Desurvire E. Classical and Quantum Information Theory: An Introduction for the Telecom Scientist
Подождите немного. Документ загружается.


14.2 Nonlinear channel 277
the SHT boundary, the more sensitive the system SER is to SNR and signal power.
Also, the more complex the error-correction codes to bring the BER to low values.
Given constraints in power and noise, in BER requirement after code correction, in
code-correction complexity, and, finally, in modulation-format technologies, the difficult
task for communications engineers is to find the right tradeoffs between academic and
practical engineering solutions.
14.2 Nonlinear channel
Shannon’s information theory and all its subsequent developments have always been
based on the assumption of channel linearity. The principle of channel linearity can be
summarized by two basic conditions:
(a) Assuming signal input to the channel with power P, the signal output power is of
the form P
= λP, where λ is a real constant that is power independent (λ<1for
lossy channels, λ = 1 for transparent or lossless channels, and λ>1 for channels
with internal amplification or gain);
(b) The channel noise N is added linearly to the signal power (additive noise), namely
the total output power is of the form P
= κ P + µN, where µ is a real constant
that is power independent.
Condition (a) implies that the signal can be increased to an arbitrarily high power level,
without any changes in the channel’s properties, as provided by information theory.
Condition (b) implies that the transmission pipe in the channel is free from any power-
dependent signal degradation effects. In the physical world, such effects are referred to
as nonlinearities. Such nonlinearities are usually not observed in vacuum or atmospheric
space.
14
However, they are observed in transmission media such as optical fibers, for
instance. The advent of high-speed optical telecommunications in the last 20 years has
come with the discovery of various types of nonlinearity, namely, Raman and Brillouin
scattering, self- and cross-phase modulation, and four-wave mixing. Such nonlinearities
cause power limitations (hence SNR and BER limitations) in fiber-optic systems. In
the following, I shall focus on fiber-optic systems as an illustration of the nonlinear
communications channel.
An optical fiber is a highly transparent transmission medium. However, optical signals
are attenuated on propagation therein, according to an exponential law P
= λP =
P exp(−αL), where L is the fiber length and α the attenuation coefficient. For lengths
of 50 km and 100 km, the relative signal attenuation or power loss is typically 90%
(λ = 0.1) or 99% (λ = 0.01) respectively. To compensate for fiber loss, fiber-optic
systems use in-line optical amplifiers. Such amplifiers are periodically placed every
50–100 km (depending on applications) to produce a signal gain g = 1/λ, such that the
14
At least with the power levels used in telecommunications. The atmosphere is made of atomic and molecular
matter, which may exhibit nonlinear absorption or scattering at certain frequencies and powers, and a vacuum
is nonlinear from the high-energy physics or quantum-electrodynamics viewpoint.

278 Gaussian channel and Shannon–Hartley theorem
signal power at the amplifier output is P
= gλP ≡ P. The amplified fiber-optic link is
said, therefore, to be transparent. However, the amplification process produces additive
noise, which accumulates linearly along the transparent link, each amplification stage
contributing to a noise increment.
Overlooking any effect contributed by the optical fiber, an amplified fiber-optic link
is intrinsically linear.
15
A simple justification comes as follows. At the end of the
link, the optical signal is converted by the receiver into a photocurrent. It can be
shown that the dominant noise component in the receiver photocurrent output is of the
form:
σ
2
= 2PN, (14.27)
where P is the optical power input to the (transparent) link and, hence, to the end
receiver, and N is the optical amplifier noise accumulated along the link, which is
power-independent.
16
The electrical signal power is of the form
P
e
= P
2
. (14.28)
The electrical signal-to-noise ratio (SNR) is, thus,
SNR =
P
e
σ
2
=
P
2
2PN
≡
P
2N
, (14.29)
whose noise denominator is now seen to be independent of the signal power. Thus, the
SNR scales linearly with the input (optical) power, which is the linearity condition for the
communication channel. But the transmission medium, the optical fiber, is intrinsically
nonlinear. It is beyond the scope of this book to detail the different types and causes of
fiber nonlinearities.
