Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.


305
protective resistances on the surfaces by an increase in pH and the precipitation of
scales with the application of cathodic protection.
The current density required for cathodic protection is related to the velocity and
supply of corrodants to the metal surface. For example, protective potential can be
achieved in a calm sea water environment with 3-5 mA/ft*, whereas 70 mA/ft2 or
more is required in the high-velocity waters of Cook Inlet in Alaska, as noted by
Bertness and Blaunt (1969).
In general, the current density required for cathodic protection of steel structures
in sea water ranges from about
5
to
80
mA/sq ft. In the mud zone, a current density
of only 1-4 mA/sq
ft
is required (Cron and Marsh, 1983, p. 1039).
Galvanic type of cathodic protection involves use of “sacrificial” anodes, such as
aluminum and magnesium. Cathodic protection current is generated when these
anodes are coupled to steel and are immersed in the same electrolyte, e.g., sea water.
These anodes are consumed upon generation of current, as electrons resulting from
the oxidation of aluminum are forced into the steel, which is below aluminum in an
electromotive series. Zinc, tin, mercury and indium are used as alloying elements of
aluminum anodes, which are typically cast on 2-in. steel pipe cores for use on
offshore platforms. These 300- to 800-lb anodes are attached by welding during the
construction of the platform, and are commonly designed to last as long as twenty
years (see Cron and Marsh, 1983, p. 1039).
In the case of impressed-current cathodic protection, alternating current is
converted to direct current by rectifiers. As compared to the galvanic cathodic
protection, electrical current is purchased as needed and fewer anodes are used. In
addition, renovation and repairs are not as difficult as the replacement of an entire
galvanic system (Cron and Marsh, 1983, p. 1039).
The disadvantage of impressed-current system is the delay in initiating cathodic
protection after the placement of platform on the seafloor.
In cathodically protecting offshore pipelines, sacrificial anodes (e.g., zinc) can be
placed in the form of bracelets along the pipeline at certain intervals. A newly-devel-
oped aluminum alloy is also operative in mud. Larger sacrificial anodes can also be
placed alongside the pipe and connected to the pipe with a cable. Cron and Marsh
(1983,
p. 1037) pointed out that insulation is an economical practice and should be
used universally except when flowlines are incorporated into a cathodic-protection
system (e.g., on offshore platforms). Decision as to whether to use cathodic
protection or not is reached after studying the leak frequency curve and logs,
expected well life, and leak repair costs. Typically, protection cost is around
US
$0.25/yr/sq ft of surface allowing for installation, maintenance, and operating
costs.
Cron and Marsh (1983, p. 1036) clearly showed that in pipelines, casings, etc. the
log of cumulative leaks is sometimes a linear function of time. Cathodic protection
can be used only for preventing the external corrosion of casing and not the internal
corrosion.
In the case of mooring chain links, much corrosion can be prevented through use
of a cathodic protection. Individual links, however, must be bonded to a cable for

306
electrical continuity. As pointed out by Cron and Marsh (1983, p.
1041),
aluminum
anodes in sufficient number attached to the buoy can protect both the chain and the
buoy.
Because of the differences in availability of dissolved oxygen, long cell action
may be set up between the lower parts
of
the casing (and the surface flowlines) and
upper parts of the casing. Thus, it is indispensable either to apply cathodic
protection to surface structures as well as to the casing or to insulate the casing
completely from large surface structures.
Comprehensive monitoring of potential of cathodic-protected structures is re-
quired to maintain effective control of corrosion.
Polarization tests are recommended for determining the current requirements for
complete protection
of
well casings and pipelines as discussed by Kubit (1968).
ROLE
OF
BACTERIA
IN
CORROSION
The influence of sulfate-reducing bacteria in the corrosion process has been the
subject of extensive investigations. The subject is complex and the reader
is
referred
to
the comprehensive treatment of the subject by Davis (1967). (Also see Chilingar
and Beeson, 1969.)
Microbial corrosion has not been significant in the corrosion of drillpipe.
