Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

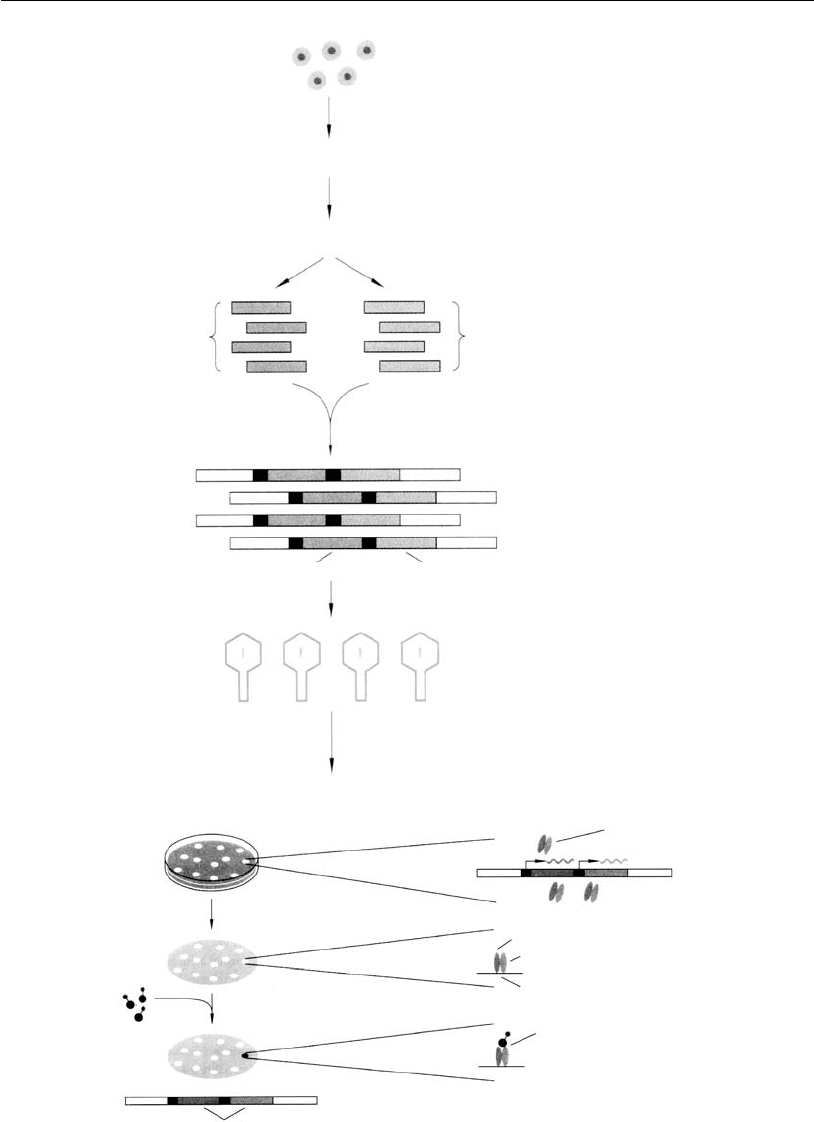
Amplified
heavy chain
cDNAs
Amplified
light chain
cDNAs
Lymphocytes from
immunized mouse
mRNA
Reverse transcriptase
cDNA
PCR PCR
Ligate H and L chain cDNAs
into expression vectorλ
H chain cDNA L chain cDNA
Package into phage
Combinatorial
phage library
Infect
H and L chains are
produced in infected cells
E. coli
H and L chains associate
to form Fab molecules
Prepare replica filter
Add labeled antigen
to filter
H and L chain-encoding cDNAs
from reactive antibody are cloned
Heavy chain
Light chain
Fab binds to filter
Fab binds
antigen
Isolate phage
DNA from
master plate
424 Animal Cell Technology
F(ab9)2 portion (Figure 17.4) normally last longer in circulation than do
smaller fragments (Fab, Fv, or scFv). If necessary, however, the half-life of
the smaller fragments can be increased by mixing them with a PEG, or by
chemical conjugation to form dimers, trimers, and tetramers (‘‘diabodies,
‘‘triabodies’’, and ‘‘tetrabodies,’’ respectively (Figure 17.4). These poly-
meric structures are obtained from the modulation of the length of the
flexible peptide bridges of the scFv modules (Hudson and Souriau, 2003;
Roque et al., 2004).