The introduction of channel nonlinearity in the field of information theory is relatively
recent (2001).
17
As we shall see, this new hypothesis is rich in implications in the analysis
of channel capacity, in particular for optical transmission systems. We shall, thus, assume
that the nonlinear channel noise, N
∗
, has the power-dependent form
N
∗
(P) = N + sP, (14.30)
where N is the linear channel noise (P = 0) and sP is the power-dependent contribution
to the channel noise, which is attributed to optical fiber nonlinearities. The nonlinearity
parameter s can be written as
s = 1 −exp
&
−
P
P
th
2
'
, (14.31)
15
As in any amplifiers, the amplification factor (gain) of optical amplifiers saturates with increasing signal
power. However, optical amplifiers deployed in transmission systems are designed to yield constant gain
regardless of signal power changes.
16
See, for instance: E. Desurvire, Erbium-Doped Fiber Amplifiers, Devices and System Developments (New
York: J. Wiley & Sons, 2002), Ch. 3.
17
P. P. Mitra and J. B. Stark, Nonlinear limits to the information capacity of optical fiber communications.
Nature, 411 (2001), 1027; for a tutorial introduction, see also E. Desurvire, Erbium-Doped Fiber Amplifiers,
Devices and System Developments (New York: J. Wiley & Sons, 2002), Ch. 3.
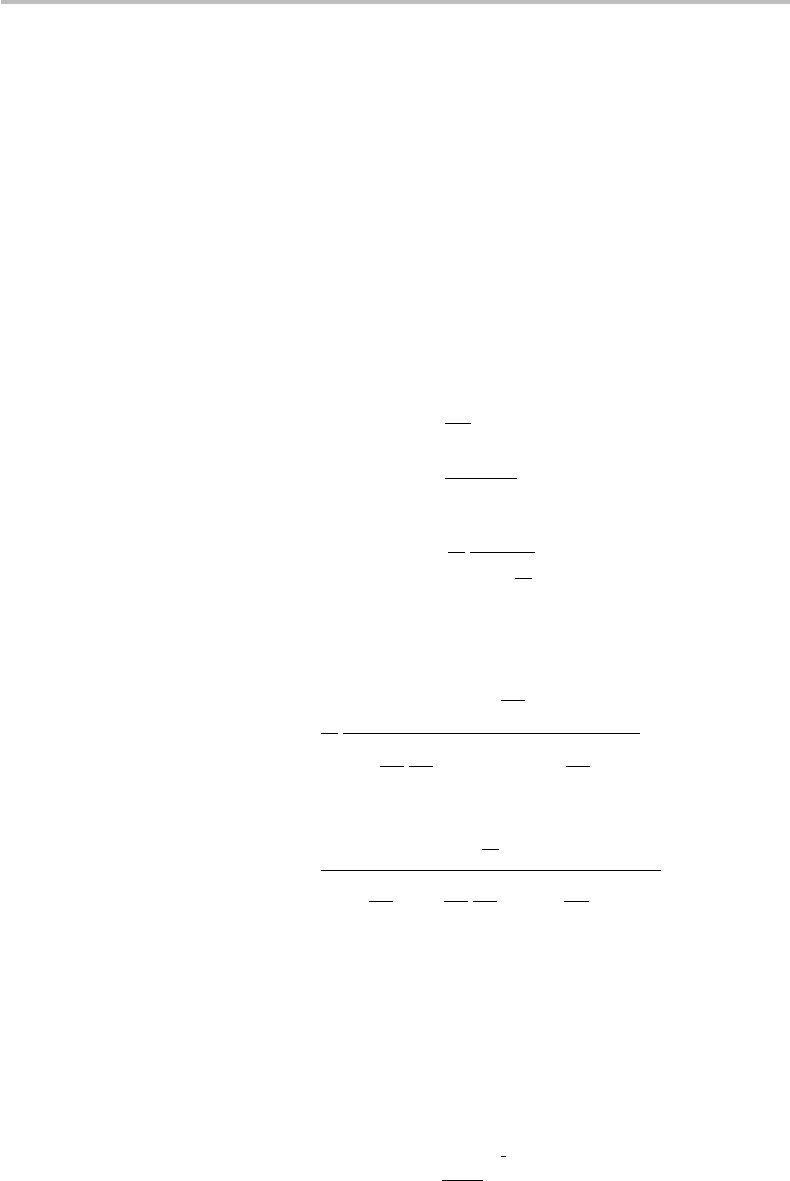
14.2 Nonlinear channel 279
where P
th
is the power threshold at which nonlinearity comes into effect. The exact
definition of P
th
in relation to the various fiber-optics link parameters can be found in the
aforementioned references. The above definition of the nonlinearity parameter s shows
that nonlinearity is negligible, or the optical channel is linear, for P P
th
or s → 0
