Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.

170 4 Ferrimagnetism
Table 4.2 Molecular field coefficients of nickel and manganese spinel ferrite (determined
semiempirically)
Fe
3C
Ni
2C
Fe
3C
O
4
Mn
0:8
2C
Fe
0:2
3C
Mn
0:2
2C
Fe
1:8
3C
O
4
Fe–Fe Fe–Ni Ni–Ni Fe–Fe Fe–Mn Mn–Mn
mol=cm
3
mol=cm
3
mol=cm
3
mol=cm
3
mol=cm
3
mol=cm
3
N
AA
–200 – – 200 180 160
N
BB
–60 –58 –60 60 59 58
N
AB
312 276 – 312 187 62
Table 4.3 Magnetic parameters of common spinel and garnet cubic ferrites
4M
s
.
0
/
4M
s
.
300 K
/
T
C
.
exp:
/
Compound n
B
.
tet
/
n
B
.
oct
/
n
B
(theor.) (G) (G) (K)
SPINEL
Fe
3
O
4
5 5 C 4 4 6,400 6,000 858
”Fe
2
O
a
3
58.33.3 5;000 4;500 948
ZnFe
2
O
4
0 5 5 0 (antiferro) paramag paramag –
CdFe
2
O
4
0 5 5 0 (antiferro) paramag paramag –
MnFe
2
O
b
4
5 5 C 5 5 7,000 5,000 573
CoFe
2
O
4
5 5 C 3 3 6,000 5,300 793
NiFe
2
O
4
5 5 C 2 2 3,800 3,400 858
CuFe
2
O
4
5 5 C 1 5 7,000 5,000 728
MgFe
2
O
c
4
5 5 C 0 0 1,800 1,500 713
Li
0:5
Fe
1:5
O
4
5 5 C 2:5 2.5 4,200 3,900 943
GARNET
Y
3
Fe
5
O
12
15 10 5 2,400 1,800 560
Typical spinel lattice parameter a
o
8:4
˚
A; density 4:5–5:5 gm=cm
3
; typical garnet (YIG)
lattice parameter a
o
12:4
˚
A; density 5:17 gm=cm
3
a
A defect spinel structure that is called maghemite and written as Fe
3C
h
1=3
Fe
3C
2=3
i
O
4
,where
represents a cation site vacancy. A more common antiferromagnetic nonspinel form is known
as hematite with designation ’Fe
2
O
3
b
Mn
2C
ions occupy the tetrahedral sublattice in the amount of 20%. The anomalously low Curie
temperature is caused by very weak
N
AB
interaction involving Mn
2C
c
The experimentally observed magnetic moment is attributed to upwards of 10% of the Mg
2C
ions
occupying the tetrahedral sublattice thereby placing more Fe
3C
in the octahedral sublattice
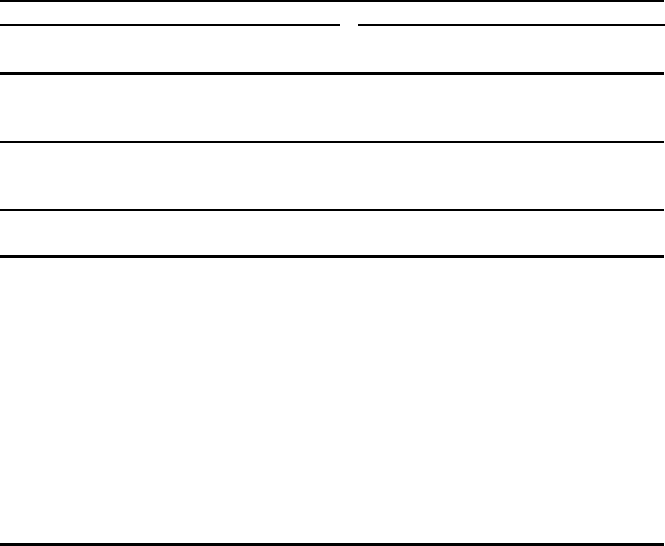
based on the experimental parameter values listed in Tables 4.2 and 4.3.InFig.4.10,
thermomagnetization characteristics from measurements are compared with the re-
sults of computations in which the molecular orbital exchange concepts developed
in Sect. 3.1 were applied to the spinel ferrite family [32]. The corresponding param-
eter values used for the calculations are listed in Table 4.4.
