Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.


165
DESIRED
PROPERTIES
OF
OILWELL
ACIDS
Each type of acid has its own peculiar chemical and physical properties. The type
of acid selected for stimulation of a particular oilwell would depend upon:
(1)
rock-dissolving capacity of acid,
(2)
spending time of acid, (3) solubility of reaction
products, (4) amount and pattern of metal corrosion, (5) compatibility of acid with
reservoir fluids, (6) density and viscosity of spent fluids, and (7) etching pattern
after acidizing (see Harp and Dobbs, 1967).
“
Rock-dissolving capacity” refers to the volume of rock that can be dissolved by
the acid. The increase in fluid conductivity of a flow channel is dependent upon the
quantity of rock dissolved from the surface of that flow channel. Harp and Dobbs
(1967) have shown that the conductivity of a fracture will vary as the cube of the
fracture width:
c,
=
4.5
x
10~~~
(5-2)
where
C,
=
fracture conductivity (darcy-ft) and
w
=
fracture width (in.). A fracture
having a width of 0.002 in. has a conductivity of 0.036 darcy-ft, whereas a fracture
having a width of 0.2 in. has a conductivity of 36,000 darcy-ft.
“Spending time” refers to the time required for an acid to expend 85-90% of its
initial “strength” (dissolving capacity). After that, the acid reaction is very slow due
to acid dilution. It is desirable to have a spending time high enough
so
that the acid
can be pushed away as far as possible from the wellbore into the formation before it
is spent. If the acid spends itself only a few inches from the wellbore, the improved
conductivity of flow channels will also end at that point. Acids with a longer
spending time offer a better opportunity for obtaining maximum and uniform
conductivity and deeper penetration of etched flow channels. This is of utmost
importance where it is necessary to create flow channels through a zone around the
wellbore damaged by the drilling fluid filtrate, etc. (“damaged zone”).
Four major reaction products, i.e., water, carbon dioxide, and calcium and
magnesium salts of that acid, are produced upon reaction of acid with reservoir
rock. The first two reaction products do not pose problems because they are
produced later along with the formation fluids. On the other hand, insoluble salts
can precipitate and plug the formation pore channels and/or fractures. Thus, a
prerequisite for any acid to be used is that the reaction salts must be soluble.
Solubility of salts varies with temperature and the quantity of similar salts already
dissolved in the brine. An additional problem arises when the acid-reaction salts
react with other ions present in the formation waters to form insoluble salts.
An acid by its very nature will react with various metals, such as iron, as shown
by the following reaction:
2HC1+ Fe
-+
FeCl
+
H
(5-3)
This process is often called “acid corrosion”. For most acids, a chemical inhibitor

166
must be added to the acid solution to aid in retarding the corrosion process. In the
case
of
many of these inhibitors, a protective thin film forms on the metal surface.
This chemical film, which serves as a barrier between the reactive acid and the
metal, tends to break down at high formation temperatures. Chemical acid corro-
sion inhibitors, however, do not provide
100%
protection from corrosion for long
periods of time.
As
pointed out by Harp and Dobbs (1967, p.
2),
a certain critical
volume of inhibitor-bearing acid must flow past the metal surface before a layer
of
inhibitor is deposited, decreasing the corrosion rate. Emulsions can form on
agitating a mixture of oil, water, and acid. If formed in flow channels, these
emulsions (1) will increase the pressure gradient required to move fluids in those
channels,
(2)
will increase fluid viscosity,
(3)
may plug formation pore channels, and
(4)
will cause a well-fluid clean-up problem. Reaction between acid and oil,
especially in the case of concentrated HCI
(28%
and higher), may also result in the
formation of sludges (precipitation of asphaltenes, etc.) in the flow channels, which
may result in plugging of the channels. Surfactants can be used to combat the
problems
of
emulsification and formation
of
sludges. Properly selected acid must
produce the least amount of emulsification and sludge, which can be economically
controlled (Harp and Dobbs, 1967).
As
pointed out by Harp and Dobbs (1967, p.
3),
the density and viscosity
of
spent acid water increase with increasing concentration of the initial acid. They
increase proportionally with the relative strength of the acid type used, because
increased quantities of calcium and magnesium salts are formed and dissolved in the
spent acid water. The increase in density of spent fluid in the case of
(1)
higher
concentrations of acid and/or
(2)
using higher ionizing (stronger) acids could create
some difficulty during the recovery of treating fluids (Harp and Dobbs, 1967, p.