(or sP N ), which, according to Eq. (14.30) gives the power-independent, additive
channel noise N
∗
(P) ≈ N . In the general case, we see from the relation N
∗
(P) =
N + sP that a fraction sP of signal power is effectively converted into nonlinear noise.
Because of energy conservation, the received signal power at the end of the link is not P,
but rather P
∗
= P(1 −s). The actual SNR is now SNR
∗
= P
∗
/N
∗
, which reduces to
SNR
∗
= P/N in the linear limit P P
th
(s → 0). We can now evaluate the nonlinear
channel capacity. According to the Shannon–Hartley theorem (SHT), Eq. (14.18), the
channel capacity ((bit/s)/Hz) is given by:
C = log
1 +
P
∗
N
∗
= log
1 +
P(1 −s)
N + sP
(14.32)
= log
1 +
P
N
1 − s
1 + s
P
N
.
Replacing the definition of the nonlinearity parameter s in the above yields:
C = log
1 +
P
N
exp
&
−
P
P
th
2
'
1 +
P
P
th
P
th
N
3
1 − exp
&
−
P
P
th
2
'4
= log
1 +
P
N
exp
P
P
th
2
+
P
P
th
P
th
N
3
exp
P
P
th
2
− 1
4
.
(14.33)
The result in Eq. (14.33) shows that in the high-power limit P/P
th
→∞, the channel
capacity reduces to C = log(1 + ε) with ε → 0, or C → 0. Nonlinearity, thus, asymptot-
ically obliterates channel capacity. Since in the linear regime channel, capacity increases
with signal power, we then expect that a maximum can be reached at some power value.
It can be shown that such a maximum capacity is reached for the optimal signal power
P
opt
as analytically defined:
P
opt
=
NP
2
th
2
1
3
. (14.34)
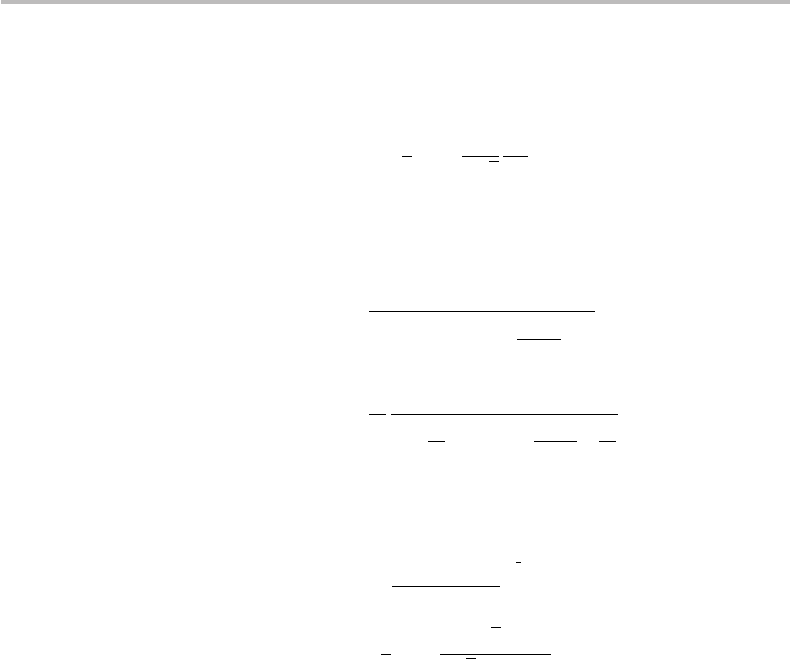
280 Gaussian channel and Shannon–Hartley theorem
Replacing this definition of P
opt
into Eq. (14.33) yields the maximum achievable channel
capacity C
max
:
C
max
=
2
3
log
2
3
√
3
P
th
N
. (14.35)
A different model that I developed,
18
which takes into account the quantum nature of
