Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.

355
0.11.
0.10
...
i
0
>
!I
>
a
$
0.09
2
LL
u
W
a
111
-
I
-
5
0.08
+
?
E
a
>
>
[L
0
u
w
VI
n
I
a06
0.05
60
80
100
120
TEMPER
ATU
RE
OF
/
Fig.
9-5.
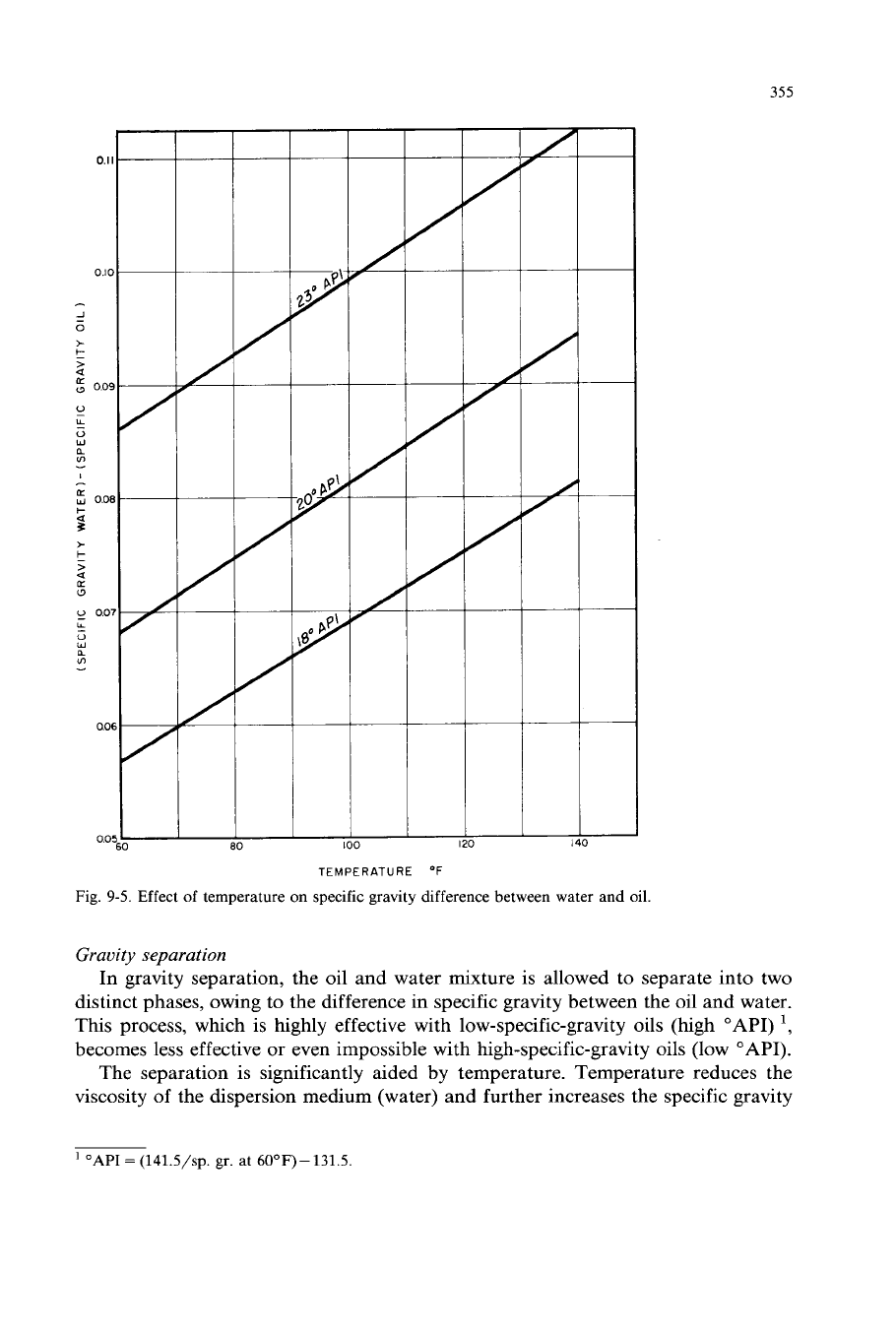
Effect of temperature
on
specific gravity difference between water and oil.