Sulfate-reducing bacteria have produced serious corrosion to well casings as re-
ported by Doig and Wachter (1951). Pitting occurred on the external surfaces of the
casing where drilling fluid was present between the casing and the wall of the hole.
The low pH of the mud and the presence of organic nutrients were favorable for the
growth of sulfate-reducing bacteria.
The pH favorable for bacterial growth ranges from
5
to 9. Bacteria will thrive in
areas
of
stagnant flow even in high-pH systems, however, provided other require-
ments, i.e., temperature and contents
of
organic nutrients, salts, and oxygen, are
satisfied.
Sulfate-reducing bacteria are anaerobic and thrive only in the absence
of
oxygen.
In
an aerated system, oxygen is depleted in stagnant areas, eg, along the walls of
mud
pits and behind casing. This allows the sulfate-reducing bacteria to grow.
Equation can be presented as follows:
H2S0,
+
8H
+
bacteria
+
H2S
+
4H20
(8-23)
Thus, hydrogen atoms formed at cathodic areas of metals are removed and are
utilized to reduce sulfates to sulfides. The end products are live bacteria and
corroded iron.
The bacterial attack of organic additives
of
drilling fluids may result in excessive
use of mud chemicals and a rapid reduction of pH. The mud will become more
corrosive under these conditions. Thus, bacteria must be controlled by the use of
high pH (e.g.,
10.5)
or bactericides.

307
Bacteria in producing operations can contribute to corrosion, plugging of injec-
tion wells, and fouling of flowlines and water-handling facilities. Most oilfield
waters contain soluble organics which can be utilized by bacteria. Bacteria including
sulfate reducers can be cultured from most produced waters and waters in waterflood
projects.
Sulfate-reducing bacteria can be abundant and yet not be active in depolarization
of
the corrosion process. Sulfate-reducing bacteria preferentially utilize organics,
which usually are abundant in oilfield waters rather than nacent (atomic) hydrogen.
The hydrogen sulfide produced by bacteria, however, causes severe pitting in the
same manner as naturally-occurring hydrogen sulfide.
Oxygen in trace amounts in water facilities with sulfate-reducing bacteria will
accelerate the pitting attack and must be removed completely.
Years of investigation of anaerobic corrosion by Starkey (1958) did not disclose
serious bacterial corrosion in the presence
of
sulfate-reducing bacteria.
The injection of waters which contain sulfate ion, such as sea water, can change
sweet corrosion condition in the reservoir into a sour one. For example, the
Wilmington oil field in California, U.S.A., initially produced sweet oil and gas. The
conditions became sour following the injection of sea water. Hydrogen sulfide
content in the produced gas increased from an average
of
trace amounts to about
1000
ppm, with progress
of
the waterflood.
Sulfate-reducing bacteria can thrive in water filter systems. The large surface
areas in filter beds are sites for bacterial growth. The bacteria can generate H,S,
which is carried with the water into the distribution system. This results in corrosion
and contamination of the water and facilities downstream of the filters.
The filter backwash cycles should incorporate thorough cleaning with detergents,
bactericides, chlorine, or steam to maintain water quality.
CORROSION
IN
GAS-CONDENSATE WELLS
Corrosion in gas-condensate wells presents serious problems which cannot be
predicted accurately.
A
rigorous corrosion control program, with conscientious
monitoring of equipment condition and failures, properties of produced fluids, and
corrosion rates, is required to maintain corrosion control.
Analysis of the problem by NGAA
(1953)
and the NACE Committee on
Condensate Well Corrosion (1958) provided a few guidelines for control of corro-
sion. The
NGAA
(1953) statistical studies showed that corrosion was likely in
condensate wells with
(1)
depths greater than
5000
ft,
(2)
bottomhole temperatures
above
160"F,
(3)
bottomhole pressures above
1500
psi, and
(4)
CO, partial
pressures above 30 psi.