amplifier noise leads to an alternative definition C
of the channel capacity:
C
= log
1 +
P(1 −s)
1 + 2N + s
1 +
1
1 − s
P
= log
1 +
P
N
1 − s
2 +
1
N
+ s
1 +
1
1 − s
P
N
.
(14.36)
The new definition of capacity in Eq. (14.36) yields different expressions for the optimal
power and maximum capacity, namely P
opt
and C
max
, as defined by:
P
opt
=
(1/2 + N )P
2
th
2
1
3
, (14.37)
C
max
=
2
3
log
&
2
√
2P
th
3
√
3(1 + 2N )
'
. (14.38)
We note from Eqs. (14.34)–(14.35) and Eqs. (14.37)–(14.38) that the optimal powers
P
opt
, P
opt
and maximal capacities C
max
, C
max
actually have very similar values. With
respect to the classical model, the quantum model of the nonlinear channel provides a
small correction to the maximum SNR of the order of 2
−1/3
≈ 0.8, which, in base-2
logarithms, represents about 30%.
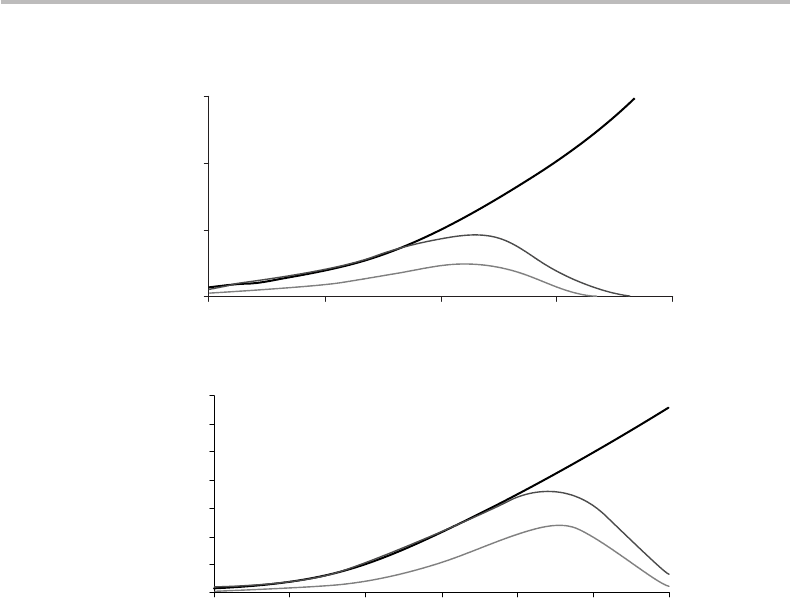
Figure 14.6 shows plots of thee nonlinear-channel capacities C, C
(according to the
two above models), as plotted vs. the linear SNR, i.e., SNR = P/N , as expressed in
decibels,
19
with different parameter choices for N and P
th
. The SHT capacity corre-
sponding to the linear-channel case is also shown. Figure 14.6(a) corresponds to the
worst case of a channel with a nonlinear threshold relatively close to the linear noise
(N = 5 and P
th
= 15). In contrast, Figure 14.6(b) corresponds to the case of a compara-
tively low linear-noise channel having a relatively high nonlinear threshold (N = 1 and
P
th
= 100). It is seen from the figure that in both cases, and regardless of the nonlinear
model (classical or quantum), the channel capacity is bounded to a power-dependent
maximum, unlike in the linear information theory where from SHT the capacity increases
as log(SNR).