It is also possible to produce bi-specific antibodies (Figure 17.4), which
have two variable regions, each with a distinct specificity, but which bind,
for example, to two adjacent epitopes of a given antigen, thus increasing
the avidity of the connection of the antigen to the antibody. Bi-specific
antibodies can also be generated so that they bind simultaneously to a
tumor antigen and a cytotoxic T lymphocyte, thus facilitating the elimina-
tion of the tumor (Hudson and Souriau, 2003). These bi-specific antibodies
can be produced by hybrid hybridomas (‘‘quadromas’’), by either chemi-
cal or genetic conjugation, as well as by the fusion of adhesive heterodimer
domains of two or more Fab modules (Roque et al., 2004).
Bi-functional antibodies can also be obtained from the fusion of natural
antibodies or recombinant antibodies with compounds that might carry
out auxiliary functions after antibody binding to its specific target (Hud-
son and Souriau, 2003; Roque et al., 2004). These compounds can be
radioactive conjugates, cytotoxic drugs, proteins, toxins, enzymes, and
viruses, any of which can have diagnostic or therapeutic uses.
17.5 Producti on systems
The development of a specific process for the production of an mAb
requires the selection of (a) a system of expression; (b) a bioprocess for
obtaining the product, (c) a purification technique, and (d) analytical
methods for determining purity and product quality. It is clear that all the
choices must be compatible. So, for example, the selection of a process
must be compatible with the expression system. Moreover, the process
chosen must consider the specific conditions of the product in relation to
the competitive market, the quality required, and the total volume to be
produced.
Thus, for diagnostic or biogeneric industries, the production cost
dominates the choices, and the optimization of the productive process is
extremely important (NAS, 1999). This is not true for the therapeutic
industry, or for products protected by patents, which are more dependent
on the regulatory requirements.
Unlike the production of other proteins from animal cells, mAbs can
also be produced in vivo by inducing ascitic tumors in laboratory animals,
Figure 17.5 (opposite)
Construction of a mAb combinatory library expressed in a bacterial system (from
Recombinant DNA, 2/e by James D. Watson, et al. # 1992 by James D. Watson,
Michael Gilman, Jan Wirkowski, and Mark Zoller. Used with permission).
Monoclonal antibodies 425

as described above. This is an option when using hybridomas (Falkenberg,
1998; Hendriksen and Leeuw, 1998). However, there is a drive worldwide
to find suitable in vitro production methods, to avoid the suffering of
laboratory animals, as well as minimizing the risks of contamination of the
final product by adventitious substances. However, in certain specific
situations, the use of an in vivo system of production is unavoidable. This
includes the following: (a) when the mAb concentrations are low
(, 5 g/ml in batch systems, 50 g /ml in hollow-fiber bioreactors, or
300 g/ml in membrane bioreactors, (b) when it has been proved that the
use of cell culture results in a loss or decrease of the specific function of
the mAb; (c) when the hybridoma line can only grow and synthesize the
product in vivo; and (d) when the quantity to be produced exceeds the
laboratory capacity of the producer (NAS, 1999). A noteworthy example
of such a product generated in vivo is the therapeutic agent OKT3
1
, which
can lose its biological activity when purified from the supernatant of a cell
culture.