Gravity separation
In gravity separation, the oil and water mixture is allowed to separate into two
distinct phases, owing to the difference in specific gravity between the oil and water.
This process, which is highly effective with low-specific-gravity oils (high
"API)
',
becomes less effective or even impossible with high-specific-gravity oils (low
"API).
The separation is significantly aided by temperature. Temperature reduces the
viscosity of the dispersion medium (water) and further increases the specific gravity
"API
=
(141.5,’~~.
gr.
at
60OF)-
131.5.

356
differential between the oil and water (Fig. 9-5). The less the specific gravity
difference between the oil and the water, the lower the rising velocity
of
oil and,
therefore, the longer the residence time required in the separator.
The subject of gravity separation has been covered in detail in an API manual in
1963 (API, 1963). The following references should be consulted when the engineer is
dealing with tanks, API gravity separators, or open ponds, because the principles
are the same in all cases: API (1951), Ingersoll (1951), Johnston and Campbell
(1957), and Brunsmann et al. (1962). Usually, utilization of these principles gives a
high degree of success in oil removal. The causes of difficulty of oil removal in the
unusual case may be the presence of emulsions or the lack of sufficient specific
gravity difference between oil and water. When the gravity separator does not give
the desired degree of oil removal or it is not feasible or economical to use a
properly-designed gravity separator, one must use the flotation principle.
Flotation
There are two distinct types of flotation processes in use today: dissolved gas
flotation and froth flotation.
Froth flotation is an adaptation
of
a beneficiation process long used in the
mining industry. Froth flotation requires the addition
of
a chemical to stabilize the
froth, whch is mechanically formed by “beating” air or gas into the water. Froth
flotation is less sensitive to overloading than dissolved gas flotation; however, it has
other shortcomings. These may limit the application of froth flotation to a specific
system and field trials are highly recommended.
Aside from the manner in which the gas bubbles are formed in the water, the two
types of flotation processes are similar. Dissolved gas flotation is a process in which
gases are dissolved into the water under pressure. Upon subsequent release
of
pressure, the evolving bubbles become attached to particulate matter and/or
oil,
and float them to the surface where they may be skimmed off (Katz, 1958, 1960;
Simonsen, 1962; API, 1963).
Flotation is a highly efficient method
of
removing suspended oil from water
when the load is less than 100 ppm and emulsions do not exist. Increasing amounts
of oil are left in the water as the incoming load increases. The flotation process has
the following shortcomings: (1) emulsions are seldom resolved, (2) suspended solids
may interfere with oil removal, (3) high incoming oil contents give rise to more oil in
the output, and
(4)
the process is very sensitive to (a) velocity or throughput,
(b)
gas/water ratio, and (c) recycle ratio.
When the flotation cell is overloaded or when emulsions are present and cannot
be prevented by remedial measures upstream, one must use supplementary chemical
treatment with the flotation cell. Certain clays are good adsorbents of oil. Adding
these clays as a slurry to the flotation cell, followed by a polyelectrolyte, results
in
a
very clear water discharge. Alum, alone or with coagulant aids, also helps an
overloaded flotation cell or one receiving emulsions.
The chemical theory, which
is
the older conventional theory, assumes that (a) the
colloids are aggregates of defined chemical structure,
(b)
the primary charge
of

357
colloid particles arises from the ionization of complex inorganic groups present on
the surfaces
of
the dispersed particles, and (c) the destabilization
of
colloids
is
due
to the chemical interactions, such as complex formation and proton transfer. This
chemical theory, however, does not explain all the processes that take place during
coagulation.
As
a result, a newer theory has been developed called the “physical
theory”
’.
This theory emphasizes the concept
of
the electrical double layer and the
significance of predominantly physical factors, such as counter ion adsorption,
reduction of zeta potential, and ion pair formation in the destabilization of colloids.
It has aided significantly in the interpretation of coagulation mechanisms and in
control
of
coagulation techniques, and has practically replaced and superseded the
older chemical theory (Black, 1948,1960; Stumm and Morgan, 1962; Riddick, 1964;
Hudson, 1965).
Filtration is necessary when the last traces of oil must be removed from the water
and when gravity separation and/or flotation are inadequate. Filtration is also used
when solids must be removed.