As the gas wells were drilled deeper (greater than
=
10,000
ft), the corrosion
problem became more complex. As a result of laboratory and field studies, Hilliard
(1980) has noted that the effects of gas composition, pressure, temperature, velocity,
and composition of produced water modify the simple relationshps between these
werq
m
PY
coTro5
3
2
I
luting
rote
CORROSION RATE
AS
FUNCTION
OF
BRINE PRODUCTION
Gas Production Range
0.4
-
0.7
MMcft/D
0
prior lo inhibition
inhibiled
KP-
153
mP),
'
I
,'SLOPE
=
KG=
a06
/
/
/
/
0
/
SLOPE
=
0.01
,
brine production
0
*'
10
20
30
40
50
60
bbl/MMcft
Gas Production Range
1.5-
3
MMcftAI
1
/
1
I
sL
I
I
I
i3
I
I
I
I
I
.OPE
=
El5
I
/
I
INHIBI'
brine
production
-
10
20
30
40
50
60
bbVMMcft
W
$2
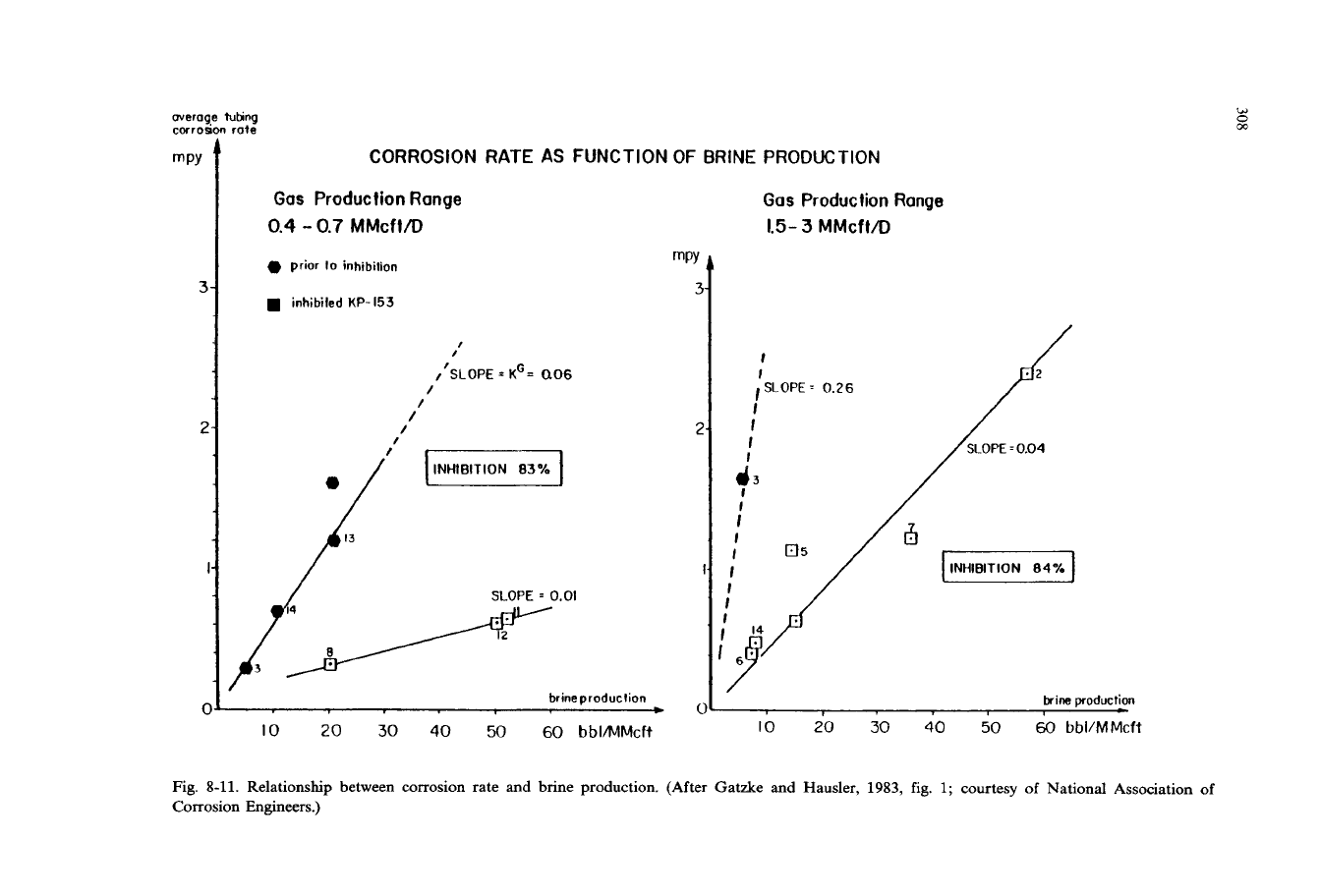
Fig. 8-11. Relationship between corrosion rate
and
brine production. (After Gatzke and Hausler, 1983, fig.