3).
Some wells, which might otherwise flow after treatment, could require swabbing and
may experience delayed returns because the reservoir pressure is insufficient to
displace the higher density water.
For
example, in a 15,000-ft hole a difference in
hydrostatic head for spent
15%
HC1 and spent
28%
HC1 would amount to 990 psi
(Harp and Dobbs, 1967, p.
3).
Whenever possible, one would prefer an acid which
produces the maximum amount of carbon dioxide and only a moderate amount of
reaction salts (Harp and Dobbs, 1967, p.
3).
The chemical dissolution of the formation rock by the action of acids is called
etching.
In general, stronger acids and acids of higher concentration (1) produce
better etched flow channels with higher conductivities,
(2)
tend to channel or etch
more erratically, and
(3)
are more effective in reservoirs containing very small
amounts of dispersed insoluble fines. The choice of acid as far as etchability is
concerned depends upon the nature and etchability characteristics
of
the individual
reservoir rock (Harp and Dobbs, 1967, p.
4).
Pillar-pocket type of etchng results in
the case of carbonate rocks which are heterogeneous, because solubilities of lime-
stone (CaCO,), dolomite [CaMg(CO,),], dolomitic limestones, and calcitic
dolomites, that may all occur in the same formation, are different. Solubility usually
decreases with increasing content of MgCO,. Kinetic models for reaction of acids
with formation minerals are presented in Table 5-11.
As
pointed out by Williams et
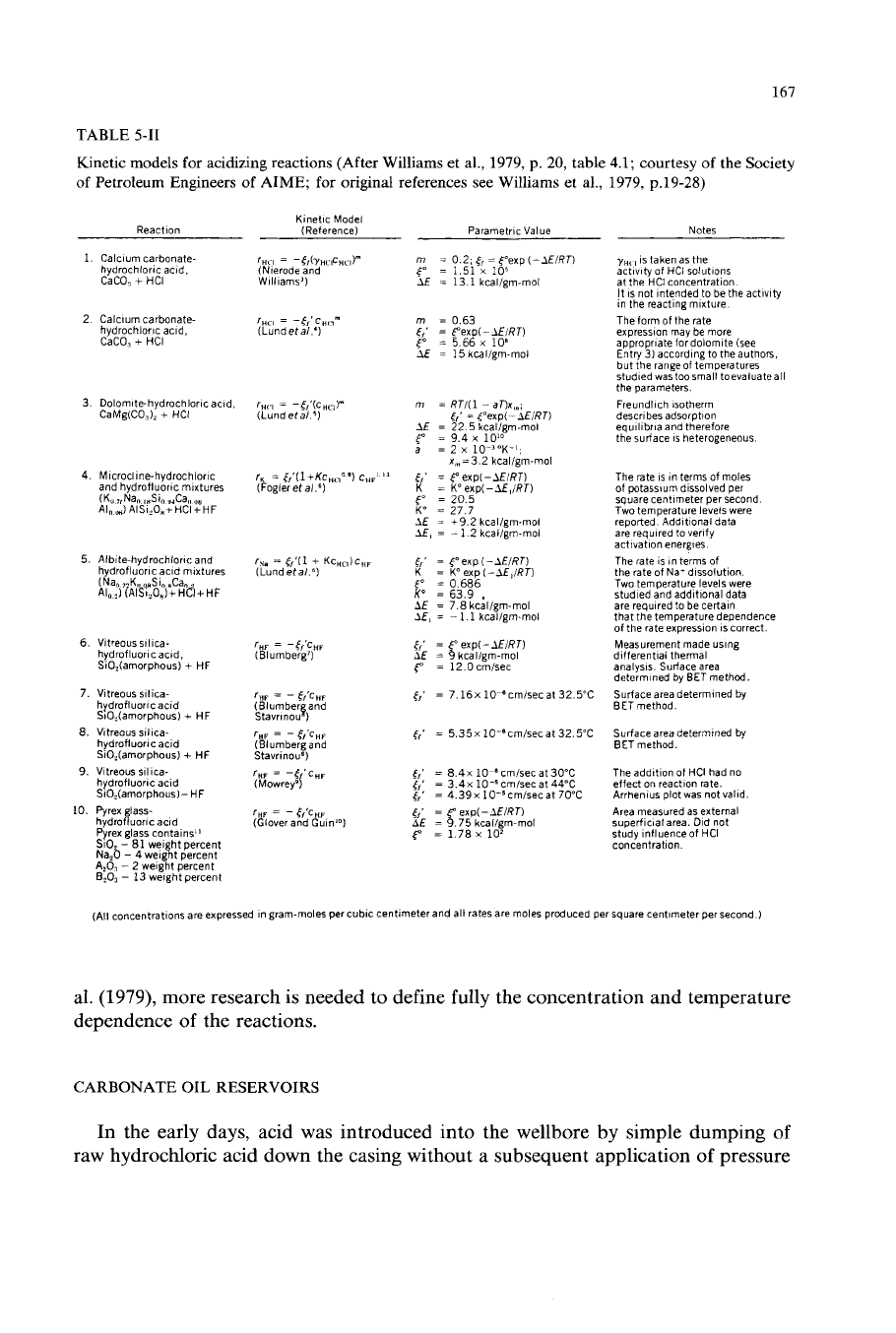
167
TABLE
5-11
Kinetic models for acidizing reactions (After Williams et al.,
1979, p.
