Dionne G.F. Magnetic Oxides
Подождите немного. Документ загружается.


Chapter 5
Anisotropy and Magnetoelastic Properties
In this chapter, we discuss the local origins of the two measurable macroscopic
effects that occur from interactions between the ionic magnetic moments and the
lattices in which they reside: magnetocrystalline anisotropy and magnetostriction.
In the preceding chapters, the focus has been on the molecular origin of the mag-
netic moments in crystal lattices. For the 3d
n
transition group in particular, the
disposition of spin alignments as determined by covalent-induced superexchange
and the randomizing effect of temperature has been reviewed. The spin system is
also influenced by geometrical shape of the specimen in which it resides (described
in Chap.1) and the symmetry of the lattice itself and its elastic properties, each of
which contribute to the anisotropy that influences the magnetization process and
other magnetic properties. In addition, large anisotropic magnetic effects can result
from asymmetry of the local crystal fields and their interactions with magnetoelastic
cations. In this sense, magnetoelasticity refers to the coupling between the magnetic
moment of the cation and local crystal field of the anion coordination. All of these
mechanisms, however, involve interaction between the spins and the elastic proper-
ties of the lattice, which can be collective, as in the case of dipole–dipoleinteractions
in fixed array of lattice sites, or individual through orbital angular momentum cou-
pling to the crystal field. The conventional macroscopic phenomenological model
is presented later in this chapter, but it is the molecular origins of these properties
where our initial attention will be focused.
Following the context established by the preceding chapters, we begin by exam-
ining the local origins of the local anisotropy. In particular, self-induced anisotropy
in the form of crystal-field distortions derived from spin–orbit coupling and the
Jahn–Teller effect will be emphasized. The underlying physics is reviewed first
through the properties of individual ions. With the single-ion concepts in hand, we
then examine the ions in an exchange-coupled ferromagnet (or ferrimagnet) to de-
termine how the macroscopic anisotropy and magnetostriction effects influence the
collective magnetization statically, and then dynamically in Chap. 6.
G.F. Dionne, Magnetic Oxides, DOI 10.1007/978-1-4419-0054-8 5,
c
Springer Science+Business Media, LLC 2009
201

202 5 Anisotropy and Magnetoelastic Properties
5.1 Quantum Paramagnetism of Single Ions
In the previous chapter, the concept of magnetic anisotropy was introduced some-
what incidentally in order to explain the variation in magnetic moment of the
rare-earth ion Ho
3C
in iron garnet through the effects of crystal fields on the to-
tal angular momentum. Although crystal-fields influence the magnetic properties
of rare-earth ions, they are critically important in the 3d
n
transition iron group se-
ries. Because the coupling to the electrostatic fields of the crystalline environment
is with the orbital angular momentum, the reason why the iron group ions are so
sensitive to their surroundings is the unshielded 3d shell. Not only do the d elec-
trons sense the electric fields of the ligands, but they also participate in the chemical
bonding by sharing their orbital states with the oxygen anion 2p electrons. Much
of the underlying theory is reviewed in Chap. 2, but the actual relation between
the electron–lattice effects and the properties of the oxides (and other families of
transition-metal compounds) was not discussed. The role of spin–orbit coupling as
the intermediary between spin and lattice and the interaction between orbit and spin
angular momentum with a magnetic field must now be examined.