For antibodies produced in vitro, as is the case for many other proteins
obtained from animal cell cultures, the main question involves the low
level of expression of these products in the culture medium. This necessi-
tates the use of large culture volumes for production, thus involving higher
costs, especially for purification. In general, the optimization of these in
vitro processes attempts to increase the concentration of the product in the
medium. This is often possible by using high cell densities. Typical values
for traditional processes are in the range of 20–100 mg/L, whereas for
optimized systems, this can rise to 4.6 g/L (Kretzmer, 2002; Wurm, 2004).
It is unusual to find an optimized generic process for obtaining mAbs
since each producer cell has a unique pattern of response to stress,
consumption of nutrients and synthesis of products and byproducts.
However, it is clear that the systems utilized for the production of mAb
do not differ in any significant way from those using animal cells for the
synthesis of other products, as can be seen in various chapters of this book
(Chapters 5, 9, 11, and 12). For this reason, only those aspects that are
especially relevant for obtaining mAbs from in vitro systems will be
presented here.
17.5.1 Cell lines
Although various systems have been developed for the production of
mAbs, only those involving cultures of animal cells will be presented in
this chapter, since this is the system used to produce most of the antibodies
on the market today (van Dijk and van de Winkel, 2001). Those of murine
origin are produced directly from hybridomas, whereas the humanized or
completely human ones come from the culture of animal cells transfected
with specific genetic sequences, which are capable of following the
patterns of glycosylation and the desired structural conformation, required
for adequate drug performance.
Hybridomas constitute the most widely used cell lines for the produc-
tion of mAbs, on both small and large scales. Section 17.4 of this chapter
presents details of methods used to construct this type of cell. However,
these cells produce antibodies that have limited therapeutic application,
426 Animal Cell Technology

due to the immunological responses to murine antibodies that often arise
in patients.
As discussed above, one solution for this problem is the genetic
manipulation of cell lines to enable them to synthesize humanized anti-
bodies. In this situation, the hybridomas serve as an important source of
gene sequences that codify antibody molecules (or their fragments),
although these are later transfected into other animal cells. In general, the
cell lines used are more robust than hybridomas, due either to their greater
stability or even a reduced tendency for apoptosis.
The Chinese hamster ovary (CHO) cell line is now being used as a
standard host cell for transfection with genes of interest for later use in the
production of recombinant antibodies (Butler, 2005). Other cells with
equivalent performance are the murine myelomas NS0 and Sp2/0, as well
as baby hamster kidney (BHK), human embryonic kidney (HEK-93), and
a derivative of the human retina (PER.C6
1
) (Chu and Robinson, 2001).
The selection of the best cell line should consider antibody productivity,
as well as cell growth rate, although these parameters frequently follow
opposite trends (Wurm, 2004). For the production of large amounts of
mAb, it is fundamental that the productivity of the selected cell line is
high. Otherwise, larger reaction volumes will be required, and the cost of
purification will be increased. A reference value for specific protein
productivity is 20 pg/cell per day.
Another important aspect involved in the selection of transfected lines is
the capacity to grow without physical support, since the scale-up of such
processes is much simpler than those designed for growth of anchorage-
dependent cells. Thus, cells that grow naturally in suspension are pre-
ferred, such as myeloma cells (Sp2/0 and NS0), or others that can be easily
adapted to this form of cultivation, such as CHO and BHK (Chu and
Robinson, 2001).
17.5.2 Basic conditions for in vitro cultivation
Culture medium
Traditionally, the production of mAbs uses complex culture media con-
taining glucose and amino acids as the main sources of carbon for cell
metabolism, as well as vitamins, micronutrients and sometimes animal
serum, usually fetal bovine serum. Chapter 5 provides a discussion on
composition of culture media and recent trends in the search for formulas
that do not require the use of animal serum, or of proteins of animal
origin. These serum-free formulations use substitutes such as peptones,
epithelial and fibroblast growth factors, hydrolysates, yeast extract, cho-
line, and inositol. For the production of mAbs, various serum-free
formulas are available, some of these developed specifically for a given cell
line (Chu and Robinson, 2001). The development of those media is easier
for non-anchorage-dependent cells, such as those used for mAb produc-
tion. Thus, approximately 50% of the antibodies for therapeutic use are
already produced using serum-free media. In some circumstances, the
elimination of serum should be accompanied by the addition of other
substances with the same shear stress protective effect of serum proteins,
Monoclonal antibodies 427

such as Pluronic
1
F68 and carboxymethylcellulose (CMC) (Wu, 1995;
Chu and Robinson, 2001).