18
E. Desurvire, Erbium-Doped Fiber Amplifiers, Devices and System Developments (New York: J. Wiley &
Sons, 2002), Ch. 3.
19
With SNR(dB) = 10 log
10
(SNR).
14.2 Nonlinear channel 281
0
1
2
3
−10 −5
0510
SNR (dB)
Channel capacity C,C´ ((bit/s)/Hz)
Linear channel (SHT)
C
´
C
(a)
0
1
2
3
4
5
6
7
−
10
−
50 5 201510
SNR (dB)
Channel capacity C,C´ ((bit/s)/Hz)
Linear channel (SHT)
C
´
C
(b)
Figure 14.6 Nonlinear channel capacities C, C
, as functions of linear signal-to-linear-noise ratio
P/N according to classical and quantum models with SHT linear-channel capacity shown for
reference (thick line), with dimensionless parameters (a) N = 5andP
th
= 15, and (b) N = 1
and P
th
= 100.
The consequences of these developments concerning nonlinear channels remain to
be fully explored. It is important to note that the assumption of channel nonlinearity
does not affect the key conclusions of Shannon’s (linear) information theory, namely the
channel coding theorem and the SHT. Indeed, error-correction codes are used in realistic
(nonlinear) optical communication systems to enhance SNR and BER performance. The
fact that in these nonlinear optical channels the noise is power-dependent does not affect
the effectiveness of such codes. The key conclusion is that the capacity limits imposed
by Shannon’s information theory still apply, despite the new complexities introduced
by channel nonlinearity, which introduces yet another upper bound. It should not be
interpreted, however, that nonlinearity defines an “ultimate capacity limit” for optical
communication channels, present or future. Indeed, optical fiber design makes it possible
effectively to reduce fiber nonlinearity by increasing the fiber core size (referred to as the
“effective area”), so as to decrease the nonlinearity threshold, P
th
, and, hence, effectively
achieve the operating conditions P P
th
(s → 0). Furthermore, there exist several
types of “countermeasures” and power–SNR tradeoffs to alleviate optical nonlineari-
ties, which it is beyond the purpose of this chapter to describe. Overall, the key conclusion
282 Gaussian channel and Shannon–Hartley theorem
is that the optical communication channel, albeit nonlinear, can be made to operate as a
linear channel, which is, therefore, essentially compliant to the capacity limits set up by
Shannon’s coding theorem.
14.3 Exercises
14.1 (M): The signal-to-noise ratio in a continuous channel is SNR =+12.5 dB. What
is the channel capacity? Suggest a Reed–Solomon block code for which the
capacity could be approached within approximately one bit.
14.2 (T): Show that the differential conditional entropy
H(Y |X ) =−
p(y|x) p(x)logp(y|x)dxdy
reduces after analytical computation to
H(Y |X ) = log
(
2πeσ
2
ch
.
14.3 (T): Show that the capacity of a Gaussian channel with NRZ/OOK modulation
rapidly converges with increasing signal power to the upper limit:
C = 1 (bit/s)Hz.
Clues:
(a) Assume a binary input signal x
i
∈ X ={x
1
, x
2
}taking the values x
1
= a and
x
2
=−a, and a continuous output y ∈ Y ,
(b) Define the mutual information as
H(X ; Y ) =
i
Y
dyp(x
i
, y,)log
p(x
i
, y)
p(x
i
)p(y)
,
(c) Define the conditional probability p(y|x
i
)as:
p(y|x
i
) =
1
σ
√
2π
exp
−
(y − x
i
)
2
2σ
2
,
where σ
2
is the channel noise variance.