5.1.1 Theory of Anisotropic g Factors
The classical approach to paramagnetism from a collection of isolated magnetic
moments m was treated in Sect. 1.2.1. From this model Curie’s law of / 1=T
was derived with versions that employed the Langevin or semiclassical Brillouin
functions. In either case, the applicable variable is m H =kT (or gm
B
HJ=kT for
the Brillouin function). In later developments spearheaded by Van Vleck, the theory
was refined to take into account an angular momentum energy level structure above
the ground state [1]. Because these excited levels have differing values of m,a
Boltzmann probability distribution can be used to determine a weighted average
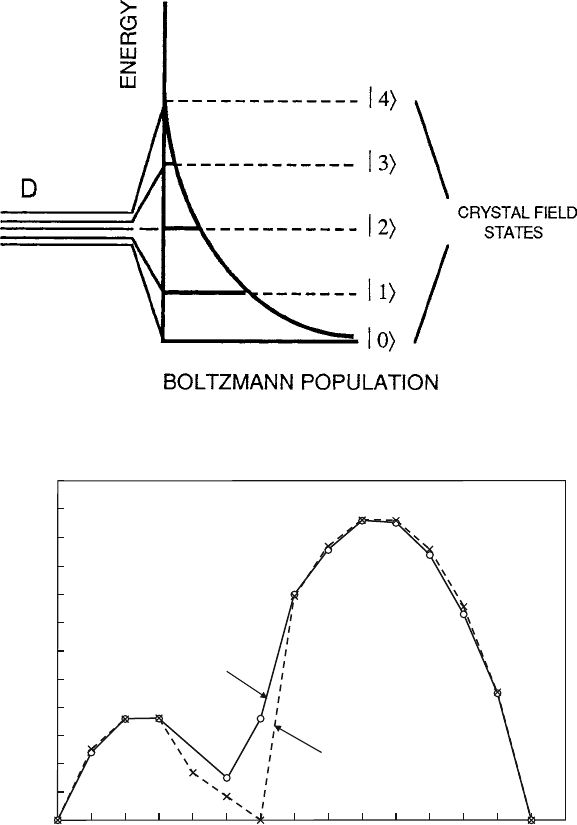
susceptibility shown schematically in Fig. 5.1 for a five-level orbital group (with
spin implied) split in a crystal field. The general expression for the paramagnetic
susceptibility of a molecule with quantum numbers L, S,andJ can be written as
D
LCS
P
J DjLS j
.2J C 1/ .LSJ / exp ŒJ .J C 1/ =2kT
LCS
P
J DjLSj
.2J C 1/ exp ŒJ .J C 1/ =2kT
; (5.1)
where .LSJ/ is the susceptibility contribution from the energy level designated
as
2SC1
L
J
. Details of the derivation of (5.1), including an expression for .LSJ/,
may be found in Griffith’s book [2].
The most successful application of this theory was carried out by Van Vleck for
the 4f
n
lanthanide series. The results are shown in Fig.5.2, where the effective
5.1 Quantum Paramagnetism of Single Ions 203
Fig. 5.1 Schematic diagram of Boltzmann population distribution function showing the relative
occupancies of the five d orbital states controlled by the exp
.
E=kT
/
n
B
= g[J(J+1)]
1/2
experiment
0
2
4
6
8
10
12
n
B
(Bohr magnetons)
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu
Fig. 5.2 Rare-earth ion magnetic moments as a function of the number of 4f
n
electrons. The
lanthanide series is used as the x-axis coordinate, which extends from 0 to 14. The experimental
data and calculated fit were reported by Van Vleck and Frank [1]. Note that the lack of agreement
with the simple theory for Eu
3C
and Sm
3C
indicates that the more complex model of (5.1)is
needed
moments are graphed in terms of Bohr magnetons n
B
D
3kT =N
A
m
B
2
1=2
,
where N
A
is Avogadro’s number. These data may be found in tabular form in
most textbooks on magnetism. Despite intense efforts to extract information from
204 5 Anisotropy and Magnetoelastic Properties
susceptibility measurement data, results of analysis based on this theory have gen-
erally fallen short of expectations. The difficulty in sorting out the individual
contributions from the ladder of energy levels with varying separations, com-
pounded by the uncertainty in the values of the Land´e g factors that are implicit
within the .LSJ/ of individual states led researchers to adopt more sophisticated
approaches in both measurement and theory. Because this necessary spectral infor-
mation is so sensitive to the effects of the crystal field, more powerful tools were
developed to probe the lowest energy states and directly measure g factors by the
Zeeman effect in many of the transition metal ions. In these situations, the analysis
applied to electron paramagnetic resonance (EPR) spectroscopy proved to be fer-
tile ground for theorists with skill in the application of group theory and quantum
mechanical perturbation theory.
