d
4
–d
3
and d
8
–d
3
combinations sketched in Fig.3.12. As pointed out above,
the former favors ferromagnetism in one state of the e
g
shell that is opposed by all
three antiferromagnetic virtual states of the t
2g
shell. In a charge-ordereddouble per-
ovskite lattice, e.g., La
3C
Ca
2C
Mn
3C
Mn
4C
O
6
, the result is largely inconclusive.
With Ni
2C
d
8
in place of Mn
4C
, however, all five orbital levels produce ferro-
magnetism that results in a Curie temperature near 300 K [29]. This subject will be
addressed further in Sect. 3.3.
Before the topic of the effective magnetic fields created by superexchange is
examined in the context of antiferromagnetism and ferrimagnetism, the implications
of tetrahedral sites and non 180
ı
bond angles should be mentioned. Recalling the
discussion of the crystal field parameter 10Dq and how it reverses sign between
octahedral and tetrahedral sites, we immediately recognize that the relative strengths
of and ¢ bonds should be expected to change since the locations of the negative
anion charges are in the directions of the body diagonals in the tetrahedral case.
Where the bonds form other than 180
ı
angles, the influence of the t
2g
orbitals is
likely to increase relative to the e
g
orbitals. These subtleties will be discussed further
as the need arises in later chapters.
3.2 Antiferromagnetism
Because superexchange is generally associated with antiparallel spins, magnetic
oxides usually feature antiferromagnetic ordering. At this point in our discussion,
it is necessary to return to the subject of molecular fields introduced in Sect. 1.3
in preparation for a more detailed account of the opposing sublattice theories of
antiferro and ferrimagnetism.
3.2.1 Superexchange and Molecular Fields
One of the most important results of the insights gained into the relation between
covalent bonding and superexchange is the increased understanding of the origins