Most of the cell culture media are formulated to attain a physiological
osmolality in the range of 270–330 mOsm/kg. Under hypo-osmotic stress,
hybridomas show a reduction in cell growth rate without any specific
increase in antibody productivity (Ryu and Lee, 1999). However, under
hyperosmotic stress, some hybridomas show physiological changes such
as an increase in cell size, reduction in specific growth rate, and increase in
specific mAb synthesis (Cherlet and Marc, 2000). The overall effect of
these changes is a function of the cell line, and may or not result in an
increase in the production of the antibody (Ozturk and Palsson, 1991;
Bibila et al., 1994). The addition of osmoprotectors such as glycine, betain,
sarcosine, glycine, and proline may attenuate the toxic effects of high
osmolality on growth, with an overall positive effect.
Influence of oxygen
Oxygen is crucial for the production of energy during phosphorylation
and for the synthesis of cell components. In most cases, adequate growth
conditions require an excess of dissolved oxygen (DO), with the optimum
dependent on the cell line, cultivation conditions, and growth phase. The
critical range of DO, that is, that which limits growth and/or mAb
production, also varies as a function of cell line and cultivation conditions,
and usually is in the range of 10–20% of air saturation.
The best system for aeration and stirring of culture medium in a
bioreactor must minimize shear stress without significantly reducing the
oxygen transfer (K
L
a) and yet avoiding the generation of foam. In some
systems, protectors such as serum, Pluronic
1
F68, PEG, dextrans, lipids,
and cholesterol, may have to be used to prevent shear stress. Sensitivity to
shear depends on the cell line, and fortunately the lines most often utilized
industrially for the production of mAbs, such as hybridomas, CHO, and
BHK-21, are among the most resistant animal cells in relation to these
hydrodynamic forces (Chisti, 2000; Chu and Robinson, 2001; Wu, 1995).
17.5.3 Cell metabolism
As discussed in Chapter 4, glucose and glutamine are the two main
substrates in culture media used in animal cell processes, for generating
intermediate components of anabolism and catabolism (Doverskog et al.,
1997). The fraction of the energy produced by the metabolism of each of
the substrates depends on the cell line. Under conditions of excess glucose,
the hybridomas normally obtain 90% of the ATP necessary for metabolic
functions from glutamine, whereas CHO cells only obtain 40% (Jeong
and Wang, 1995).
An excess of glucose and amino acids, especially glutamine, generates
lactate and ammonia, respectively, and in many cases, these compounds
inhibit the growth of animal cells and the formation of the products of
interest, although the rate of mAb formation is not necessarily affected
(Schneider et al., 1996; Doverskog et al., 1997). Large quantities of these
metabolites in the culture medium can also diminish the quality of the
428 Animal Cell Technology

final product. Some cells can eliminate some of the metabolites by excret-
ing them in the form of alanine, which is a non-toxic byproduct.
Lactate reduces the internal pH of the cells or of the culture medium, as
well as increasing the osmolality of the medium. The mechanisms of
toxicity of ammonia are less well understood (Schneider et al., 1996). The
critical concentrations of these compounds depend on the cell line and the
culture conditions. Typical critical values for ammonia in the production
of mAbs are in the range of 2–10 mM (Schneider et al., 1996), whereas for
lactate the range is much broader, varying from 1 to 300 mM.