Removal
of
solids (filtration)
Filtration must be viewed as a clean-up or “polishing” operation, as it is not
economical for removal of large amounts of solids or oil from water. The choice of a
filter is based upon the following factors: (1) quality of effluent desired,
(2)
amount
of
suspended solids,
(3)
nature
of
suspended solids, (4) capital cost versus oper-
ational cost,
(5)
space available, (6) weight of installation (offshore platform loading
considerations),
(7)
flexibility of operation desired, (8) salvage value, (9) degree of
mobility
of
equipment desired, (10) variability in quality of incoming water, and
(1
1) degree of reliability of operation required.
Conley (1965) has presented an excellent discussion on the interrelation of the
various components of a water treatment plant, showing how to determine the
optimum treatment practice and optimum sizing of the various components for
greatest economy.
The classic concept of water filtration is the use of the slow sand filter in which a
layer of solids (filter cake) is built up on the top layer of sand. This layer is known
as the “Schmutzdecke”. (It is composed of the solids causing the turbidity in the
water and bacterial growth). The filter cake concept applies only to the slow sand
filter. The rapid sand filters build a collected solids zone inside the top layers
of
the
filter media.
Until recently all domestic water plant installations have had sand filters, either
slow or rapid.
A
revolution in water filtration has occurred in the last four decades.
This revolution had its origin in the need for mobile treatment plants for use by the
armed forces during World War
11.
Portable diatomaceous earth filters were devised
and used extensively by the armed forces. In recent years the utility of these
diatomaceous earth filters has led to a determined assault on the problems of water
’
A more appropriate name will be “physicochemical theory”

358
filtration. In recent years more papers have been published on water filtration and
coagulation than on any other phase of water treatment.
The following filters are being used at the present time:
(1)
slow sand filters,
(2)
rapid sand filters; (a) gravity sand filters; (b) pressure sand filters, and
(3)
d’
iatoma-
ceous earth filters. Each filter has its proper place in water treatment.
Slow
sand filters
The slow sand filter has been superseded by the rapid sand filter in all new
installations built in recent years, because the slow sand filter requires very large
surface area for its operation, is inflexible, and is not backwashable. Regeneration of
the filter bed requires physical removal of the top layer of sand with the “Schmutz-
decke”, which is done with shovels.
Rapid sand filters
Rapid sand filters are divided into two main types:
(1)
gravity and
(2)
pressure
filters. The principles of the two types
of
filters are identical. The pressure filter is
operated at elevated pressures, thus prolonging the filter cycle and/or increasing the
rate of flow of water through the filter. Gravity filters are commonly operated at
2
GPM/sq ft
’,
whereas pressure filters are opearted at
3
GPM/sq ft and higher.
The rapid sand filter is operated with clarification ahead of the filter. This step
reduces the load on the filter, allowing longer filter runs and high quality effluent at
higher flow rates. Rapid sand filters have a layer of sand on layers of graded gravel
and do not utilize a “Schmutzdecke” layer for the filtration action. Instead, the
particulate matter is adsorbed on the sand in the layers below the surface.
A
considerable amount of support for the adsorption of solids causing turbidity as the
predominant removal mechanism of rapid sand filters was gained from the report of
O’Melia and Crapps
(1964)
in their study on the chemical aspects of filtration.
Rapid sand filters are customarily operated with sand on top
of
a graded gravel
bed.
A
considerable amount of interest, however, has been shown in some areas in
the use of sized coal in place
of
sand. Coal has the advantage of lower density,
occupying greater volume per unit weight, and, more important, requiring lower
velocity of the backwash water to suspend the coal bed during the washmg or
scrubbing cycle. Coal, however, is soft and abrades rapidly, with reduction in
particle size. This results in losses during the backwash cycle and, consequently, coal
replacement is much more frequent than that
of
sand.
A
skid-mounted bank of three high-rate rapid sand filters ready for shipment to
the field is presented in Fig.
9-6.