Magnetite (lodestone) is a naturally occurring normal spinel of chemical formula
Fe
3C
Fe
3C
Fe
2C
O
4
. Because the electrical conductivity introduced by the full
complement of octahedral-site Fe
2C
ions that can transfer electrons among the Fe
3C
neighbors (a process called polaronic electron hopping), magnetite is more a vehicle
for studying the electrical properties of oxides than a practical magnetic insulator
4.3 Ferrimagnetic Oxides 171
Fig. 4.10 Thermomagnetism characteristics of basic spinel ferrites: (a) measured curves inspired
by Fig. 32.7 in reference [24], and (b) computations by a molecular-orbital model [32]
Table 4.4 Spinel ferrite molecular field coefficients (determined from molecular-orbital theory)
B-site Ion-S
M
N
AA
mol=cm
3
N
BB
mol=cm
3
N
AB
mol=cm
3
Li
1C
Fe
3C
5=2 150 60 C273
Fe
3C
5=2 200 60 C300
”-Fe
3C
5=2 170 60 C282
Mn
2C
5=2
a
135 51 C178
Mn
2C
5=2
b
200 32 C222
Fe
2C
2 200 25
c
C240
Co
2C
3=2 200 58
c
C269
Ni
2C
1 200 90
c
C330
a
Standard site distribution Fe
0:2
3C
Mn
0:8
2C
Mn
0:2
2C
Fe
1:8
3C
O
4
b
Fully inverted site distribution Fe
3C
Mn
2C
Fe
3C
O
4
c
Values are corrected for t
2g
–t
2g
ferromagnetic direct exchange which decreases monotonically as
the shell fills with paired spins. For Fe
3C
Fe
2C
Fe
3C
O
4
(magnetite), N
BB
was adjusted to 25
from 44 mol=cm
3
to account for ferromagnetic double exchange
[33]. It is also one of the best hosts for studying the magnetic contribution of the
Fe
2C
ion, which can cause important magnetoelastic and dielectric effects. One in-
teresting feature of this compound is the order-disorder transition of Fe
2C
–Fe
3C
ions in the B sublattice that occurs at T 120 K. Below this temperature, the crys-
tal structure undergoes a phase transition from cubic to orthorhombic symmetry,
lending further credence to the proposition that the magnetoelastic Fe
2C
3d
6
ions
undergo a J–T or S–O condensation that stabilizes the transfer electrons in their po-
laronic traps and causes an anomalous decrease in conductivity. This general subject
is discussed further in Chap. 8.
Lithium ferrite contains the most iron ions next to magnetite and is the ferrite
compound with the largest Curie temperature .T
C
940 K/. As can be recognized
immediately from the chemical formula Fe ŒLi
0:5
Fe
1:5
O
4
, 25% of the B sublat-
tice is diluted by the Li
1C
ions. Nonetheless, the net number of Bohr magnetons
is 2.5, which is sufficient to produce a room-temperature 4M 3;500 G. To
lower the moment, magnetic dilution can be accomplished by direct Al
3C
in the
B sublattice or more commonly by substitutions of 0:5Li
1C
C Ti
4C
! 1:5Fe
3C
with chemical formula Fe
1t=2
Li
t=2
ŒLi
0:5
Fe
1:5t
Ti
t
O
4
. As the site distributions
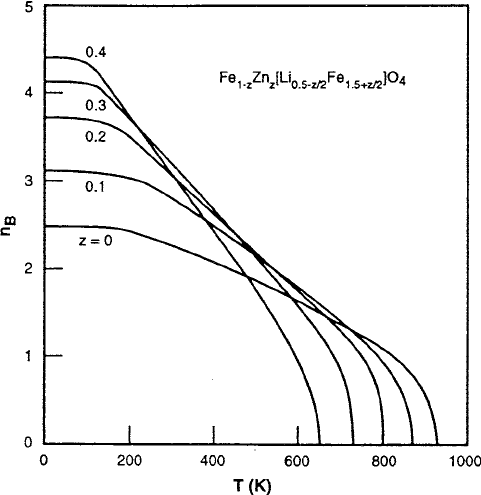
172 4 Ferrimagnetism
Fig. 4.11 Calculated lithium-zinc ferrite thermomagnetization curves
indicate, when the B sublattice is diluted with Ti