1;
courtesy of National Association
of
Corrosion Engineers.)
309
0.6
0.4
0.2
0.1
0.06
0.04
0.m
0.01
KG
mwMMef+
H20
bbl
prior
to
inhibition
EL0
I
Gas
Roducth
MMcft/O
I
-
123456
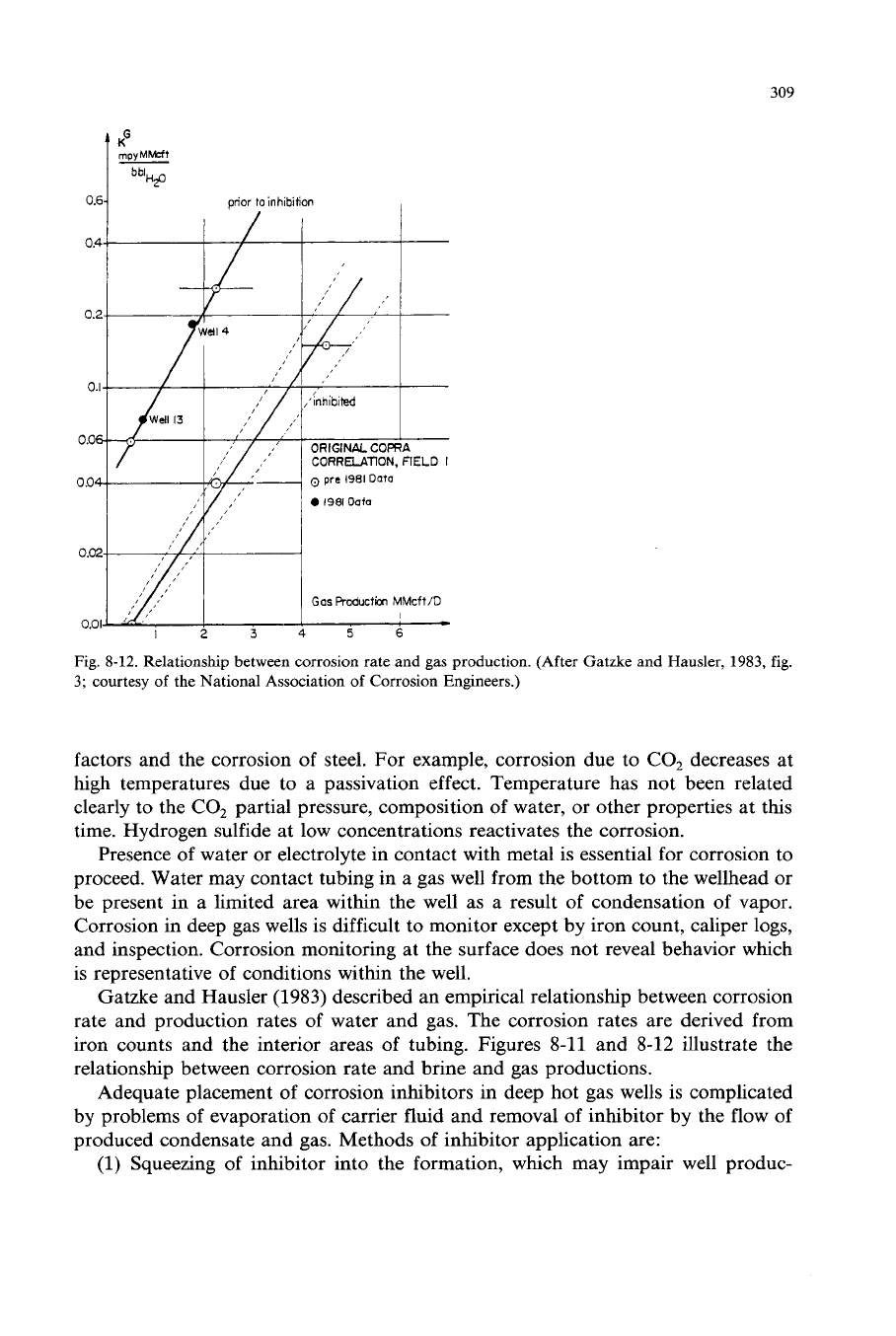
Fig.