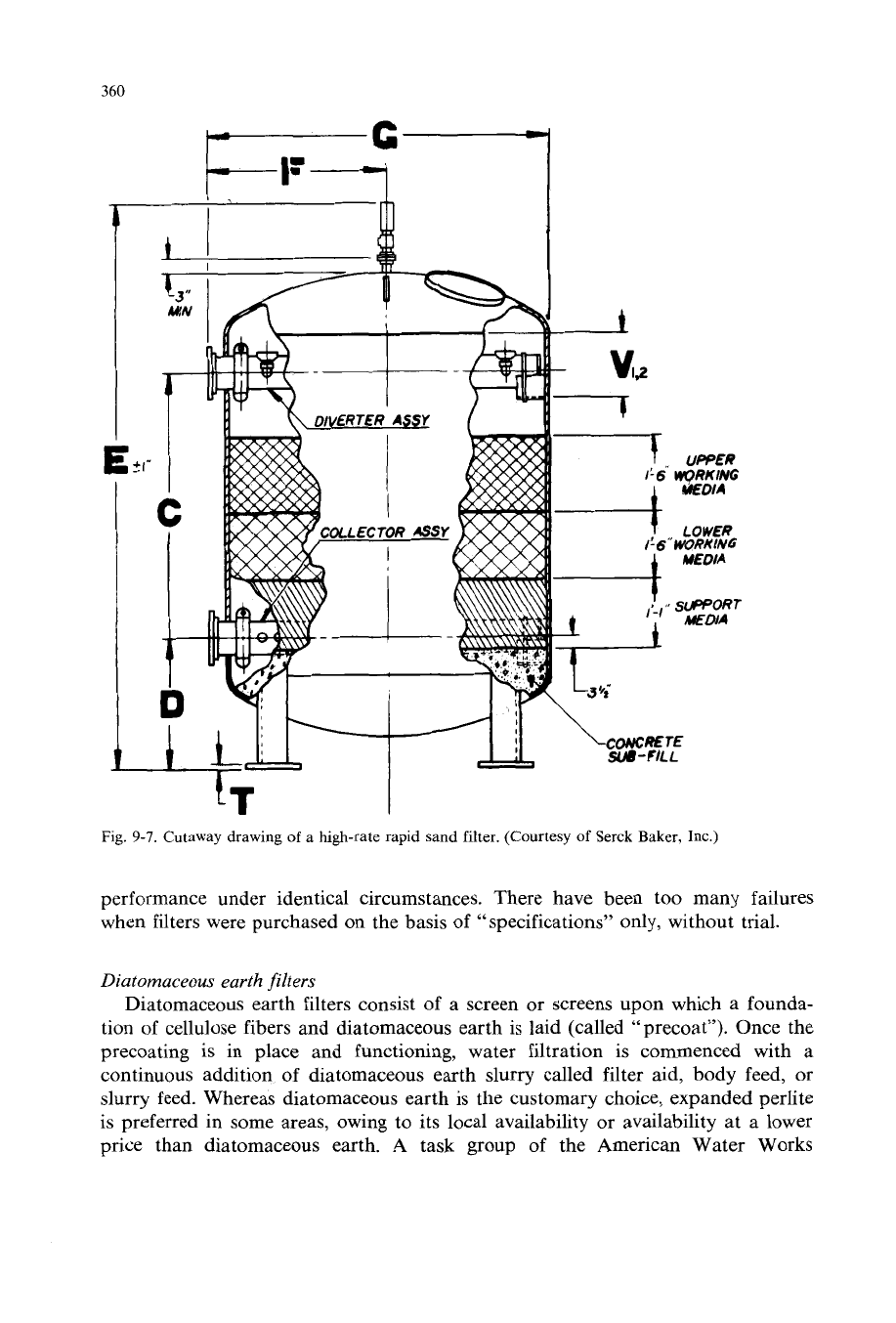
Figure
9-7
is a cutaway drawing of a hgh-rate
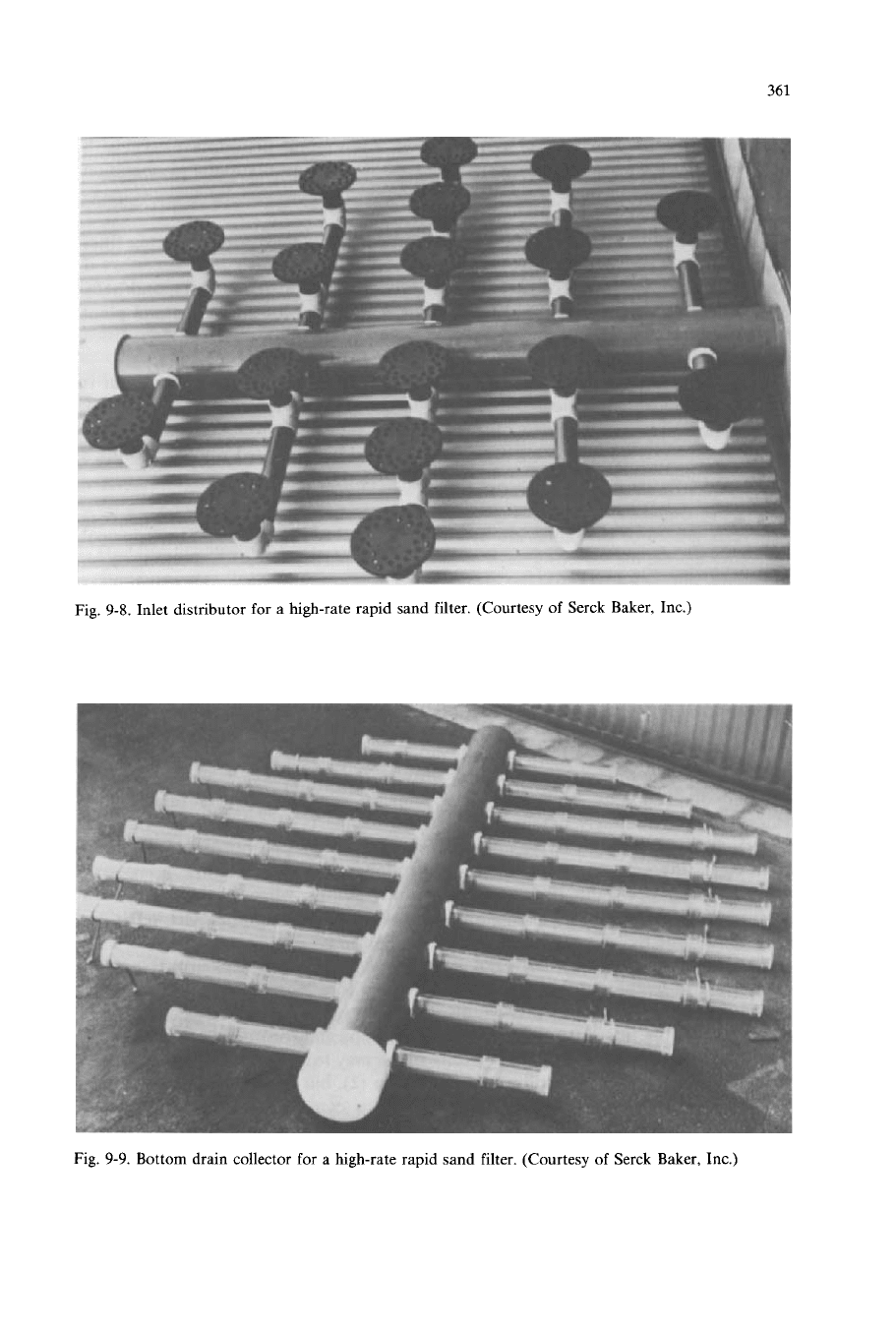
rapid sand filter showing the internals and the media. Figure
9-8
shows the inlet
distributor, whereas Fig.
9-9
shows the bottom drain collector for a high-rate rapid
sand filter. The openings are spaced to obtain an equal flow through each.
High-rate rapid sand filters
High-rate rapid sand filters have been developed in the
1960’s
(Udwin,
1971).
’
GPM
or
gpm
=
gallons
per
minute;
1
gal
=
0.003785
cu
m
=
3785.43
cu
cm.
359
Fig.
9-6.
Skid-mounted bank
of
three
high-rate rapid sand filters. (Courtesy
of
Serck Baker, Inc.)
Rates exceeding
10
gal/min/sq ft with effluent quality of less than 0.2 mg/l of
suspended solids are not uncommon. The high filtration rate is
achieved by
optimizing inlet and discharge header flows in order to obtain uniform flow through
the entire filter bed. Dual-media beds are common and some triple-media beds are
in use.
Dual-media may consist of coal on top of sand or coal on top of garnet.
Triple-media beds consist of all three, i.e., coal, sand, and garnet.
A
combination of
particle size and specific gravity serves to adjust the mass of the particles of each
media
so
that minimal intermixing of the layers takes place during backwashing.
The use of multiple media permits either higher bed loadings or longer filter runs.
Normally, the coarsest particles are at the inlet of the filter bed.