4C
ions, the extra Li
1C
ions
required for electrical charge neutralization replace Fe
3C
ions in the A sites. To in-
crease the moment, Zn
2C
! 0:5Fe
3C
C0:5Li
1C
are substituted into the A sublattice
according to Fe
1z
Zn
z
Li
0:5z=2
Fe
1:5Cz=2
O
4
. This modification also increases the
Fe
3C
content of the B sublattice, producing the initial rise in magnetization shown
in Fig. 4.4, but it also causes a reduction in T
C
because of the decreased net num-
ber of Fe
3C
A
–Fe
3C
B
interactions shown in the curves of Fig. 4.11. The results are
from a molecular field analysis [23] and are also listed in Table 4.1 and discussed
in Appendix 4A. The uses of Li ferrite have become mainly in microwave appli-
cations because of its high T
C
made possible by the larger amounts of Fe
3C
in
both sublattices and generally good dielectric properties in the microwave bands.
Its higher 4M capabilities make it particularly attractive in the millimeter-wave
bands (above 35-GHz frequencies). The development of Li ferrite of good ceramic
quality was delayed by the high volatility of Li. When these materials are subjected
to temperatures above 1;000
ı
C, Li
2
O is lost and the compound becomes iron-rich
with the result that diamagnetic ’-Fe
2
O
3
hematite phase precipitates and Fe
3C
is
reduced to Fe
2C
in the B sublattice, causing charge transfer that lowers the resistiv-
ity. To make dense microwave-quality Li ferrite, bismuth oxide .Bi
2
O
3
/ is added as
a flux to lower sintering temperatures [34]. Unfortunately, the tendency of Bi
2
O
3
to
segregate along grain boundaries also causes a deterioration in mechanical integrity.
4.3 Ferrimagnetic Oxides 173
Nickel ferrite Fe
3C
Ni
2C
Fe
3C
O
4
is an inverted and generally well-behaved
spinel with Ni
2C
occupying almost half of the octahedral B sublattice. A small
amount .<5%/ can be expected to find its way into the tetrahedral A sites under
thermal equilibrium conditions, causing magnetoelastic and spin–lattice relaxation
effects. Because Ni
2C
carries a spin value S D 1 in addition to an effective Land´e
g factor greater than 2 (see Sect. 5.1) nickel ferrite can offer higher magnetizations.
Since Ni
3C
occurs only occasionally and in special situations (e.g., Li
1C
-substituted
NiO) [35, 36], the incidence of Fe
2C
is usually negligible, and dielectric prop-
erties are excellent for microwave applications. Because the e
g
orbitals are half
filled, this ion presents a significant covalent transfer integral b and consequently
a strong superexchange coupling to neighbors of its own kind and to the adja-
cent Fe
3C
ions of both sublattices. Similar to Li ferrite its magnetic moment can
be reduced, in this case typically by direct substitutions of Al
3C
for Fe
3C
ions,
which initially favor B-site dilution according to the theoretical analysis reported by
Borghese [12].