20,
table
of
Petroleum Engineers of AIME; for original references see Williams et al.,
Kinetic Model
Reaction (Reference) Parametric Value
I.
Calcium carbonate
hydrochloric acid,
CaCO,
+
HCI
rHCl
=
(Nierodeand
Williams'l
m
6"
=
1.51
x
lo5
AE
=
13.1 kcallgm-moi
=
0.2,
5,
=
cexp
(-1ElRT)
2
Calciumcarbonate-
rHo
=
-z,'c,,," m =063
hydrochloric acid, (Lund el
a/
'1
f;
=
pexp(-1E/RJl
=
5
66
x 10'
5
=
15kcallgm-mol
CaCO,
+
HCI
3.
Dolomitehydrochloricacld,
rHC,
=
-(,'(c,,,l"
m
=
RTI(1
-
aT)x,,;
CaMg(CO.,l,
+
HCI (Lundel
(,'
=
6"exp(-1E/RTl
1E
=
22.5 kcallgm-mol
a
=
2
x
10-'"K-l;
5"
=
9.4 x 1010
x,,,=3.2
kcallgm-mol
4. Microclinehydrochloric
rK
=
(,'ll+K~,,,~~l cllF'
''
6,'
=
pexpl-LEIRTl
and hydrofluoric mixtures (Fogleref
K
=
K"exp(-AE,/RJl
(K,, ,,Nan ,,Si,.,Ca,,
,Jb
=
20.5
Al, AISi:O,+HCI
+HF
6:
=
27.7
AE
=
+9.2
kcallgm-mol
AE,
=
-
1.2
kcallgm-mot
5.
Albite-hydrochloricand
rXs
=
.$'I1
+
Kc,,,Ic,,,
(;
=
5"exp(-AE/RT)
hydrofluoric acid mixtures (Lund eta/.6)
K
=
K"
exp
l-IE,/RTl
INa
K
Si Ca,.,
Al, 9")7~AfS708")$ Hd
+
HF
1E
=
7.8 kcaligm-mol
A€,
=
-1.1
kcallgm-mol
6.
Vitreous sil ica-
rMF
=
-f,'cwr
hydrofluoric acid, IBlumberg'I
SiO,(amorphousl
+
HF
7.
Vitreous silica-
8.
Vitreous silica-
rHF
=
-
e'cHr
rHF
=
-
f,'cHF
hydrofluoric acid
(
Blumberg and
SiO,(amorphousl
+
HF Stavrinou
I
hydrofluoric acid (Blumbergand
SiO,(amOrphouSI
+
HF Stavrinou'l
9. Vitreous silica-
rHF
=
-&'c,,
hydrofluoric acid (Mowrey9)
SiO,(amorphousl- HF
10
Pyrex glass-
=
-
4'cH,
hydrofluoric acid (Glover and G~in'~1
Pyrex glass contains"
SiO
-
81 wei ht percent
Na,b
-
4 we& percent
A
0
2
weight percent
B:O:
I
13
weight percent
(;
=
gexpI-A-ElRTl
AE
=
kcaligm
mol
p
=
12 Ocmisec
f,'
=
7.16~10-~cm/secat32 5°C
&'
=
5.35~
iO-'cmisecat32.5"C
5'
=
8.4~ lO-*cmIsec at 30°C
4'
=
3.4x10-5cm1secat44"C
&'
=
4.39~ 10-5cmlsecat 70°C
exp(
-
1EIRTl
tE
1
c75
kcaligm-mol
p
=
1.78~ 10'
4.1;
courtesy
of
the Society
1979, p.19-28)
Notes
Freundlich isotherm
describes adsorption
equilibria and therefore
the surface
IS
heterogeneous
The rate is
in
terms of
moles
of potassium dissolved per
square centimeter per second
Two temperature levels were
reported Additional data
are required to verify
activation energies
The rate
1s
in
terms of
the rate of Na- dissolution
Two temperature levels were
studied and additional data
are required to be certain
that the temperature dependence
of the rate expression is correct
Measurement made using
differential thermal
analysis Surface area
determined by BET method
Surface area determined by
BET method
Surface area determined by
BET method.