Other types of high-rate rapid sand filters have been developed and promoted.
A
popular type is the upflow filter in which the inlet is at the bottom and the flow of
water is up through the bed. Whereas advantages are claimed for upflow filters, one
must be aware of their sensitivity to hydraulic shock, which can cause unloading of
collected solids into the filter effluent. In another type of filter, the inlet is at the
center, with flow occurring both upwards and downwards from the middle
of
the
bed.
It is strongly advised that filters be purchased based on either field trial or
360
Fig.
9-7.
Cutaway drawing
of
a high-rate rapid sand filter. (Courtesy
of
Serck
Baker,
Inc.)
performance under identical circumstances. There have been too many failures
when filters were purchased on the basis of “specifications” only, without trial.
Diatomaceous earth filters
Diatomaceous earth filters consist of a screen or screens upon which a founda-
tion of cellulose fibers and diatomaceous earth is laid (called
“
precoat”). Once the
precoating is in place and functioning, water filtration is commenced with a
continuous addition of diatomaceous earth slurry called filter aid, body feed,
or
slurry feed. Whereas diatomaceous earth
is
the customary choice, expanded perlite
is preferred
in
some areas, owing to its local availability or availability at a lower
price than diatomaceous earth. A task group of the American Water Works
361
Fig.
9-8.
Inlet distributor for a high-rate rapid sand filter. (Courtesy of Serck Baker, Inc.)
Fig.
9-9.
Bottom drain collector for a high-rate rapid
sand
filter. (Courtesy
of
Serck Baker, Inc.)

362
Association led by Baumann (1965) prepared a report on diatomite filters, with
many references. This report covers in detail the design, installation, and operation
of diatomite filters in large-scale plants. It should be consulted for general back-
ground information.
Baumann and LaFrenz (1963) reported on the extreme need for optimizing filter
design, as water production costs can be four to five times higher than necessary if
other than optimum conditions are used for flow rate, terminal head loss, and body
feed. Automatic operation proved to be most economical for all waters and plant
siLes. Maintenance of an automated plant, however, can be a problem if qualified
personnel are not readily available.
In a paper presented by Bell (1962), a considerable amount of information is
made available about various aspects of design relating to hydraulic velocities,
septum considerations, adequacy of filter cleaning, precoating technique, and princi-
ples of continuous slurry feeding. This paper should be consulted when designing a
diatomite filter.
Selection
of
diatomite
Several grades of diatomite are available
for
use as a filter aid. The principal
difference among the various grades of diatomite is particle size distribution, which
causes the difference in filtration properties. The finest size filter aid, which gives
the lowest flow rate, is used for removing tight emulsions and ultrafine colloids. The
coarsest grade of diatomite (high flow rate), on the other hand, produces water of
good clarity when the
“
turbidity” is due to relatively coarse particles.
The common shortcoming of inadequately prepared diatomaceous earth is short
filter cycles, resulting from the rapid buildup in filtration pressure. The final
selection of a filter aid should be made only on the basis
of
field trials, inasmuch as
one is striving to optimize the following variables: filtration pressure, filter effluent
clarity, filter cycle time, and rate of flow through the filter.
When diatomaceous earth filters are properly run, they deliver hgh-quality
water. One can routinely obtain filtered water having 0.2 ppm suspended solids.
Unfortunately, it is very easy to plug wells on using improperly operated diatoma-
ceous earth filters. Some of the causes
of
filter failure are as follows:
(1)
Leaving open the line to hgh-pressure pumps when backwashng and
precoating filter.
As
a result, large amounts of fiber and diatomaceous earth are
injected into the wells.
(2)
Dropping off of the filter cake and precoat from the screen, in whole or in
part, owing to temporary shutdown of the filter or to a momentary pressure surge.
Subsequent operation of the filter results in all slurry feed and all suspended solids
going through the filter into the high-pressure pumps and/or into the wells.
(3)
Inadequate precoating of the screens which may leave holes in the precoating.
This results in the same condition as described in
(2),
but to a lesser degree.
(4)
Leaving a backwash valve open, partially or completely, after backwashing
the filter. This results in partial or complete bypassing
of
the filter.
This
is worse
than having no filter at all, because, in addition to bypassing the filter, filter aid is
supplied continuously to the water.

363
(5)
Mechanical damage to the septum allowing precoat and body feed to pass
through.