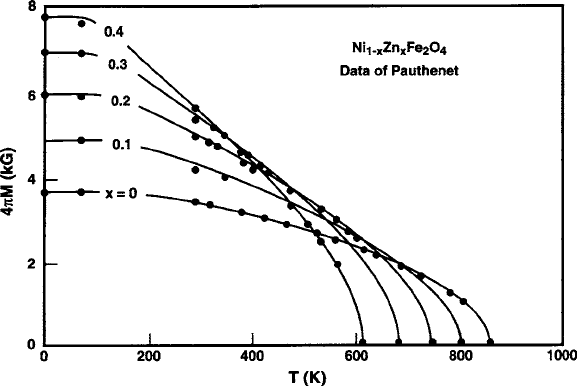
The magnetic moment can also be raised by the Zn
2C
ions diluting the A sublat-
tice (similar to the LiZn ferrite case shown in Fig. 4.4, with the attendant increase in
the sensitivity of 4M to temperature resulting from the decrease in T
C
,asshownin
Fig. 4.12 [37]. The peak maximum magnetization of 5;000 G at room temperature
can be obtained with Zn replacing Ni, but occupying 35% of the tetrahedral sites,
i.e., Fe
3C
0:65
Zn
2C
0:35
Ni
2C
0:65
Fe
3C
1:35
O
4
. An additional benefit of Zn for ma-
terials preparation is a reduction in the solid-state reaction (sintering) temperature.
As examined in Chap. 5, a drawback to nickel ferrite can be the large negative
100
magnetostriction constant that renders the anisotropy and hysteresis-loop properties
Fig. 4.12 Thermomagnetism curves of nickel-zinc spinel ferrite, showing the effects of Zn
2C
dilution of the minority tetrahedral A sublattice. Note decrease in Curie temperatures. Data are
from Pauthenet [37]
174 4 Ferrimagnetism
of the material highly stress-sensitive. The origin of this property could be the
unquenched orbital momentum of the A-sublattice Ni
2C
ground state, which is dis-
cussed in Sect. 5.3. Manganese in the 3C state helps to compensate magnetostriction
through its local Jahn-Teller <100> axial expansion effect [38], and should be used
as an additive where square hysteresis loops are desired. Although the Curie tem-
perature of Ni ferrite is not as high as that of Li ferrite, the material in ceramic
form is generally considered to be of better quality in a microstructural sense, i.e.,
it is denser and has more uniform grain size. Because of these properties, as well
as because of its wide range of magnetization values, Ni ferrite ranks as one of the
most-used ferrite families.
Manganese ferrites are mainly normal with site distributions indicated by the
formula Mn
2C
0:8
Fe
3C
0:2
Mn
2C
0:2
Fe
3C
1:8
O
4
.TheMn
2C
ion has a larger radius
than most of the ions of the 3d
n
series, 0.80
˚
A instead of 0:65
˚
A. To accommo-
date this larger cation, the lattice parameter is increased with the result that some
of the b
2
=U factors of the covalent bonding are reduced, thereby increasing the
N
BB
coefficient leading to a Curie temperature that is the lowest of all of the basic
spinel ferrite families, as shown in Fig. 4.10. This effect is reflected in the values of
the molecular field coefficients N
ij
, which reveal weaker interactions that involve
Mn
2C
, as shown in Table 4.2 where large reductions occur in the N
AB
coefficients
for Fe-Mn and Mn-Mn [24]. These results support the conclusions of Simsa and
Brabers [39] that a canting angle of 54
ı
among the Mn
2C
ions in the B sublattice
accounts for an initial deficiency in the magnetic moment at T D 0 K.
For microwave applications, dielectric losses due to poor dielectric properties
stem from the charge transfer mechanism that stabilizes Fe
3C
in B sites, i.e.,
Fe
2C
C Mn
3C
! Fe
3C
C Mn
2C
. There are also two possible polaronic conduc-
tion opportunities here: Fe
2C
$ Fe
3C
C e
and Mn
2C
$ Mn
3C
e
. In general,
the Curie temperatures of this family are unacceptably low for applications with
power dissipation effects that are likely to increase the temperature. Similar to Li
and Ni ferrites, T
C
is reduced further when Zn
2C
is added to increase 4M .
Magnesium ferrite is mainly inverted with a typical formula Mg
2C
0:1
Fe
3C
0:9
Mg
2C
0:9
Fe
3C
1:1
O
4
. The magnetization and permeability are too low for most
low frequency applications, but the family has proven to be useful for certain mi-
crowave regimes in situations where the high temperature sensitivity is tolerable. In
these cases, manganese is added for the purpose of suppressing any formation of
Fe
2C
through the charge transfer equation Fe
2C
CMn
3C
! Fe
3C
CMn
2C
[40,41].