(d) Show that the channel capacity is a function of the “bit SNR” parameter
S
2
≡ a
2
/(2σ
2
) and numerically determine the limit for increasing S
2
.

15 Reversible computation
This chapter makes us walk a few preliminary, but decisive, steps towards quantum
information theory (QIT), which will be the focus of the rest of this book. Here, we shall
remain in the classical world, yet getting a hint that it is possible to think of a different
world where computations may be reversible, namely, without any loss of information.
One key realization through this paradigm shift is that “information is physical.” As we
shall see, such a nonintuitive and striking conclusion actually results from the age-long
paradox of Maxwell’s demon in thermodynamics, which eventually found an elegant
conclusion in Landauer’s principle. This principle states that the erasure of a single bit
of information requires one to provide an energy that is proportional to log 2, which,
as we know from Shannon’s theory, is the measure of information and also the entropy
of a two-level system with a uniformly distributed source. This consideration brings
up the issue of irreversible computation. Logic gates, used at the heart of the CPU in
modern computers, are based on such computation irreversibility. I shall then describe the
computers’ von Newman’s architecture, the intimate workings of the ALU processing
network, and the elementary logic gates on which the ALU is based. This will also
provide some basics of Boolean logic, expanding on Chapter 1, which is the key to the
following logic-gate concepts. As I shall describe, a novel concept for logic gates (and,
hence, their associated circuits or networks), can be designed to provide truly reversible
computation.
15.1 Maxwell’s demon and Landauer’s principle
The QIT story begins with a strange machine devised in 1871 for a thought experiment
by the physicist J. C. Maxwell. Such a machine – if one were ever able to construct it –
would violate the second principle of thermodynamics! As Maxwell describes in his
theory of heat:
1
One of the best established facts in thermodynamics is that it is impossible in a system enclosed
in an envelope which permits neither change of volume nor passage of heat and in which both the
temperature and the pressure are everywhere the same, to produce any inequality of temperature
or of pressure without the expenditure of work. This is the second law of thermodynamics, and
1
See http://en.wikipedia.org/wiki/Maxwell%27s_demon; http://users.ntsource.com/∼neilsen/papers/demon/
node3.html.
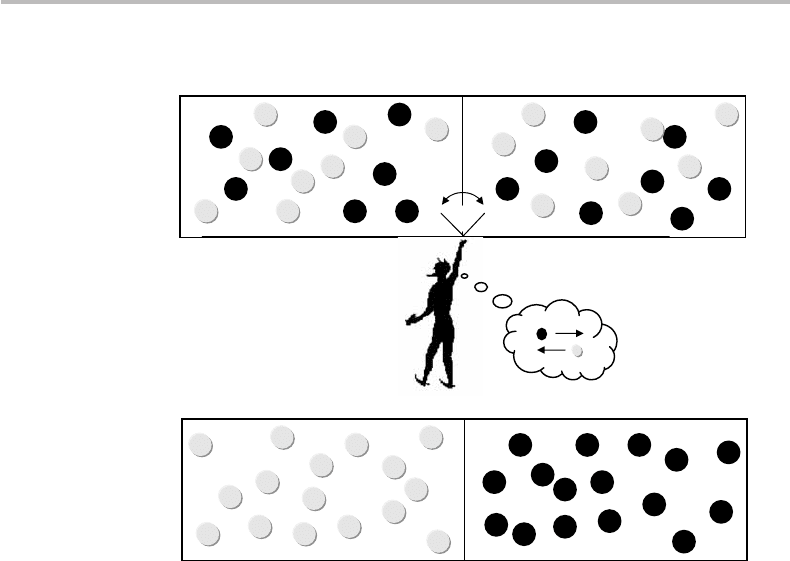
284 Reversible computation
T
T
(a)
(b)
T
´<
TT
´´>
T
A
B
A
B
Figure 15.1 Maxwell’s demon thought experiment: (a) initially, the gas molecules are evenly
spread between two parts A and B (dark = fast, clear = slow), with homogeneous temperature
T , and (b) after the demon sorted molecules according to speed, showing lower temperature T
in
part A and higher temperature T
in part B.