8-12.
Relationship between corrosion rate and
gas
production. (After Gatzke and Hausler,
1983,
fig.
3;
courtesy
of
the National Association
of
Corrosion Engineers.)
factors and the corrosion of steel. For example, corrosion due to CO, decreases at
high temperatures due to a passivation effect. Temperature has not been related
clearly to the CO, partial pressure, composition of water, or other properties at ths
time. Hydrogen sulfide at low concentrations reactivates the corrosion.
Presence of water or electrolyte in contact with metal is essential for corrosion to
proceed. Water may contact tubing in a gas well from the bottom to the wellhead or
be present in a limited area within the well as a result of condensation of vapor.
Corrosion in deep gas wells is difficult to monitor except by iron count, caliper logs,
and inspection. Corrosion monitoring at the surface does not reveal behavior whch
is representative of conditions within the well.
Gatzke and Hausler
(1983)
described an empirical relationship between corrosion
rate and production rates of water and gas. The corrosion rates are derived from
iron counts and the interior areas of tubing. Figures
8-11
and
8-12
illustrate the
relationship between corrosion rate and brine and gas productions.
Adequate placement of corrosion inhibitors in deep hot gas wells is complicated
by problems of evaporation of carrier fluid and removal of inhibitor by the flow of
produced condensate and gas. Methods of inhibitor application are:
(1)
Squeezing of inhibitor into the formation, which may impair well produc-

310
tivity.
deep wells.
(2)
Batch treatment down the tubing, which may not reach the corrosive areas in
(3)
Continuous injection of inhibitor through a separate line to bottom.
The continuous injection method with appropriate inhibitor and carrier fluid is
the most effective procedure. A detailed discussion of corrosion characteristics and
control practices in deep hot gas wells was presented by Annand
(1981).
INHIBITORS AND PASSIVATORS
An inhibitor is a chemical substance
or
a mixture which effectively decreases
corrosion when added to an environment (usually in small concentration). A
passivator, on the other hand, is an inhibitor which appreciably changes the
potential of a metal to a more cathodic or noble value.
Anodic inhibitors for iron in water are soluble hydroxides, chromates, phos-
phates, carbonates, and silicates. These substances increase anodic polarization,
probably by helping to form (or to repair) a protective film on the metal surface.
Cathodic inhibitors for iron partially immersed in water are magnesium, zinc,
and nickel salts. As
0,
is reduced at cathodic areas, the pH is increased, resulting in
the precipitation of Mg(OH),, Zn(OH),, or Ni(OH), over the cathodic surface as a
fairly adherent porous deposit. Thus the reaction is slowed down because
0,
must
diffuse through these deposits in order to reach the cathodic surfaces. In waters
containing CO,, the calcium salts act similarly by precipitating CaCO, on the
cathodic areas as a result of increasing pH.