As a consequence, this family is usually referred to as the magnesium-manganese
ferrites. Final properties of ceramic versions are sensitive to the firing schedule be-
cause of the uncertain ionic site dispositions. The principal virtues of this hybrid
family are its square hysteresis loops and insensitivity to stress made possible by the
presence of Mn
3C
ions. The compensation of magnetostriction is believed to result
from a combination of J–T effects in B sites and a minority of S–O effects in A sites.
Cobalt ferrite is also an inverted spinel of formula Fe
3C
Co
2C
Fe
3C
O
4
, with
Co occupying the B sites. For reasons that will be explained in subsequent chapters,
this compound is of interest primarily for its magnetoelastic properties from which
the phenomena of magnetocrystalline anisotropy and magnetostriction arise. Like
Ni
2C
, it can also feature a g factor greater than 2 in octahedral sites. Divalent cobalt
4.3 Ferrimagnetic Oxides 175
in a B site is a classic spin-orbit stabilized ion that favors a compressive distortion
along the c axis of the ligand octahedron, analogous to Ni
2C
in tetrahedral sites.
Unfortunately, these properties are generally undesirable for many conventional ap-
plications. Moreover, the strong coupling of Co
2C
orbital states to the lattice is
responsible for large power losses when this ferrite is used as a microwave propaga-
tion medium. In small amounts, however, Co
2C
ions can be beneficial for adjusting
anisotropy and magnetostriction, and in controlling relaxation effects that influence
peak power limits.
Copper ferrite is another spinel that features a magnetoelastically active con-
stituent Cu
2C
, which like Mn
3C
is a classic Jahn-Teller ion in an octahedral site.
Also similar to Mn
3C
, magnetoelastic and relaxation effects from a small fraction of
Cu
2C
in tetrahedral sites would also be expected from unquenched L in the upper T
2
triplet. The formula is generally inverted, in the form of Fe
3C
Cu
2C
Fe
3C
O
4
,but
can in theory assume a monovalent state in Cu
1C
0:5
Fe
3C
2:5
O
4
analogous to Li fer-
rite, but with part of the Cu
1C
ions occupying tetrahedral A sites [31]. As in the case
of Ni
2C
and Co
2C
in octahedral sites, the g factor of the Cu
2C
ion can be greater
than 2, but because of its low spin value S D 1=2, Cu ferrites are not of high perme-
ability and are rarely considered for microwave applications because their chemistry
and microstructure in ceramic form are difficult to control. At high temperatures
copper has a volatility comparable to that of lithium. Sintering temperatures are
lower than typical for spinels, and the attainment of good stoichiometry is a chal-
lenge. Furthermore, because of the strong Jahn-Teller tendencies, the Cu
2C
ion can
drive the lattice symmetry tetragonal, as it does in Fe
3C
Cu
2C
Fe
3C
O
4
(and also
the superconducting cuprate perovskite host compounds such as La
3C
2
Cu
2C
O
4
),
and will also produce strong positive magnetostrictive effects when used as an ad-
ditive to cubic lattices. Another drawback is the polaronic conduction mechanisms
similar to those of Mn ferrite (Cu
2C
$ Cu
3C
C e
and Cu
2C
$ Cu
1C
e
)
[42]. One interesting possibility is the use of monovalent copper in A sites to pro-
duce higher magnetization compounds for microwave applications. This subject
is discussed in Appendix 4B. Table 4.5 lists a qualitative summary of spinel fer-
rite magnetization properties as well as magnetoelastic effects that are discussed in
Chap. 5.
4.3.2 Garnet Ferrites fc
3
g
Œ
a
2
.
d
3
/
O
12
The garnet family is the best-behaved chemically and structurally of all ferrimag-
netic oxides. From a magnetic standpoint, it is also the most thoroughly investigated.
Following the first synthesis of the iron garnets by Bertaut and Forrat [43], care-
ful analysis of the crystal structure was carried out by Geller and Gilleo [44, 45].