Frequently, it is advisable to install strainers
or
in-line filters downstream of a
diatomaceous earth filter to prevent mistakes or problems which could result in
plugging
of
wells.
REMOVAL
OF
DISSOLVED GASES
In addition to oil and solids removal, sometimes dissolved gases also must be
removed. The commonly encountered dissolved gases and resulting problems are as
follows:
Gas: Problem:
(I)
Hydrogen sulfide
(2)
Oxygen
(3)
Carbon dioxide
Corrosion, precipitation of iron
sulfide
Corrosion, bacterial growth
Corrosion
Undesirable dissolved gases may be removed from water by
(1)
aeration, by
spraying or cascading,
(2)
vacuum degassing,
(3)
countercurrent gas stripping, and
(4)
chemical treatment, in the case of presence
of
oxygen
or
low amounts of
hydrogen sulfide. The choice of the specific method is determined by the amount
of
contaminant and economics.
Aeration
Spreading water in order to create large surface areas for contact with air may
release unwanted gases, such as carbon dioxide and hydrogen sulfide, and take on
less objectionable gases, such as oxygen. The equipment to be used may be slat-type
cooling towers, spray nozzles, or high-speed breakers to achieve mechanical division.
EQUIPMENT CONSIDERATIONS
Equipment required for the collection, treatment, and distribution of injection
water for oilfields is a major consideration when determining the economic feasibil-
ity of various processes. Inasmuch as the water is usually aggressive and may require
treatment with aggressive chemicals, careful consideration should be given to the use
of corrosion-resistant materials, low-maintenance operations, and to a fairly high
degree of automation for reduction
of
plant upsets and operating labor costs.
Pipelines
Pipelines in the past have been normally made of bare steel. Massive replace-
ments and/or hgh maintenace costs of bare steel pipelines have led many operators

364
to alternatives. Some have concluded that properly treating the water requires more
commitment than they or their personnel are willing to give. Thus, they have
decided that pipelines must be made from a corrosion-resistant material, having
resistance greater than that of steel. The following altenatives have been used:
(a) Internally plastic lined steel pipeline with special attention to joints.
(b) Fiberglass reinforced plastic pipe, prefabricated and joined in the field.
(c) Plastic pipelines, such as PVC, may be satisfactory if the temperature of
produced water is low, the pipelines are not subject to external or internal shock,
and the pressures are low.
(d) Cement-asbestos pipelines, whch are resistant to corrosion if made
of
type-5
cement and are autoclave-cured. In special cases, the pipelines may be epoxy lined.
Again there
is
a pressure limitation.
(e) Cement-lined pipe.
In general, consideration should be given to the convenience of rapid dismantling
and relocation of the lines, as in the case of cement-asbestos pipelines, to the types
of epoxies and plastics to be used based on actual tests with the fluids handled, and
to
the requirements and costs of additional facilities, such as cathodic protection of
steel pipelines. External corrosion of pipelines is covered in Chapter 17.
The design
of
the pipeline should take into consideration: (a) the possibility of
hydraulic shock and surge, which may rupture the pipeline, (b) the venting of air
pockets, (c) methods
of
testing for leaks, (d) the use of cleanout pigs for the removal
of scale and other fouling materials, and (e) the use of surface-mounted pipelines
with attendant expansion problems versus buried and restrained pipelines. Many
instances of hydraulic rupture of steel pipelines are known where an inadequate
analysis
of
pipeline transients was made during the design phase. Cases are also
known where thermal expansion has caused inadequately buried pipelines to liter-
ally jump out of the ground.
Separators
The type
of
separators selected depends upon the origin and volume of sus-
pended materials to be removed, the aggressiveness
of
the waters, and the value of
the land on which they are to be located.
Volume and origin
of
suspended material to be removed
Normally, when the waters are to be injected for secondary recovery, the content
of suspended solids must be low, in some cases below
0.2
ppm. When produced
waters are combined with sea water, a heavy precipitate of barium sulfate may be
formed. The settling of ths precipitate may require a large sedimentation pond with
up to
72
hr retention time. This will also enable gravity separation
of
oil suspen-
sions.
Sedimentation ponds are made of reinforced concrete having sufficient depth to
allow a reasonable collection of sediment before being taken out of service for
cleaning. Generally, two or more separate ponds should be provided
so
that when