An insufficient concentration of an anodic inhibitor in a system that is under
cathodic control intensifies the attack on small, localized areas. This results in
pitting and early perforation. The required concentration of anodic inhibitor de-
pends on the concentrations
of
ions such
as
chloride or sulfate, which interfere with
the formation of passivating films. Other factors which are considered in determin-
ing the required concentration include:
(1)
agitation
of
the liquid,
(2)
the composi-
tion of the environment,
(3)
stresses in the metal,
(4)
composition of the metal,
(5)
contact with dissimilar metal, and
(6)
temperature.
Certain inhibitors change the electrochemical potential of a metal to more
cathodic or noble value. These inhibitors are called passivators, and they are
frequently anodic inhibitors. The cathodic inhibitors are not likely to act as
passivators. The iron will stay bright indefinitely in water containing a sufficient
amount of chromate (anodic inhibitor).
The pigments which are used in priming paints usually contain passivators. For
example, zinc chromate is a pigment which is soluble enough in water and diffuses
through the paint film bringing a small concentration of chromate ions, CrO:--, to
the metal surface. An appreciably less soluble chromate, such as lead chromate, does
not have this property.
The chromate ions form a thin protective film on the surface. This film is
composed of an insoluble iron compound such as iron chromate or a ferric-chromic

311
oxide mixture. According to the electron configuration theory, the chromate ions
form an adsorbed layer on iron. This film absorbs and shares electrons
of
the
surface iron atoms and satisfies secondary valence forces; however, it does not
disrupt the metal lattice. Thus, the metal surface becomes less reactive and more
noble in the galvanic series.
Sodium nitrite (NaNO,) is used as a passivator in oil pipelines and renders the
iron several tenths of a volt more noble than it was originally. The nitrite ion is
oxidizing in nature and, therefore, acts like chromates or other oxidizing passivators
to reduce corrosion.
Organic inhibitors are used for corrosion control in most producing operations.
Generally the inhibitor affects both the anodic and cathodic reactions by adsorption
on the metal surface.
The use of electrochemical methods to evaluate corrosion inhibitors under
laboratory and field conditions is discussed by Martin (1979, 1982).
The reader is referred to the excellent discussion of fundamentals
of
inhibitors by
Hackerman and Snavely (1971).
SAMPLE PROBLEMS
‘
Problem
8-1
A plant engineer has a problem of protecting the inside
of
a water tank from
corrosion, by “cathodic protection”. The tank is a vertical cylinder 10 ft high and 8
ft in diameter. This tank contains water to a depth
of
8 ft. The engineer installed
suitable anodes in the tank and connected it to the negative terminal of a rectifier
output. By inspection, water analysis, and former experience, he determined that a
total current of 5.06 A would stop the corrosion. Assuming that this current would
equal the sum of all corrosion currents (local cell currents) due to corrosion, and if
cathodic protection were not applied, what would be the tank life in years? The
original tank wall thickness is
0.300
in. and the tank has to be scrapped when the
wall thickness is reduced to
0.200
in. Both the tank bottom and walls in contact with
the water are being corroded.
Assume uniform corrosion and that iron corrodes as Fe
-+
Fe2+
+
2e-.
The charge on an electron is 1.59
x
C
(C
=
A
X
sec). One ampere flowing
for one second dissolves anodically, or deposits cathodically, 1.11800 mg of silver.
Avogadro’s number
=
6.06
X
Density of steel
=
7.8 g/cc.
’
Courtesy
of
Professor
J.S.
Smatko, formerly
of
Chemical Engineering Department, University
of
Southern California, Los Angeles, California.

312
Solution
:
Amount of steel corroded:
o‘loo
0.419 ft3.
(a) Bottom of tank
=
V,
=
7
X
-
=
(b) Sides
=
V,
=
(2
x
T
x
4)
X
-
o.loo
x
8
=
1.676
ft3.
(c) Total volume corroded
=
V,
+
V,
=
0.419
+
1.676
=
2.095 ft3.
(d) Total weight of steel corroded
=
2.095
X
28,400
X
7.8
=
465,000 g.