The three cation sites dodecahedral c, octahedral a, and tetrahedral d are shown in
relation to a section of the crystallographic unit cell in Fig. 4.13. It can be readily
seen that the principal symmetry axes of the a and d sites do not conform to the cu-
bic cell axes <100>, <111>,and<110>, in marked contrast to the spinels which
have the A and B sites neatly packed into the cubic cell. Only the octahedral site

176 4 Ferrimagnetism
Table 4.5 Initial effects of dilutant ions in inverted spinel ferrite A
3C
B
3C
B
2C
O
4
Ions Site M
s
jK
1
jjK
1
=M
s
jj
s
=K
1
j
Al
3C
,Ga
3C a
B
.
A
/
++ ––
Mg
2C b
B ++ ––
Li
1C
C 2Ti
4C c
A C 2B ++ ––
Zn
2C
A * – + –
Co
2C d
B – +
!
*+
!
**
!
+
Mn
3C e
B –– – +
!
*
Fe
2C f
B – ** ** –
a
Al
3C
and Ga
3C
occupy both octahedral and tetrahedral sites, but opposite to the garnets, they
initially have strong preference for the octahedral sites in the inverted spinels
b
Mg
2C
is the divalent-ion basis for the magnesium ferrite family
c
Li
1C
C2Ti
4C
combination is used in Li ferrite. The added Li
1C
replaces Fe
3C
in the tetrahedral
sites, but the net result is a lowering of M
s
by the 2Ti
4C
ions diluting the octahedral sublattice
d
Co
2C
is used in very small amounts to compensate K
1
. It will cause K
1
to pass from negative
to positive at about 0.01–0.02 ions per formula unit. Because it is spin-orbit stabilized, it is also a
fast-relaxing ion
e
Mn
3C
is a Jahn-Teller ion that influences magnetostriction by adding a positive contribution to
the large
100
constant that makes the average value
s
pass through zero at a concentration of
about 0.2 ions per formula unit
f
Fe
2C
is a spin-orbit stabilized ion that is a natural constituent of spinel ferrites. It enhances the
negative anisotropy constant by favoring the h111i-axis extensions, and thereby increases the
111
magnetostriction constant. In combination with host Fe
3C
ions, it can provide a hopping electron
conduction mechanism that is detrimental to high-frequency applications
has one of its trigonal axes aligned with a lattice <111> direction [46]. An even
more important difference is that the tetrahedral sites are more numerous than the
octahedral sites in the ratio of 3:2, thereby making the d sublattice dominant in con-
tributing to the magnetization. As will be shown in Chap. 5, this feature allows the
magnetoelastic properties to be varied over a wider range.
Dielectric properties of the garnets are generally superior to those of the spinels,
due in part because all of the cations are naturally in the 3C state, thereby affording
less opportunity for Fe
2C
to form. Magnetocrystalline anisotropy and magnetostric-
tion are also manageable quantities. Moreover, there are a plethora of substitutions
available for all three sites that may be used to tailor magnetic, microwave, and op-
tical properties. Because the system is more refractory than the spinels, chemical
stability is generally higher among conventional compounds, i.e., those based on yt-
trium or lanthanum series ions in the c sublattice. Garnet systems with lower sinter-
ing temperatures are the families with cation combinations
f
Y; Ca
g
ŒFe; Zr.Fe; V/
and
f
Bi; Ca
g
ŒFe; Zr.Fe; V/, and are used where lower magnetizations are accept-
able and the high sublimation rate of Bi at solid reaction or sintering temperatures
can be tolerated.