17.5.4 Bioreactors and operation mode
Various combinations of bioreactors and operation mode have been used
for the production of mAbs in several systems of expression, as shown in
Chapter 9. All cells utilized for the production of mAbs grow in suspen-
sion. Those that did not initially have this capacity have been adapted (as
is the case for CHO and BHK) (Butler, 2005). This results in a large
number of options for production systems. Cells with this characteristic
are easily cultivated in stirred-tank reactors, which have been scaled up to
a volume of 10 000 L (Chu and Robinson, 2001; Kretzmer, 2002). This
kind of bioreactor provides excellent homogeneity, facility for the imple-
mentation of control techniques, and the principles of scaling up are
relatively well known. Other kinds of bioreactors for the production of
mAbs are also available, such as air-lift, with volumes up to 1000 L, and
also fixed-bed bioreactors (Moro et al., 1994; Irving et al., 1996; Kretzmer,
2002).
Products with less demand, such as those used in diagnosis, are devel-
oped in small-scale systems such as T-flasks, rollers, and hollow-fiber
bioreactors (Kretzmer, 2002). The reduced size of these production
systems makes it possible to operate various units in parallel to obtain
different products. A small increase in scale can be reached by the multi-
plication of units.
Hollow-fiber bioreactors constitute an optimized production system
where it is possible to achieve higher cell concentrations (10
7
to 10
8
cells/
ml), and the product concentration can reach a level of 0.7–2.3 g/L, which
is similar to what can be obtained with ascitic fluid (Hendriksen and
Leeuw, 1998). This system can operate for over 3 months without affecting
cell viability, but presents problems with mass transport, and the forma-
tion of nutrient gradients, which require specific solutions (Kretzmer,
2002).
The operation mode used in the industry is predominantly batch, given
the simplicity and facility of control, good reproducibility, lower indices
of contamination, and low production costs (Xie and Wang, 1997). How-
ever, this operation mode has some disadvantages, the low cell and product
concentration, because it permits the accumulation of ammonia and
lactate, or the exhaustion of essential nutrients (Xie and Wang, 1997,
2006). Typical antibody concentration values in a batch system using a
stirred-tank bioreactor or air-lift are in the range of 100 g/ml.
Two approaches are possible to maximize the concentration of anti-
bodies in the culture. The first is based on an increase in the specific
Monoclonal antibodies 429

production rate by adding epidermal or fibroblast growth factors, IL-2
(Jang and Barford, 2000), butyric acid (Cherlet and Marc, 2000), or cyclic
nucleotides (Dalili and Ollis, 1988). Moreover, the addition of thymidine,
the reduction or elimination of serum, suboptimal concentrations of oxy-
gen, a reduction in pH, and the increase in osmolarity are situations that
can disturb growth, resulting in an increase in the specific antibody
production rate (Ozturk and Palsson, 1991; Reddy et al., 1992).
The second focus is based on obtaining greater cell concentrations for
longer time periods to maximize the production of mAbs (Duval et al.,
1991; Bibila and Robinson, 1995). In general, at least one of the following
factors would discourage growth and/or production: (a) exhaustion of
nutrients, especially certain essential amino acids, vitamins, glucose or
serum (Glassy et al., 1988; Duval et al., 1991; Jo et al., 1993a, 1993b; Hiller
et al., 1994); (b) inhibition due to the formation of toxic byproducts, such
as lactate and ammonia (Ozturk et al., 1992); and (c) inadequate concen-
tration of dissolved oxygen. Satisfactory results can be obtained simply by
increasing the concentration of nutrients and promoting an equilibrium of
salts to guarantee adequate osmolality (Jo et al., 1993a). However, there is
no way of avoiding the constant accumulation of inhibiting metabolites,
which ends up leading the process to collapse.
The fed-batch operation mode has been widely used in the industry due
to the simplicity of operation and control possibilities, easy scaling-up,
and efficiency in obtaining high cell and product concentrations (Jo et al.,
1993b; Bibila and Robinson, 1995; Xie and Wang, 1997; Sauer et al., 2000).