If we now return to the general perturbation Hamiltonian for an ion in a crys-
talline environment introduced as (4.38) for the lanthanide series, H
1
D H
LS
C
H
cf
C H
h
C H
ex
, we can examine the effects of the remaining interactions that in-
fluence the magnetic moment of an isolated ion. For the individual ions of the 3d
n
transition series H
ex
does not apply, and the relation is changed to
H
1
D H
cf
C H
LS
C H
h
D H
cf
C L S C m
B
.L C g
e
S / H ; (5.2)
D H
cf
C L S C m
B
g
ij
S
i
H
j
;
where g
e
D 2 is the g factor for an electron and g
ij
is the tensor that results
from the orbital quenching and its attendant anisotropy effects from the crystal
field symmetry. Since the effects of H
cf
on the orbital states have already been
dealt with in Chap. 2 for D and F terms of the iron group, we can now continue
with an examination of the next perturbations for this series: spin–orbit coupling
L S and the combined effect of the orbital and spin moments in a magnetic field
m
B
.L C 2S / H . Smaller energy terms involving spin–spin and electron–nuclear
spin interactions that produce hyperfine spectral structure will be omitted from this
discussion.
For the 3d
n
series, we recall from Chap. 2 that an undistorted octahedral crys-
tal field will stabilize either a triplet (from a free-ion D-state d
1
and d
6
, from
an F -state d
2
and d
7
), a doublet (from a D-state d
4
and d
9
), or a singlet (from
an F -state d
3
and d
8
). However, in cases where the ground state is expected to
be degenerate based on the host lattice symmetry, local ligand distortions usually
occur spontaneously to lower the symmetry. In the context of magnetocrystalline
anisotropy, it is the extent to which the crystal field lifts the orbital degeneracy that
determines the magnetoelastic properties. To this end, we will review the various
electronic structures that exist in the transition metal ions residing in ligand fields of
initially cubic symmetry.
Analysis of any of these electronic configurations is solvable by conventional
degenerate perturbation theory with the use of high-speed digital computation tech-
nology once the appropriate Hamiltonian matrices are set up. To demonstrate the
5.1 Quantum Paramagnetism of Single Ions 205
origin of single-ion anisotropy as reflected in the variation of the g factor with mag-
netic field direction, a detailed examination of the simplest case will be presented to
sensitize the reader to the important physics issues involved in these critical spin–
lattice interactions.
5.1.2 Conventional Perturbation Solutions
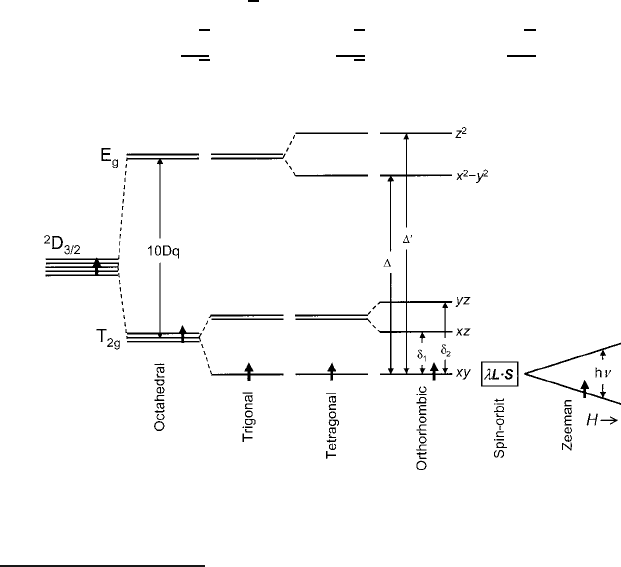
For the general case of a single d electron in an octahedral crystal field with an or-
thorhombic distortion dominated by a z-axis contraction,
1
the energy level structure
isshowninFig.5.3. For cases where the free-ion term is a
2SC1
D
J
, the appearance