Yttrium iron garnet is the basic magnetic host of this family, and can be altered by
a variety of substitutions. Al
3C
and Ga
3C
ions are used to reduce the magnetization
by forming solid solutions of Y
3
Fe
5
O
12
(YIG) with either Y
3
Al
5
O
12
(YAG) or
Y
3
Ga
5
O
12
(YGG). With these two ions, the dilution begins in the d sublattice and
gradually spills over to the a sites [47,48]. For this reason Al
3C
(or Ga
3C
)areused
to reduce the magnetization, as shown in Gilleo’s data [16]ofFig.4.14. To increase
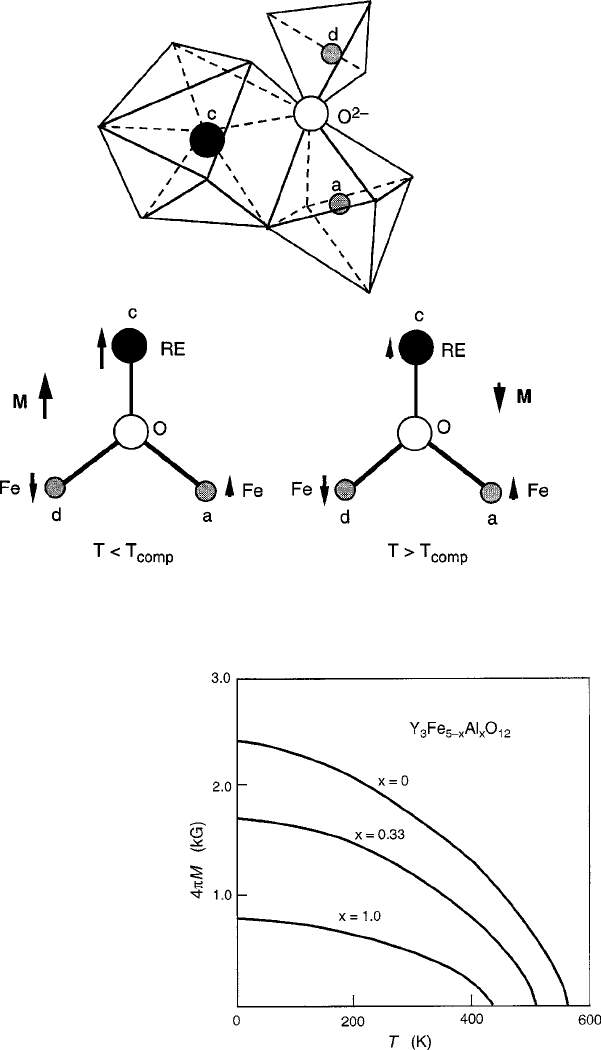
4.3 Ferrimagnetic Oxides 177
Fig. 4.13 Garnet crystal structure with bond angle diagrams, below and above the compensation
temperature T
comp
that occurs when the c-sublattice is occupied by heavy rare-earth ions of the
4f
n
series
Fig. 4.14 Thermomagnetism
curves of yttrium aluminum
iron garnet ferrite, showing
the effects of Al
3C
net
dilution of the majority
tetrahedral d sublattice. Data
are from Gilleo and Geller
[16]
178 4 Ferrimagnetism
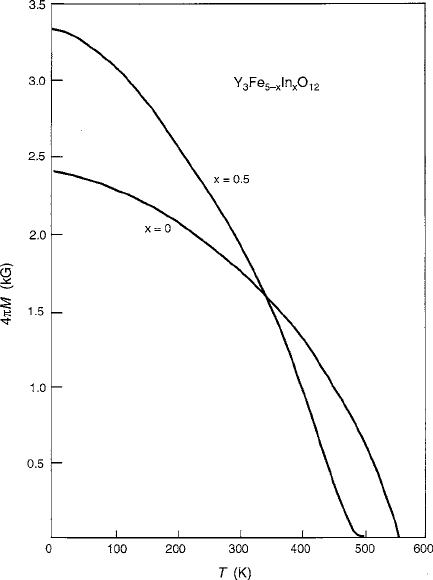
Fig. 4.15 Thermomagnetism curves of yttrium indium iron garnet ferrite, showing the effects of
In
3C
dilution of the minority octahedral a sublattice. Compare with Fig. 4.14. Data are from Gilleo
and Geller [16]
magnetization, the larger ions In
3C
(or Sc
3C
) can be used up to a concentration level
of about 0.5 per formula unit because they occupy a sites almost exclusively. With
the greater reduction in the number of J
ad
exchange couplings by a-site dilution;
however, the Curie temperature drops quickly with dilution, similar to the case of
Zn in the A sites of spinels. Examples of these effects are shown in the data of
Fig. 4.15.