The addition of HCI had
no
effect
on
reaction rate.
Arrhenius plot was
not
valid.
Area measured as external
superficial area. Did not
study influence of
HCI
concentration
(All concentrations are expressed
in
gram-moles per cubic centimeter and all rates are moles prcduced per square Centimeter per second
)
al.
(1979),
more research is needed to define fully the concentration and temperature
dependence
of
the reactions.
CARBONATE OIL RESERVOIRS
In the early days, acid was introduced into the wellbore by simple dumping
of
raw hydrochloric acid down the casing without a subsequent application
of
pressure

168
(acid soak). Carbonate formations, which demonstrate good response to acidizing,
comprised (1) limestones, (2) dolomites, (3) dolomitic limestones, and (4) calcitic
dolomites. The latter two contain CaCO,, MgCO,, and CaMg(CO,), in various
proportions. The chemical equation for the reaction between hydrochloric acid and
limestone is as follows:
2HC1
+
CaCO,
-
CaC1,
+
H20
+
CO,
(2
x
36.47) (100.09) (110.99) (18.02) (44.01)
(5-4)
The numbers under the chemical formulas represent the molecular weights of the
compounds and, therefore, the relative weights of substances which react or are
formed as products or reaction. Thus, 73 lb of hydrochloric acid reacts with 100 lb
of calcium carbonate to form
111
lb of calcium chloride, 18 lb of water and 44 lb of
carbon dioxide. Commercially available acid is diluted with water and has 35% HCl
by weight. At this strength, 1000 gal of HC1 will dissolve approximately 4710 lb of
CaCO,. The most frequent concentration of HC1 used is 15%, but concentrations
ranging from 3% to 32% have been used for stimulation. Hydrochloric acid has a
specific gravity of 1.075 at
20°C.
In 1000 gal of 15% by weight of HC1, there are
1344.8 Ib
(=
1000
X
8.34
X
1.057
X
0.15) of hydrochloric acid. Tlvs volume of acid
would react with 1842.2 lb
[
=
(184.3/146)
x
1344.81 of limestone. On assuming that
the average density for limestone is 170 lb/ft3, 10.84
ft3
of limestone will be
dissolved by the acid.
The reaction of hydrochloric acid with dolomite is similar to that with limestone,
except for the formation of magnesium chloride salt as shown in the following
equation:
4HC1
+
CaMg(CO,),
-
CaC1,
+
MgCl,
+
2H20
+
2c0,
(5-5)
(4
X
36.47) (184.3) (110.99) (95.3) (2
X
18.02) (2
X
44.01)
The reaction products are either water soluble or gaseous.
Hydrochloric acid has the greatest dissolving power for carbonate formations
followed
by
formic acid and then acetic acid. Inasmuch
as
in carbonate reservoirs,
under reservoir conditions, organic acids do not react to completion with either
limestone or dolomite, a given volume of acid will dissolve less rock than that
indicated by the chemical equations (Williams et al., 1979,
p.
14). In order to
determine the correct volume of acid required, one can use Table 5-111 which
presents the fraction of acid that reacts before chemical equilibrium is reached at
the reaction conditions (ile., formation temperature and pressure and concentration
of reaction products).
1'
In Table 5-111,
j3
is defined as the weight (or mass) of rock dissolved per unit
weight (or mass) of acid reacted:

169
TABLE
5-111
Dissolving
power
of
various acids
(After
Williams
et
al.,
1979,
p.