The increase in productivity is due to the manipulation and exploration of
the physiological state of the cells, redistributing the available resources
and rerouting the flow of metabolites. This avoids the synthesis of toxic
byproducts and, consequently, prolongs the phase of product accumula-
tion. However, it is inevitable that a progressive reduction in viability will
arise from problems of accumulation of toxic byproducts and high
osmolality.
The continuous process is the only way of avoiding the accumulation of
inhibitors, since it guarantees their constant removal at the same time as it
supplies the system with the nutrients necessary for the performance of
the cells. If perfusion is used, with cell retention, the system provides a
high cell density that is controllable, without gradients, and which can
maintain optimal conditions of nutrient concentration and toxic by-
products.
References
Abbas AK, Lichtman AH, Pober JS (2000), Cellular and Molecular Immunology, 4th
Ed., WB Saunders, Philadelphia.
Bibila T, Robinson DK (1995), In pursuit of the optimal fed-batch process for
monoclonal antibody production, Biotechnol. Prog. 11:1–13.
Bibila T, Glazomitsky K, Ranucci CS, Robinson DK, Silberklang M, Buckland BC,
Aunins JG (1994), Monoclonal antibody process development using medium
concentrates, Biotechnol. Prog. 10:87–96.
Butler M (2005), Animal cell cultures: recent achievements and perspectives in the
production of biopharmaceuticals, Appl. Microbiol. Biotechnol. 68:283–291.
430 Animal Cell Technology

Cherlet M, Marc A (2000), Stimulation of monoclonal antibody production of
hybridoma cells by butyrate: evaluation of a feeding strategy and characterization
of cell behavior, Cytotechnology 32:17–29.
Chisti Y (2000), Animal cell damage in sparged bioreactors, Trends Biotechnol.
18:420–432.
Chu L, Robinson DK (2001), Industrial choices for protein production by large-scale
cell culture, Curr. Opin. Biotechnol. 12:180–187.
Cotton RG, Milstein C (1973), Fusion of two immunoglobulin-producing myeloma
cells, Nature 244:42–43.
Dalili M, Ollis DF (1988), The influence of cyclic nucleotides on hybridoma growth
and monoclonal antibody production, Biotechnol. Lett. 10:781–786.
De St Groth FS, Scheidegger D (1980), Production of monoclonal antibodies: strategies
and tactics, J. Immunol. Methods 35:1–21.
Doverskog M, Ljunggren J, Ohman L, Haggstron, L (1997), Physiology of cultured
animal cells, J. Biotechnol. 59:103–115.
Duval D, Demangel C, Jolain KM, Miossec S, Geahel I (1991), Factors controlling cell
proliferation and antibody production in mouse hybridoma cells: I. Influence of
the amino acid supply, Biotechnol. Bioeng. 38:561–570.
Emanuel PA, Dang J, Gebhardt JS, Aldrich J, Garber EAE, Kulaga H, Stopa P, Valdes
JJ, Dion-Schultz A (2000), Recombinant antibodies: a new reagent for biological
agent detection, Biosens. Bioelectron. 14:751–759.
Falkenberg FW (1998), Monoclonal antibody production: problems and solutions,
Res. Immunol. 149:542–547.
Glassy MC, Tharakan JP, Chau PC (1988), Serum-free media in hybridoma culture
and monoclonal antibody production, Biotechnol. Bioeng. 32:1015–1028.
Hendriksen CFM, Leeuw W (1998), Production of monoclonal antibodies by the
ascites method in laboratory animals, Res. Immunol. 149:535–542.
Hiller GW, Clark DS, Blanch HW (1994), Transient response of hybridoma cells in
continuous culture to step changes in amino acid and vitamin concentrations,
Biotechnol. Bioeng. 44:303–321.