of the crystal-field energy levels in the ground-state occupancy diagram conforms
to the actual energy states of a multiple d -electron ion (as opposed to the
2SC1
F
J
cases to be reviewed in subsequent sections). The ground term is indicated by
2
D
3=2
and the eigenvectors of the crystal-field states are listed as j0i; j1i; j2i; j3i,andj4i,
where from (2.12)
j
4
i
D d
z
2
D d
0
D Y
0
2
D
1
2
3z
2
r
2
;
j
3
i
D d
x
2
y
2
D
p
3
p
2
.d
2
C d
2
/ D
p
3
p
2
Y
2
2
C Y
2
2
D
p
3
2
x
2
y
2
;
Fig. 5.3 3d
1
energy-level model in an orthorhombic crystal field, showing spin stabilization in a
Zeeman splitting of the ground-state Kramers doublet
1
In other situations, the distortion can be an expansion, which is manifested by the orbital functions
with a z-dependence lying below the in-plane x-ory-dependent states. Note also that a trigonal
[111]-axis expansion is compatible with a [100]-axis contraction.
206 5 Anisotropy and Magnetoelastic Properties
j
2
i
D d
yz
D i
p
3
p
2
.d
1
C d
1
/ D i
p
3
p
2
Y
2
1
C Y
1
2
D
p
3yz; (5.3)
j
1
i
D d
xz
D
p
3
p
2
.d
1
d
1
/ D
p
3
p
2
Y
2
1
Y
1
2
C
p
3xz;
j
0
i
D d
xy
Di
p
3
p
2
.d
2
d
2
/ Di
p
3
p
2
Y
2
2
Y
2
2
D
p
3xy:
The solutions of these perturbation effects will be carried out in two steps: first,
the eigenstates of H
LS
term will be determined and a new ground state will be
found; then the Zeeman term will be applied to determine the anisotropy of the
magnetic moment which will appear in the inequality of the elements of the g
tensor. The problem will be analyzed in terms of the Ti
3C
-substituted hydrated
alum salt
2
Rb
1C
Al
3C
; Ti
3C
.SO
4
/
2
2
12H
2
O from three approaches, begin-
ning with the most general [3]. First, degenerate theory will be applied whereby
a full perturbation matrix is established for the elements hkjH
cf
C L S jni,
where hkj and jni are eigenfunctions from the list in (5.3) with the spin states in-
cluded. Since each orbital state has a twofold spin degeneracy .m
s
D˙1=2/ this
exercise involves a total of ten wavefunctions, requiring the diagonalization of a
10 10 matrix. For the spin–orbit contributions a convenient form of the operator
is L S D L
z
S
z
C .1=2/ .L
C
S
C L
S
C
/.
Details of the solution are given in Appendix 5A, where the secular equation
is shown to be separable into two identical functions that yield degenerate energy
states, each representing a twofold spin degeneracy (known as Kramers doublets,
which occur in ions with odd numbers of d electrons). The resulting ground state
eigenfunctions from (5.72)are
j
e
0
C
i
D a
ˇ
ˇ
0;
1
2
˛
C b
ˇ
ˇ
1;
1
2
˛
C c
ˇ
ˇ
2;
1
2
˛
C d
ˇ
ˇ
3;
1
2
˛
; (5.4)
j
e
0
i
D a
ˇ
ˇ
0;
1
2
˛
C b
ˇ
ˇ
1;
1
2
˛
c
ˇ
ˇ
2;
1
2
˛
d
ˇ
ˇ
3;
1
2
˛
;
where the highest energy j4i level has been dropped to simplify the calculation with
only a small loss in accuracy, are then used for the 2 2 ground state Zeeman matrix
according to hkjm
B
.L C 2S / H jni.