13,
table
3.4;
courtesy
of
the Society
of
Petroleum Engineers
of
AIME)
Xt
5 10 15 30
plu0**
Percent Percent Percent Percent
~-
-~
~~~ ~
~~
Limestone (CaCO,, calcite:
praco.,
=
2.71
grnicc)
Hydrochloric(HC1)
1.37 0.026
0.053
0.082 0.175
Formic (HCOOH)
1.09 0.020 0.041
0.062 0.129
Acetic (CHJOOH)
0.83 0.016 0.031
0.047 0.096
~-
Dolomite
-
ICaMg(CO,,),:
pl.ah,re,c'0,,2
=
2.87
gmiccl
Hvdrochloric
1.27 0.023 0.046 0.071 0.152
Formic
1.00
o.oi8
0.036 0.054 0.112
Acetic
0.77 0.014 0.027 0.041 0.083
*Data fororganicacids have not been correctedforequilibrium
I*
o,,,,,
mass rock dissolved
r,iass pure acid reacted
ix
=
-volume rockdissolved
volumeacid solution reacted
where
M,,,
=
molecular weight of mineral,
S,
=
stoichiometric coefficient
of
mineral,
Ma
=
molecular weight of acid, and
Sa
=
stoichiometric coefficient of acid.
In the case of pure limestone and
100%
hydrochloric acid, the dissolving power,
plo0
=
100.09
X
1/36.47
X
2
=
1.372 (wt of rock dissolved/wt of acid reacted).
In the case of 15% by weight HC1, the dissolving power,
PIS,
is equal to:
PIS
=
p,,,,,
x
0.15
=
0.206.
In terms of volume
of
rock dissolved per volume of acid reacted, the dissolving
power,
X,
is equal to:
X=
(sp. gr. acid
x
P)/sp. gr. carbonate
(5-7)
For
example, in the case
of
15% HC1 solution (sp. gr.
=
1.07) and pure limestone
(sp. gr.
=
2.71),
X,,
=
1.07
X
0.206/2.71
=
0.082 (volume of acid dissolved/volume
of
acid reacted).
Porosity of the rock is
not
included
in
these calculations.
Also,
inasmuch as
organic acids do not react completely, the acid will dissolve less rock than is
indicated in Table 5-111.
SANDSTONE
RESERVOIRS
Acidizing treatments in sandstone formations normally employ a mixture of
hydrochloric and hydrofluoric acids (Williams et al., 1979, p. 16). The primary
purpose
of
acidizing sandstone formations
is
to dissolve the fine particles such as
clays that plug the flow channels of the formation near the wellbore. These fines are
170
often introduced into the formation during the drilling operations from the drilling
fluid. Clays present in the formation may also swell upon contact with a mud-filtrate
or water that has a different concentration of ions from that
of
the original
formation water. For example,
if
Na/Ca ratio of mud filtrate is higher than the
Na/Ca ratio of the formation water, clays can be converted to Na-base clays which
swell more than Ca-base clays.
As pointed out by Williams et al. (1979, p. 16), the chemical reactions between
the hydrofluoric acid and silica or calcite in the rock matrix are comparatively
simple, whereas the reactions with clays and/or feldspars are complex. Thus,
chemical formulas such as those used in carbonate reactions are not easily de-
veloped. Williams et al. (1979, p. 16) have presented the following equations for the
reactions
of
hydrofluoric acid and various minerals which are found in sandstone
reservoirs:
Reaction with silica:
SiO,
+
4HF
+
SiF,
+
2H,O
SiF,
+
2HF
+
H,SiF6
Reaction with silicates (feldspar or clays):
Na,SiO,
+
8HF
+
SiF,
+
4NaF
+
4H,O
2NaF
+
SiF,
+
Na ,SiF6
2HF
+
SiF,
+
H,SiF6
(5-8)
(5-9)
(5-10)
(5-11)
(5-12)
Reaction with calcite:
CaCO,
+
2HF
+
CaF,
+
H,O
+
CO,
(5-13)
The dissolving power of hydrofluoric acid can thus be computed as in the case
of
carbonates. Hydrochloric acid is not appreciably reactive with sand and clay
minerals and, therefore, is not included in the dissolving-power calculations. In a
study of the complex reactions involved in sandstone acidizing, Labrid (1975) found
that for silica, the main product of the reaction is fluorsilicic acid accompanied by a
small amount of colloidal silica. In the first stage, feldspar and clay solubilization
takes place by a uniform alteration of its crystalline lattice. This is followed by a
progressive extraction
of
aluminum from the lattice in the form of fluorinated
complexes. Williams et al. (1979, p. 17) pointed out that at least seven fluorine
complexes
of
aluminum (from A1,F;- to A1F2+ are thought to be formed by the
contact of hydrofluoric acid with clay minerals.