One Faraday
(=
96,500
C)
will corrode
1
g equ of a metal.
Amount corroded
=
465,000 g.
1
g equ of iron
=
55.85/2.
Current
=
5.06 A
=
5.06 C/sec.
Duration of corrosion
=
465’000
365
x
24)
=
10
yrs.
TX
82
12
12
96’500
=
31.8
x
lo7
sec
=
31.8
x
lo7
(1/3600
x
(55.85/2)
X
5.06
Problem
8-2
A steel water tank was to be protected by “cathodic protection” with
DC
current
applied
so
that the tank would have negative polarity. The tank was 12 ft tall, open
at the top, and was 10 ft in diameter. The wall thickness was 0.38 in. The practical
life of the tank was considered at an end when any part reached 0.18 in. in
thickness. The current flow was adjusted
so
that
0.05
A/ft2 was impressed.
Accidentally, connections were made backwards and the tank was made anodic
(deliberately corroded). The error was not noted for 2 yrs, the blame going to an
imperfect system and corrosive solutions. Assuming that the reaction is Fe
+
Fe2+
+
2e-
at 100% efficiency and that 26.8 A-hr are capable
of
dissolving 27.93 g of
iron, how much iron dissolved in 2 yrs? What was the wall thickness after 2 yrs?
What fraction of the tank’s life was used up?
Solution:
Wall area (wetted)
=
10~
X
12
=
377 ft2.
Area of tank bottom
=
T
X
52
=
78.54 ft2.
Total area
=
377
+
78.54
=
455.54 ft2.
Specific weight of Fe
=
7.8
X
62.4
=
490 Ib/ft3.
454
X
26.8
Electrochemical equivalent of Fe
=
2
yrs
=
2
x
24
x
365
=
17,500
hrs.
Current
flow
=
455.54
X
0.05
=
22.78 A.
A-hr in 2 yrs
=
22.78
X
17,500
=
398,000.
398’000
1000
Fe dissolved
=
Thickness loss
=
12
X
1.868/455.54
=
0.0492 in.
Fractional life loss
=
(0.0492
X
100)/(0.38
-
0.18)
=
24.6%.
Wall thickness after
2
years
=
0.380
-
0.0492
=
0.331 in.
27’93
‘Oo0
=
2.297 lb/1000 A-hr.
2.297
=
915 lb
or
915/490
=
1.868 ft3.

313
Problem
8-3
A sacrificial magnesium anode is used to protect a section of a steel gas pipeline.
It weighs 70 Ib and must be replaced when the remaining magnesium reaches
10
Ib
in
weight. During the protection job, only one-half of the magnesium corrodes in
such
a
way as to deliver current to the pipe. The other half is self-corroded without
any benefit to the pipe. How long would this anode last,
if
3 A flow continuously?
Atomic weight of Mg
=
24.32.
1
Faraday
=
96,500 C
=
26.8 A-hr.
Solution
:
Wt. loss
of
anode usefully
=
___
-
-
30
lb
or
30
X
454
=
13,550 g.
Corrosion reaction: Mg
+
Mg2+
-t
2e-
1
Faraday
=
96,500
C
=
26.8 A-hr and is the electricity produced by
1
g equ of
70
-
10
2
usefully corroding Mg.
Amount
of
g
equ corroded
= =
1114.
13,550
24.32/2
A-hr
=
26.8
X
1114
=
29,900.
hr/year
=
24
x
365
=
8760.
Life
=
29,900/(8760
X
3)
=
1.13 yrs.
Problem
8-4
A
steel vessel has a 0.5-in. thick wall. During use, it corrodes. Its internal area
is
40 ft2. Assuming
a
uniform corrosion rate, the following facts are to be noted: The
plant chemist found that the product, processed in the vessel, contained
1.0
g of
Fe(OH), in 150 gal of solution processed during each run; 24 runs per day were
made. Steel has a density of 7.7 g/cc. Find the corrosion rate expressed as
mg/m2/day (m.m.d.), also as in. penetration per year (i.p.y.). What is the life
of
this
vessel
if
it is considered worn out when the average wall thickness reaches 0.375 in?