Horibata K, Harris AW (1977), Mouse myelomas and lymphomas in culture, Exp. Cell
Res. 60:61–77.
Hudson PJ, Souriau C (2003), Engineered antibodies, Nat. Med. 9:129–134.
Huhalov A, Chester KA (2004), Engineered single chain antibody fragments for
radioimmunotherapy, Q. J. Nucl. Med. Mol. Imag. 48:279–88.
Irving RA, Hudson PJ, Goding JW (1996), Construction, screening and expression of
recombinant antibodies, In: Goding JW (Ed.), Monoclonal Antibodies: Principles
and Practice, 3rd Ed., Academic Press, Manchester, pp. 424–464.
Jang JD, Barford JP (2000), Effect of feed rate and antibody production in the fed-
batch culture of murine hybridoma cells, Cytotechnology 32:229–242.
Jeong YH, Wang SS (1995), Role of glutamine in hybridoma cell culture: effects on cell
growth, antibody production, and cell metabolism, Enzyme and Microbial Tech-
nology 17:47–55.
Jo EC, Kim DI, Moon HM (1993a), Step-fortifications of nutrients in mammalian cell
culture, Biotechnol. Bioeng. 42:1218–1228.
Jo EC, Park HJ, Kim DI, Moon HM (1993b), Repeated fed-batch culture of
hybridoma cells in nutrient- fortified high-density medium, Biotechnol. Bioeng.
42:1229–1237.
Karpas A, Dremucheva A, Czepulkowski B H (2001), A human myeloma cell line
suitable for the generation of human monoclonal antibodies, Proc. Natl Acad. Sci.
U S A 98:1799–1804.
Ko
¨
hler G (1981), The technique of hybridoma production, In: Immunological Meth-
ods, vol. II, Academic Press, pp. 285–308.
Ko
¨
hler G, Milstein C (1975a), Continuous cultures of fused cells secreting antibody of
Monoclonal antibodies 431

predefined specificity, Nature 256:495–497.
Ko
¨
hler G, Milstein C (1975b), Derivation of specific antibody-producing tissue culture
and tumor lines by cell fusion, Eur. J. Immunol. 6:511–519.
Ko
¨
hler G, Shulman MJ (1978), Cellular and molecular restrictions of the lymphocyte
fusion, Curr. Top. Microbiol. Immunol. 81:143–148.
Kretzmer G (2002), Industrial processes with animal cells, Appl. Microbiol. Biotech-
nol. 59:135–142.
Lin MZ, Teitell MA, Schiller GJ (2005), The evolution of antibodies into versatile
tumor-targeting agents, Clin. Cancer Res. 11:129–38.
Little M, Kipriyanov SM, Le Gall F, Moldenhauer G (2000), Of mice and men:
hybridoma and recombinant antibodies, Immunol. Today 21:364–370.
Littlefield JW (1964), Selection of hybrids from matings of fibroblasts in vitro and their
presumed recombinants, Science 145:709–710.
Maki R, Traunecker A, Sakano H, Roeder W, Tonegawa S (1980), Exon shuffling
generates an immunoglobulin heavy chain gene, Proc. Natl Acad. Sci. U S A
77:2138–2142.
Moro AM, Rodrigues MTA, Gouveia MN, Silvestri MLZ, Kalil JE, Raw I (1994),
Multi-parametric analyses of hybridoma growth on glass cylinders in a packed-
bed bioreactor system with internal aeration. Serum-supplemented and serum-free
media comparison for MAb production, J. Immunol. Methods 176:67–77.
NAS (1999), Monoclonal Antibody Production, National Academic Press, Washing-
ton, DC.
Nelson PN, Reynolds GM, Waldron EE, Ward E, Giannopoulos K, Murray PG
(2000), Demystified monoclonal antibodies, J. Clin. Pathol. Mol. Pathol. 53:
111–117.