3
To compute the Zeeman splittings for a particular direction of H , we deter-
mine the matrix elements by applying the appropriate operators, recalling that
L
x
D .1=2/ .L
C
C L
/ and L
y
D – .i=2/ .L
C
L
/ and likewise for the spin
operators. In each case the Zeeman matrix is diagonalized and the expressions for
the energies of the split spin doublet are subtracted to give E
i
D g
i
m
B
H
i
, from
2
In this lattice, the ligands of the Ti
3C
ions are an octahedron of H
2
O molecules that mimic
O
2
ions.
3
For this exercise we consider only the lowest Kramers doublet (Zeeman state) of the three orig-
inating from the T
2g
of the cubic field. Implicit in this choice is that the ground state is a singlet
that results from either an axial field component of the appropriate sign or a Jahn–Teller effect.

5.1 Quantum Paramagnetism of Single Ions 207
which expressions for g
z
, g
x
,andg
y
in terms of the a, b, c,andd coefficients can be
deduced, as shown in Appendix 5A. To solve for the g factors, values are assigned
to the crystal field splittings ı
1
, ı
2
,and;avaluefor must also be assigned,
usually the free-ion value. Conversely, if g factors are found from measurement,
paramagnetic resonance at microwave frequencies in many cases, the reverse pro-
cedure could be followed to determine the energy splittings. This method was used
to compute energy-level splittings from measured g factors for a series of Ti
3C
-
substituted alum salts starting with Rb
1C
Al
3C
.SO
4
/
2
2
12H
2
O, and the results
are included in Table 5.1. Among these compounds are two types of crystal field:
for Rb, K, Tl, and Na alum, orthorhombic with complete splitting of all five d or-
bitals that produces three g factors [3–6], and for the Cs alum-based compounds, a
trigonal field directed along the lattice <111> axes produces a ground state singlet
and two excited doublets as depicted in Figs. 2.13 and 5.3 (for ˛>60
ı
)[7], [8]. In
each case the degenerate method outlined in Appendix 5A was employed. In the Cs
alum cases, accuracy of fits between theory and experiments were limited by the ap-
proximations to the theory because the values of the lower energy splittings was on
the order of 200 cm
1
so that the assumption that =ı < 1 could not be justified, i.e.,
off-diagonal elements are as large as the diagonal ones. Among the orthorhombic
alums, variations in the g factors and their interpreted lower symmetry orbital split-
tings were discussed by Dionne and MacKinnon [6] in terms of the possible effect
on the crystal fields by differing locations of the .SO
4
/
2
radicals. The ionic radius
of the large monovalent cation relative to that of the Al
3C
host ion was correlated
Table 5.1 Anisotropic g factors and crystal field splittings of Ti
3C
d
1
in octahedral sites.
(
50
D154 cm
1
)
Ti
3C
host
Radius
(
˚
A)
Theory g
z
g
x
g
y
ı
1
cm
1
ı
2
cm
1
cm
1
Refs.
Rb alum 1.48 SpinHam 1.895 1.715 1.767 1,070 1,310 11,500 [4]
nondeg. ”””1,0501,32017,000[4]
degen.”””1,0501,32020,300
a
[4]
Tl Alum 1.40 degen. 1.938 1.790 1.834 1,462 1,843 20,300 [5]
K Alum 1.33 degen. 1.975 1.828 1.897 1,780 2,950 20,300 [6]
Na Alum 0.95 degen. 2:00 1:86 1:86 >2;000 >2;000 20,300 [6]
Ti
3C
host Radius
(
˚
A)
Theory g
jj
g
?
ı
cm
1
cm
1
Refs.