171
DISTANCE
FROM
WELLBORE-
Fig. 5-1. Precipitation zone in sandstone acidizing. (After Labrid, 1975, modified by Williams
et
al., 1979,
fig. 3.5, p.
18;
courtesy of the Society
of
Petroleum Engineers
of
AIME.)
Labrid (1975) developed a model for the dynamics of equilibrium in the sand-
stone acidizing process.
As
shown in Fig.
5-1,
near the wellbore, HF and HC1
provide a solubilizing environment for the formation materials. In the zone de-
lineated by the cross-hatching in Fig.
5-1,
the solubility of silica compounds
[assumed to be Si(OH,)] is exceeded, resulting in precipitation. Fluoraluminates
precipitate farther downstream, with dissipation of the reactant HF. When sand-
stone is first contacted with
HF,
permeability reductions occur as a result
of
the
precipitation of silica and fluoraluminates (see Labrid, 1975). Williams et al. (1979,
p. 18) have suggested that this mechanism along with a reduction of permeability
due to mechanical effects may be responsible for the permeability drop.
ACIDIZING TREATMENTS
The two main benefits of acidizing are
(1)
dissolution of foreign or formation
materials in the wellbore that may be plugging or partially blocking the flow
channels through which the formation fluids are flowing, and
(2)
enlargement
of
the
formation flow channels, which increases the permeability near the wellbore. The
increase in well fluid productivity after treatment is the measure of success for an
acid job. It depends upon
(1)
correct recognition of subsurface problems that are
causing restriction in the flow of fluids into the wellbore, and
(2)
selection of the
proper acidization plan (acid type and volume to be used along with proper
additives). There are four broad categories of acid treatment for oil wellbores:
(1)
acid soak or acid washing,
(2)
matrix acidizing,
(3)
acidizing through pre-existing
fractures, and
(4)
high-pressure acidizing (acid fracturing; see Williams et al., 1979,
for a detailed treatment).
Acid
soak or
acid
washing
Acid washing is a process of removing scales from the oilwell or opening up of
perforations. The acid can be placed (spotted) into the wellbore at a desired position
and allowed to react with the scale (or formation), or it is circulated back and forth
across the casing perforations or formation face. The intent of this type of treatment
is to clean the surfaces of the wellbore and equipment by acid reaction without

172
penetrating into the formation near the wellbore. The tools can vary from simple
equipment, such as tubing to spot
a
small quantity of acid in the wellbore, to
complex tools which enable circulation of acid within the wellbore. Circulation of
acid within the wellbore is used to accelerate the dissolution process by increasing
the transfer rate
of
unspent acid to the wellbore surface and/or formation face
(Williams et al., 1979, p.
5).
Matrix acidizing
Matrix acidizing consists of injecting acid into the flow channels of the formation
(intergranular porosity, intragranular porosity, and/or vugs) at a pressure below
that which could cause fracturing. This technique is designed to radially penetrate
the formation near the wellbore, enlarging the flow channels and dissolving the
particles that
might
be plugging the pore spaces.
This
technique is useful where
formation damage due to the swelling of clay particles in pore channels with
consequent plugging has occurred. The plugging of formation could also occur as a
result of penetration of drilling solids into the formation. This method is also used
where high-pressure injection could create fractures that would break natural flow
barriers, such as shale, that must be maintained to prevent water
or
gas production.