it is undoubtedly true as long as we can deal with bodies only in mass, and have no power of
perceiving or handling the separate molecules of which they are made up. But if we conceive
a being, whose faculties are so sharpened that he can follow every molecule in its course, such
a being, whose attributes are still as essentially finite as our own, would be able to do what is
at present impossible to us. For we have seen that molecules in a vessel full of air at uniform
temperature are moving with velocities by no means uniform, though the mean velocity of any
great number of them, arbitrarily selected, is almost exactly uniform. Now let us suppose that
such a vessel is divided into two portions, A and B, by a division in which there is a small hole,
and that a being, who can see the individual molecules, opens and closes this hole, so as to allow
only the swifter molecules to pass from A to B, and only the slower molecules to pass from B to
A. He will thus, without expenditure of work, raise the temperature of B and lower that of A, in
contradiction to the second law of thermodynamics.
A basic illustration of Maxwell’s machine is provided in Fig. 15.1. As Maxwell
describes it, the machine consists of a gas container or “vessel,” which is separated
by a wall into two chambers, A and B. The gas absolute temperature T is uniform,
2
2
To recall, the absolute temperature is expressed in units of kelvin (K). It has a one-to-one correspondence
with the Celsius (C) temperature scale, with absolute zero being 0 K =−273
◦
C and the melting-ice
temperature being 0
◦
C =+273 K.

15.1 Maxwell’s demon and Landauer’s principle 285
and is given by the formula E = (3/2)k
B
T , where E is the average kinetic energy of
the gas molecules (k
B
= Boltzmann’s constant). Both temperature and energy represent
macroscopic measures, as individual molecules don’t have a “temperature” and have
different velocities of their own, some moving slower and some moving faster. As the
figure shows, an imaginary, witty but not malicious creature (later dubbed “Maxwell’s
demon”) guards a trapdoor, which allows individual molecules to pass from A to B or
the reverse, without friction or inertia. The demon checks out the molecules’ velocities
and allows only the faster ones (E
> E) to pass from A to B, and only the slower ones
(E
< E) to pass from B to A. After a while, most of the fast molecules are found in B,
while most of the slow ones are in A. As a result, the gas temperature in A (T
)islower
than that in B (T
), with T
< T < T
.
The simultaneous heating of part B and cooling of part A, which is accomplished by
the demon without deploying energy or work, is in contradiction to the second law of
thermodynamic. This second law stipulates, in its simplest formulation, that: “Heat does
not flow spontaneously from a cold to a hot body of matter.” Since heat is a measure
of the average kinetic energy of the gas, it has a natural tendency to diffuse from hot to
cold bodies, and not the reverse. Another formulation of the second law is that: “In an
isolated system, entropy as a measure of disorder or randomness can only increase.” Let
us have a closer look at this entropy notion. In Appendix A, we have seen that a system
of particles that can be randomly distributed in W arrangement possibilities into a set
of distinct boxes, called “microstates,” is characterized by an entropy H. This entropy
represents the infinite limit, normalized to the number of particles, of the quantity log W .
Hence, the greater W or H , the more randomly distributed the system, which justifies
the notion of entropy as a physical measure of disorder. By separating and ordering the
gas molecules into two distinct families (slow and fast), the demon decreases the global
randomness of the system, since there is a smaller number of microstate arrangements W
for each of the initial molecules, the system’s entropy log W is, thus, decreased, which is
indeed in contradiction to the second law because the demon apparently did not perform
any work.
At the time, the conclusion reached by Maxwell to solve this paradox was that the
second principle only applies to large numbers of particles as a statistical law, i.e., to
be used with certain confidence at a macroscopic, rather than at a microscopic level.