CsTi Alum 1.69 Degenerate 1.24 0.93 200 20,300 [8]
” 1.19 0.70 ” ” [8]
” 1.17 0.23 ” ” [8]
CsAlum 1.69 Degenerate 1.25 1.14 300 20,300 [7]
Al
2
O
3
– – 1.067 <0:1 5.19
a
The value D 20;300 cm
1
was measured by optical absorption in an aqueous solution [10],
where the ligands are octahedra of water molecules. This situation is close to that of oxygen ligands
because of the relative location of the O in the H
2
O molecule. To obtain the measured value of
g
z
D 1:895 by the (corrected) degenerate method, D 22;000 cm
1
208 5 Anisotropy and Magnetoelastic Properties
with the magnitude of the apparent orthorhombic departures of the crystal field from
its normal alum trigonal symmetry that is seen only in the Cs alum case. One result
that supported this contention was the observation that Cs(TiAl) alum crystals could
be grown from aqueous solution over the full range of solid solution, while the rest
of the family with the apparent orthorhombic distortions would accept only small
concentrations of Ti as substitutions for Al.
If we assume that lower symmetry exists from local site distortions to produce
a singlet ground state as depicted in Fig. 5.3, we can also treat the problem as a
conventional nondegenerate case whereby the matrix elements of interest become
the off-diagonal ones hkjL S jni for use in the standard relation
ˇ
ˇ
n
0
˛
D
j
n
i
X
k¤n
j
k
i
h
k
j
L S
j
n
i
E
k
E
n
; (5.5)
where jni refers to j0; C1=2i and j0; 1=2i and jki is any of the other eigenfunc-
tions in (5.2) with the jC1=2i and j1=2i spin functions added. Applying the
matrix elements from Appendix 5A, we obtain for the ground states of (5.5)
j
e
0
C
i
D
ˇ
ˇ
0;
1
2
˛
i .=2ı
1
/
ˇ
ˇ
1;
1
2
˛
.=2ı
2
/
ˇ
ˇ
2;
1
2
˛
C i .=/
ˇ
ˇ
3;
1
2
˛
; (5.6)
j
e
0
i
D
ˇ
ˇ
0;
1
2
˛
i .=2ı
1
/
ˇ
ˇ
1;
1
2
˛
C .=2ı
2
/
ˇ
ˇ
2;
1
2
˛
i .=/
ˇ
ˇ
3;
1
2
˛
:
To calculate the Zeeman energy splitting of the Kramers doublet je
0
˙i we follow
the same procedure for the degenerate solution above to produce matrix elements
for the z direction
H
11
D
1
4
2
2ı
1
ı
2
2
4
1
ı
1
2
C
1
ı
2
2
C
2
2
m
B
H
z
;
H
22
DH
11
; (5.7)
H
12
D H
21
D 0:
By solving the secular equation and relating E D 2H
11
D g
z
m
B
H
z
,andthen
repeating the procedure for the x and y directions, the diagonal elements of the g
tensor can be expressed as
g
z
D 2
8
2
ı
1
ı
2
2
2
1
ı
1
2
C
1
ı
2
2
C
2
2
2
;
g
x
D 2
2
ı
1
C
2
2
1
ı
1
2
1
ı
2
2
C
2
2
ı
2
2
2
2
; (5.8)
g
y
D 2
2
ı
2
C
2
2
1
ı
2
2
1
ı
1
2
C
2
2
ı
1
2
2
2
:
5.1 Quantum Paramagnetism of Single Ions 209
As listed in Table 5.1 for Rb alum, splitting energies for the measured g
z
D 1:895,
g
x
D 1:715,andg
y
D 1:767 are ı
1
D 1;050, ı
2
D 1;320,and D 17;000 cm
1
[3,4].