Williams et al. (1979) listed the following reservoir
or
well conditions that are
necessary to obtain successful “matrix” acidizing results:
(1)
adequate natural
permeability must have existed
to
produce the fluids prior to damage of the
reservoir,
(2)
some degree of formation damage must be present,
(3)
sufficient
reservoir pressure must be present to force these hydrocarbons to the wellbore, and
(4)
hydrocarbons must exist in sufficient quantity for economic production. The
second condition is often difficult to evaluate quantitatively. It is ideal to determine
the wellbore damage from pressure buildup data. If such pressure data is not
available, however, other parameters may provide possible evidence
of
damage, such
as production anomalies. The first requirement is important because many matrix
acidizing failures are attributed to the lack
of
adequate initial in-situ permeability to
produce sufficient fluids. Acid fracture treatment under high pressure must be
considered in the latter case, as discussed in the following section. Even after
meeting the above requirements, some reservoirs do not exhibit successful results
after acidizing, because of improper reactions of acids with the formation fluids
and/or rock or precipitation of byproducts causing plugging. Proper selection
of
acid (strength and type) and its additives together with proper placement of acid
can prevent or minimize these problems.
The production increase by matrix acidizing is due to the total or partial removal
of the damaged zone near the wellbore. It can be estimated if the radius
of
the
damaged zone, its permeability, and the original in-situ formation permeability are
known. Pressure buildup data enables estimation of this information. Williams et al.
(1979, p.
5)
showed a method of estimating the productivity improvement possible
by using matrix acidizing. They considered the simplified radial system as shown in
Figs.
5-2
and
5-3:
a zone of reduced permeability,
k,,
extends from the wellbore
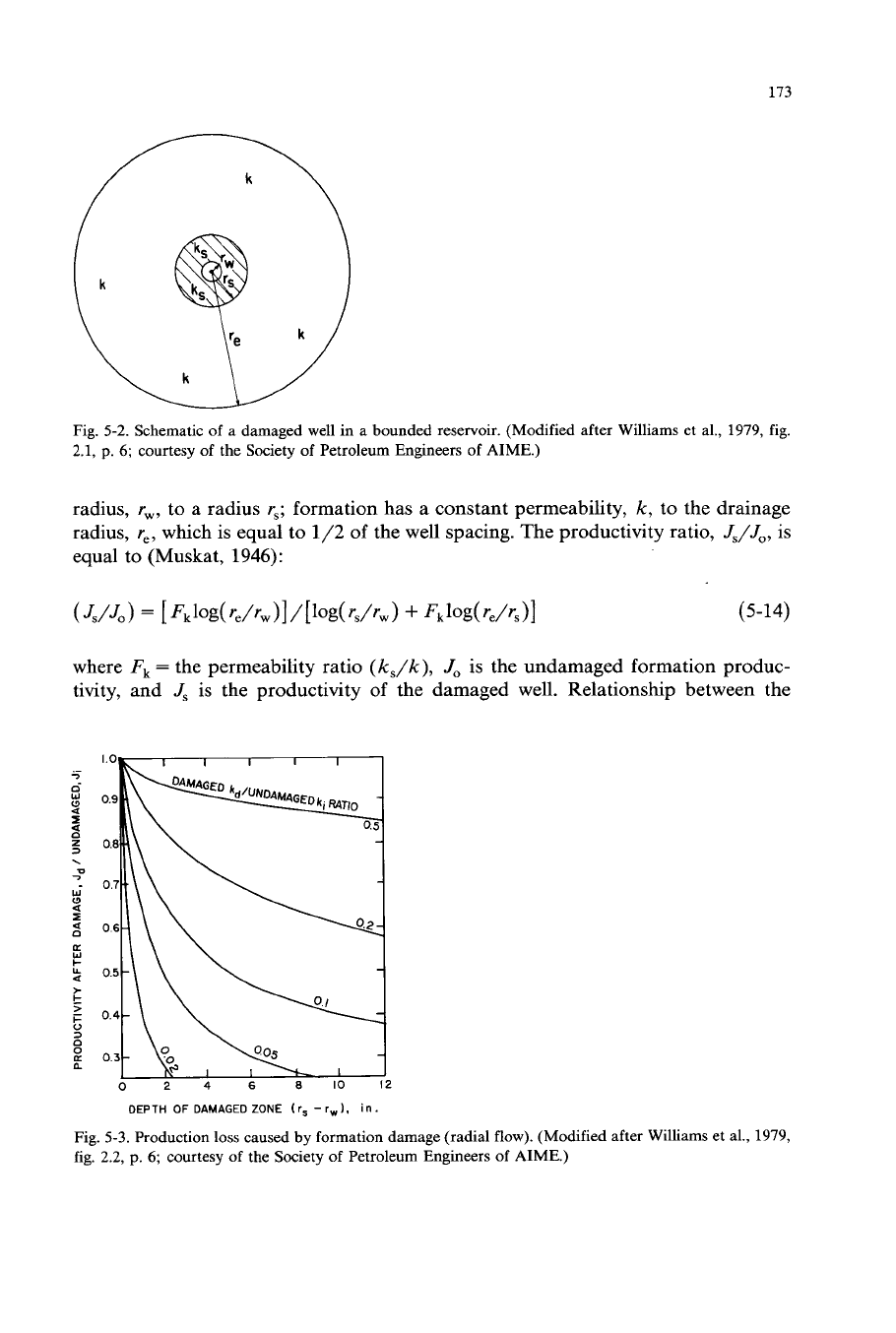
173
Fig. 5-2. Schematic
of
a
damaged well in a bounded reservoir. (Modified after Williams et al., 1979, fig.