Yttrium-calcium iron garnet is a common modification of the basic YIG compo-
sition that has been considered for use in special microwave and magneto-optical
applications with vanadium diluting d sites or zirconium a sites. The advantages of
this family lie in two features: (1) V
5C
and Zr
4C
occupy exclusively their respective
sites, which means that the adjustments in magnetization can be achieved without
unwanted dilution of the opposite sublattice that would cause a further decrease in
Curie temperature; and (2) calcium, vanadium, and zirconium are less expensive
chemicals than the alternative yttrium, lanthanum, indium, or scandium. A further
benefit of the use of calcium actually comes from the reduced amounts of yttrium

4.3 Ferrimagnetic Oxides 179
Table 4.6 Initial effects of dilutant ions in garnet ferrites
n
c
3C
3
oh
a
3C
2
i
d
3C
3
O
12
Ions Site M
s
jK
1
jjK
1
=M
s
jj
s
=K
1
j
Al
3C
,Ga
3C a
d
.
a
/
++ +, * –
2Ca
2C
C V
5C b
2c C d ++ + –
Ca
2C
C Ge
4C b
c C d ++ + –
Ca
2C
C Si
4C b
c C d ++ + –
In
3C
,Sc
3C b
a
Ca
2C
C Zr
4C b
c C a *+ ++ *
Ca
2C
C Sn
4C b
c C a *+ ++ *
Co
2C
C Si
4C c
a C d *+ ++ *
Mn
3C d
a –– – +
!
*
Bi
3C
,Pb
2C e
c – +
!
*+
!
**
!
+
Fe
2C f
a ** ** – **
a
Al
3C
and Ga
3C
occupy both octahedral and tetrahedral sites, but initially have strong preference
for the tetrahedral sites in the garnets. Ga
3C
reduces M
s
more efficiently and can raise K
1
=M
s
b
Sr
2C
can be used instead of Ca
2C
in c sites, as well as La
3C
instead of Y
3C
for lattice parameter
adjustments
c
Co
2C
is used in very small amounts to compensate K
1
. It will cause K
1
to pass from negative
to positive at about 0.02 ions per formula unit. Because it is spin-orbit stabilized, it is also a fast-
relaxing ion
d
Mn
3C
causes a Jahn-Teller distortion that adds a positive contribution to the magnetostriction
constants and makes the average value pass through zero at a concentration between 0.05 and 0.1
ions per garnet formula unit
e
Bi
3C
and Pb
2C
are important for magnetooptical applications but can increase microwave losses
f
Fe
2C
is a spin-orbit stabilized ion that occurs as a natural impurity. It enhances the negative
anisotropy constant by favoring the h111i-axis extensions, and thereby increases the
111
mag-
netostriction constant. In combination with host Fe
3C
ions, it can provide a hopping electron
conduction mechanism that is detrimental to high-frequency applications
or lanthanum because these rare-earth elements can contain fast-relaxing impurities
that are magnetically lossy to microwaves. One other ion that is used particularly for
magneto-optical application is Bi
3C
for reasons that will be explained in Chap. 7.
This ion, however, is extremely large and does not freely substitute for Y
3C
over
the full range. In fact, the compound Bi
3
Fe
5
O
12
(BIG) may exist only as films of
submicron thickness scale [49].
Table 4.6 presents a qualitative summary of magnetic and magnetoelastic prop-
erties of YIG-based garnets similar to that given for the spinels in Table 4.5.Itis
appropriate to mention at this point that in all of the magnetic garnets and spinels,
magnetoelastic properties can be tailored by select ions identified previously. To re-
duce the negative anisotropy, very small amounts of Co
2C
paired with a tetravalent
diamagnetic ion can be substituted. To offset the negative magnetostriction, Mn
3C
ions can be substituted directly for Fe
3C
. This subject will be discussed in Chap. 5.
Other ions of the lanthanide group can be used generously in the c sublattice to
form what amounts to a distinct set of ferrimagnetic compounds. Because the ionic
spins that are introduced to the c sublattice exchange couple to the iron in the d and
a sublattices, a wide variety of thermomagnetic, magnetoelastic, microwave, and
optical properties can be created in these rare-earth iron garnets.