Ozturk SS, Palsson BO (1991), Growth, metabolic, and antibody production kinetics
of hybridoma cell culture: 2. Effects of serum concentration, dissolved oxygen
concentration, and medium pH in a batch reactor, Biotechnol. Prog. 7:481–494.
Ozturk SS, Riley MR, Palsson BO (1992), Effects of ammonia and lactate on
hybridoma growth, metabolism, and antibody production, Biotechnol. Bioeng.
39:418–431.
Pernis B, Chiappino G, Kelus AS, Gell PGH (1965), Cellular localization of immuno-
globulins with different allotypic specificities in rabbit lymphoid tissues, J. Exp.
Med. 122:853–877.
Reddy S, Bauer KD, Miller WM (1992), Determination of antibody content in live
versus dead hybridoma cells: analysis of antibody production in osmotically
stressed cultures, Biotechnol. Bioeng. 40:947–964.
Reichert J, Pavlou A (2004), Monoclonal antibodies market, Nat. Rev. 3:383–384.
Roque ACA, Lowe CR, Taipa MA (2004), Antibodies and genetically engineered
related molecules: production and purification, Biotechnol. Prog. 20:639–654.
Ryu JS, Lee GM (1999), Application of hypoosmolar medium to fed-batch culture of
hybridoma cells for improvement of culture longevity, Biotechnol. Bioeng.
62:120–123.
Sakano H, Maki R, Kurosawa Y, Roeder W, Tonegawa S (1980), Two types of somatic
recombination are necessary for the generation of complete immunoglobulin
heavy-chain genes, Nature 286:676–83.
Sauer PW, Burky JE, Wesson MC, Sternard HD, Qu L (2000), A high yielding, generic
fed-batch cell culture process for production of recombinant antibodies, Biotech-
nol. Bioeng. 67:565–597.
Schneider M, Marison IW, von Stockar U (1996), The importance of ammonia in
mammalian cell culture, J. Biotechnol. 46:161–185.
Shulman M, Wilde CD, Ko
¨
hler G (1978), A better cell line for making hybridomas
secreting specific antibodies, Nature 276:269–270.
Steinitz M, Klein JG, Koskimies S, Makel O (1977), EB virus-induced B lymphocyte
432 Animal Cell Technology

cell lines producing specific antibody, Nature 269:420–422.
Tonegawa S, Maxamt AM, Tizardt R, Bernard O, Gilbertt W (1978), Sequence of a
mouse germ-line gene for a variable region of an immunoglobulin light chain,
Proc. Natl Acad. Sci. U S A 75:1485–1489.
Vaisbourd M, Ignatovich O, Dremucheva A, Karpas A, Winter G (2001), Molecular
characterization of human monoclonal antibodies derived from fusions of tonsil
lymphocytes with a human myeloma cell line, Hybrid Hybridomics 20:287–92.
van Dijk MA, van de Winkel JGJ (2001), Human antibodies as next generation
therapeutics, Curr. Opin. Chem. Biol. 5:368–374.
Vijayalakshmi MA (1989), Pseudobiospecific ligand affinity chromatography, Trends
Biotechnol. 7:71–76.
Watson JD, Gilman M, Witkowski J, Zoller M (1998), Recombinant DNA, 2nd Ed.,
Scientific American Books, New York.
Wu J (1995), Mechanisms of animal cell damage associated with gas bubbles and cell
protection by medium additives, J. Biotechnol. 43:81–94.
Wurm FM (2004), Production of recombinant protein therapeutics in cultivated
mammalian cells, Nat. Biotechnol. 22:1393–1398.
Xie L, Wang DIC (1997), Integrated approaches to the design of media and feeding
strategies for fed-batch cultures of animal cells, Trends Biotechnol. 15:109–113.
Xie L, Wang DIC (2006), Fed-batch cultivation of animal cells using different medium
design concepts and feeding strategies, Biotechnol. Bioeng. 95:270–284.
Monoclonal antibodies 433