In the first place, Maxwell’s paradox was never intended to challenge the second law.
Yet, it kept puzzling scientists for generations with many unsolved issues. In 1929, for
instance, L. Szil
´
ard observed that in order to measure molecular velocities, the demon
should spend some kind of energy, such as that of photons from a flashlight, to spot the
slow or fast molecules, and then decide from the observation whether or not to open the
trapdoor. Each of such measurements and the generation of photons would then increase
the system’s entropy, which should balance out the other effect of entropy decrease.
In 1951, L. Brillouin observed that in order to distinguish molecules by use of light
photons, the photon energy would have to supersede that of the ambient electromagnetic
radiation, thus bringing a quantum of heat k
B
T into the system. To move the trapdoor,
the “demon” must also be material. Thus, some form of heat transfer should occur during
the process, resulting in the heating of both the demon and the gas, canceling the cooling

286 Reversible computation
effect of molecule separation. In 1963, R. Feynmann observed that the demon would
not be able to get rid of the heat gained in his molecule measurements, ending up in
“shaking into Brownian motion” with the inability to perform the task!
Most of the above conjectures, along with the multiplication of improved “demon
engines,” contributed to turn Maxwell’s initial paradox into an ever-deepening enigma.
This is how the concept of information entropy came into the picture. Szil
´
ard considered
a demon engine processing just a single gas molecule. If the demon knew where the
molecule was located, then he could move a frictionless piston to confine it where
it belonged, without any work expenditure! Then the pressure effect of the molecule
on the piston could be used to generate work, such as lifting a weight. The intuitive,
yet not consolidated idea, was that the entropy bookkeeping could be balanced should
some positive entropy be associated with the information concerning molecules or the
demon’s memory itself. Either the demon keeps track of any of its binary decisions
(open trapdoor to molecule coming from A or B) or it erases them. In the first case,
the demon’s memory space expands, which goes together with an entropy increase
(number of accessible memory states in the demon’s mind). At some point, however,
the memory space is exhausted, and information must be erased. In the second case,
the memory is cleared one bit at a time prior to the next binary decision. Whether the
memory is cleared at once or bit after bit after each demon operation, the action of
erasure is irreversible.
3
According to thermodynamics, irreversible processes generate
positive entropy. Therefore, the erasure of information must increase the entropy of
the surrounding environment. Such a revolutionary concept was initially suggested by
J. von Neumann
4
and then formalized in 1961 by R. Landauer. The key conclusion
takes the form of Landauer’s principle (or the Landauer bound or limit,orthevon
Neumann–Landauer bound):
The energy dissipated into the environment by the erasure of a single bit of information is at least
equal to k
B
T log 2. The environment entropy increases by at least k
B
log 2.
Landauer’s principle, thus, provides a lower bound on the energy dissipation and
entropy increase associated with information erasure. It is not coincidental that this
minimum is proportional to log 2 (natural logarithm). Without pretending to provide a
formal demonstration of this result, consider the following. The memory to be erased
can be viewed as a binary source X =
{
1, 0
}
having a uniform distribution, i.e., p(1) =
p(0) = 1/2 (the probability that the memory bit is either 1 or 0). As we have seen in
Chapter 3,theself-information associated with any of the two events x ∈ X is I (x) =
−log
e
[p(x)] ≡−log
e
(1/2) = log
e
2, as expressed in nats. The source’s entropy H (X)
corresponds to the average information
I (x)=−p(1) log
e
[p(1)] − p(0) log
e
p[(0)]
= 0.5log
e
2 + 0.5log
e
2 = log
e
2,
3
For a given memory address, the initial state is the bit value 0 or 1. Once cleared, the bit value is reset to 0
(for instance). From this final state, it is not possible to decide whether the initial state was the bit value 0
or 1, and so on for all previously recorded states, which illustrates the irreversibility of the erasure process.
4
See http://en.wikipedia.org/wiki/Landauer%27s_principle.