5.1.3 The Spin Hamiltonian for 3d
n
Ions
To complete this instructional example, it is important that we introduce albeit
briefly the “spin” Hamiltonian that was developed by Pryce and Abragam as an
approximation to the nondegenerate model outlined in Sect. 5.1.2 [9]. This method
was used to analyze the paramagnetic resonance spectrum of multiple-d -electron
ions with an orbital singlet ground state, in particular the
4
F -state Cr
3C
ion that
was the key to the early ruby maser and laser demonstrations. If the excited orbital
states of the crystal field splittings are high enough to be ignored in following calcu-
lations, the approach differs from those of the two conventional methods in that the
perturbation matrix elements are computed for a combination of spin–orbit and Zee-
man interactions, hkjH
1
jniDhkjLS Cm
B
.L C 2S / H jni, with the result that
the spin S is treated as the only angular momentum quantum number and the con-
tribution of L becomes absorbed in the g factors, consistent with (5.8) but with less
precision. Since the je
0
i states are singlets that carry no orbital angular momentum,
i.e., S-state behavior, the only first-order nonzero elements will be he
0
j2m
B
SHje
0
i
and the second-order correction will simplify to
E
.1/
D
X
n¤0
jh
e
0
j
L S C m
B
H L
j
e
n
ij
2
E
n
E
0
: (5.9)
After collecting all first- and second-order terms that are linear in m
B
H ,(5.9) can
be reduced to
E
.
1/
D 2m
B
ı
ij
ij
H
i
S
j
; (5.10)
where
ij
D
X
n¤0
h
e
0
j
L
i
j
e
n
ih
e
n
j
L
j
j
e
0
i
E
n
E
0
: (5.11)
From (5.9) we see that the matrix elements used are only those linked to the ground
state, thereby substantially simplifying the labor involved. With this approximation
only the terms to first order in appear, and
g
z
D 2
8
;
g
x
D 2
2
ı
1
; (5.12)

210 5 Anisotropy and Magnetoelastic Properties
g
y
D 2
2
ı
2
:
For Rb alum measured g factors are fitted to splitting energies of ı
1
D 1;070,
ı
2
D 1;310,and D 11;500 cm
1
, as listed in Table 5.1. Note that only the larger
cubic field component deviates significantly from the measured reference value
of 20;300 cm
1
[10].
For ions with more complicated spin multiplets, i.e., S>1=2,suchasCr
3C
and
Ni
2C
, higher-order terms in become important for spectral analysis and a more
general form of (5.10) can be expressed as [9]
E
.2/
D 2m
B
ı
ij
ij
H
i
S
j
2
ij
S
i
S
j
; (5.13)
leading to the general form of the spin Hamiltonian expressed to second order in as
H
s
D m
B
g H S C DS
z
2
C E
S
x
2
S
y
2
C
1
6
a
S
z
4
C S
x
4
C S
y
4
; (5.14)
where the anisotropic g is now expressed in tensor format. The zero-field splitting
parameters D and E (both functions of
ij
) can be determined from experiment and
can be used to deduce information about the electronic structure of the ions in their
crystal field. Included in this version of the spin Hamiltonian is the fourth-order
term characterized by the a parameter which becomes significant in S-state ions
3d
5
(Fe
3C
,Mn
2C
)and4f
7
(Gd
3C
,Eu
2C
) for which the first-order orbital angular
momentum contributions are absent. The subject of S-state ions will be reviewed
further in Sect. 5.2.5.
Equations (5.8)and(5.12) expose the role of spin–orbit coupling in determining
the magnitude and anisotropy of the magnetic moments in these transition-metal
ions. The ratios of the constant to the various orbital level splittings can dictate
not only the degree of anisotropy of the magnetic moment as the magnetic field is
applied to different directions, but also whether the moment increases or decreases
as in this case of Ti
3C
ion. According to Table 5.2,the constants increase in mag-
nitude and reverse sign in the upper half of the 3d
n
series, thereby alerting us to the
fact that g factors greater than 2 as well as increased anisotropy is to be expected
from the ions with larger d -electron populations. Through the rest of the series, the
Aufbau principle is employed to show how the peculiarities of the orbital ground
states of the various ions are related to their magnetoelastic properties.
5.1.4 The Crystal-Field Hamiltonian for 4f
n
Ions
For the lanthanide series, analysis of isolated ion magnetic properties in solids fol-
lows the same perturbations approach as that for the iron group. The hierarchy of
perturbations is totally different, however. In the 4f
n
ions spin–orbit coupling ex-
ceeds that of the 3d
n
group by at least a factor of 3. Moreover, crystal field effects