2.1,
p.
6;
courtesy
of
the Society of Petroleum Engineers of AIME.)
radius,
r,,
to a radius
r,;
formation has a constant permeability,
k,
to the drainage
radius,
re,
which is equal to
1/2
of
the well spacing. The productivity ratio,
J,/J,,
is
equal to (Muskat,
1946):
where
Fk
=
the permeability ratio
(k,/k),
J,
is the undamaged formation produc-
tivity, and
J,
is the productivity of the damaged well. Relationship between the
DEPTH
OF
DAMAGED
ZONE
(
rs
-
rw
I,
in
.
Fig. 5-3. Production loss caused by formation damage (radial flow). (Modified after Williams et al., 1979,
fig. 2.2,
p.
6;
courtesy
of
the Society
of
Petroleum Engineers of AIME.)

174
depth of damaged zone
(r,
-
r,)
and
JJJ,
ratio for various values of
k,/k
is shown
in Fig. 5-3 for values of
(r,
-
r,)
ranging from
0
to 12 inches for a well having a
drainage radius,
re,
of 660 ft. For example, if the damaged zone extends
10
in. into
the formation and the permeability ratio is
0.1,
then the
JJJ,
will only be
0.4.
If
acidizing will remove all of this damage, there will be a 2.5-fold increase in
production rate (see Williams et al., 1979, p. 6).
In matrix acidizing of carbonate reservoirs, HC1 is normally utilized. Large and
sinuous flow channels, commonly referred to as “wormholes”, are created because
of the high rate of reaction of acid with the limestone or dolomite. These channels
start at the casing perforation, penetrating from a few inches to several feet into the
formation depending upon the volume and rate of acid injected. In matrix acidizing
of carbonates, therefore, acid tends to treat only the damaged portions of the
reservoir near the wellbore. Consequently, no significant stimulation is created
above that achieved by the removal of the wellbore damage (Williams et al., 1979, p.
7)-
High-pressure
acidizing
In high-pressure acidizing (with fracturing), acid is injected into the formation at
a pressure high enough to fracture the formation or open existing fractures.
Stimulation is significant only
if
the newly-formed, highly conductive fracture
remains open after treatment. The main purpose of this technique is to create flow
paths into the undamaged portions of the reservoir formation and thus increase the
drainage surface area into the wellbore (Williams et al., 1979, chapter 2). It is the
most widely used acidizing technique for stimulating carbonate reservoirs (lime-
stones and dolomites). According to Williams et al. (1979, p. 7), a pad of fluid is
injected into the formation at a rate higher than that which the reservoir matrix will
accept. When the pressure exceeds the compressive earth stresses and the rock‘s
tensile strength, the rock fails by fracturing. The fracture length and width increase
with continued fluid injection. Further injection of acid into the wellbore and then
into the fracture further widens the fracture by dissolving the carbonates. In order
for the treatment to be successful, the widened fracture must remain open after the
pressure is reduced and the well is placed back on production.
OILWELL ACID ADDITIVES
As
mentioned previously, certain chemicals are added to commercial acids
(1)
to
reduce emulsification of acid and other fluids in the formation, (2) to alter the rock
surface wettability to improve the acid attack or to achieve better cleanup, (3) to
reduce friction drop through tubing and other well equipment,
(4)
to divert acid
flow from one zone to another,
(5)
to tie up ions in soluble ion complexes
so
that
they do not precipitate and form insoluble salts, (6) to avoid the sludging of certain
highly asphaltic oils,
(7)
to reduce the rate of fluid loss from the fracture into the
matrix, and
(8)
to reduce the acid attack on metallic equipment.