Solution:
Area
=
40
X
0.0929
=
3.72 m2.
Fe(OH), produced per day
=
1.0
x
24
=
24 g.
Wall thickness
loss
=
0.5
-
0.375
=
0.125 in. or 0.125
X
2.54
=
0.318 cm.
Mol wt. Fe(OH),
=
106.85.
Fe metal loss/day
=
-
55'85
X
24
=
12.55 g/day.
106.85
12'55
'Oo0
=
3375 mg/m2/day.
3.72
Corrosion rate
=
Wt. loss/year
=
12.55
x
365
=
4580 g/year.
4580
VOI. loss/year
=
7
=
595 cm3.
=
0.016 cm or
-
o.016
- -
0.0063 i.p.y.
2.54
595
1.1
Thickness loss/year
=
0.125
Life
=
-
=
19.85 yrs.
0.0063
3.72
x
104

314
Problem
8-5
A coated and wrapped steel pipeline (500-miles long) having 18-in 0.d. is to be
protected by cathodic protection. Sacrificial magnesium anodes are used to supply
the electric current. The specific resistivity of the soil is 1000 f2-cm. The anodes are
grouped in stations, each station having
5
anodes weighing 17 lb each. The
5
anodes
of each station are “planted” on 10-ft centers in a string paralleling the pipeline,
and 10 ft away from the pipeline. Under these conditions a current density
of
0.01
mA/ft2 of pipe surface is obtained. The anode stations are placed 2 miles apart;
and the anodes in each station are connected in parallel with 10 PVC (polyvinyl
chloride) covered copper wire. These clusters are then connected by a 4 PVC
covered copper cable to a welded stud on the pipe. Each anode is surrounded by a
backfill composed of
5%
Na,SO,, 20% bentonite clay, and 75% gypsum. Installation
costs per mA-yr equal magnesium costs per mA-yr; magnesium costs $0.55/lb.
Assuming that only one-half of the planted magnesium actually yields useful current
for protection, find:
(a) Current flow per station.
(b)
Life of installation.
(c) Magnesium weight loss per station per year.
(d)
Cost
of installation.
(e) Cost of protection per ft2 of pipe per year.
Solution
:
Number of stations
=
500/2
-
1
=
249.
Wt. of Mg per station
=
5
X
17
=
85 lb.
Total weight of Mg for pipeline
=
85
X
249
=
21,164 lb.
Total cost for Mg
=
21,165
X
0.55
=
$11,640.75.
Area of pipe protected by one station
=
1.5
X
3.1416
X
5280
X
2
=
49,800 ft2.
Current per station
=
49,800
X
0.01
=
498
mA.
Electricity generated per year
=
498
x
24
x
365
=
4365
x
lo3
mA-hr
or
4365 A-hr.
Inasmuch as 26.8 A-hr are produced by 24.32/2 g of Mg at 100% efficiency, then
electrochemically useful Mg
=
4365
X
24.32/26.8
X
2
=
1980 g/station or 5.72 lb/
station.
Actually, twice as much magnesium corrodes per year; therefore, the lifetime
=
85/2
X
5.72
=
7.41 yrs.
Actual weight loss/station/year
=
2
X
1980
=
3960 g or 2
X
5.72
=
11.44 lb.
There are two possible interpretations concerning installation costs per year:
(a) If installation cost is equal to the cost of Mg effective in producing current
only, it would be $11,640.75/2
=
$5,820.37. Installation cost
+
Mg cost
=
5,820.37
+
11,640.75
=
$17,461.12.
(b)
If
installation cost is equal to the total cost
of
Mg used, then it would be
$1 1,640.75.
Installation cost
+
Mg cost
=
11,640.75
+
11,640.75
=
$23,281.50.
Interest charges, maintenance cost, overhead, and inspection costs should be added
to the above figures.
