Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

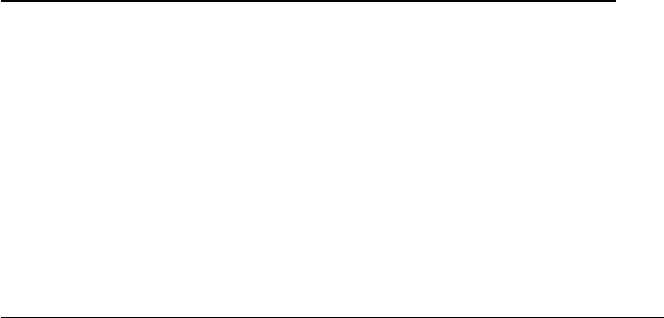
18
Viral vaccines: concepts,
principles, and
biopr ocesses
Isabel Maria Vicente Guedes de Carvalho Mello,
Mateus Meneghesso da Conceic¸a
˜
o, Soraia Attie Calil Jorge,
Pedro Estilita Cruz, Paula Maria Marques Alves,
Manuel Jose
´
Teixeira Carrondo, and Carlos Augusto Pereira
18.1 Introduction
Animal cell culture technology has always played a major role in the
development of virology. Significant progress in the propagation of viruses
and the consequent development of viral vaccines were only made possible
after the 1950s, upon the establishment of cell culture technology. Animal
cell culture gradually substituted live animals in the preparation of viral
antigens used in vaccination, such as the vaccines against smallpox and
rabies. At the same time, the production of viral antigens in cell cultures
led to considerable progress in bioprocesses technology. Cell culture
bioprocesses are now well established in bioreactors of up to 12 000 L.
Recent developments in virology and cell culture technology have
allowed research and development laboratories to engage in molecular
manipulation of viruses and cells for bioprocess production of viral gene
products. Examples are the establishment of recombinant viral vectors for
expression in animal cells that have significant potential in producing
recombining vaccines and treatment by gene therapy. Thus, a recombinant
vaccine against hepatitis B virus (HBV) has been developed and several
others are in the final phases of clinical trials.
This chapter concerns the development of cell culture technology for
viral vaccine production, which is related to: (a) a need for prophylaxis
and/or treatment of the most important viral diseases, such as AIDS,
hepatitis C, influenza, and papillomavirus; (b) the establishment of current
molecular technologies, and (c) a reduction in risk factors for the manip-
ulation of live viral particles.
Cell culture technology for the production of viral products is focused
on establishing protocols for recombinant products of low risk to prevent
viral diseases. In many countries, viruses are increasingly important as
biological agents for the control of agricultural pests (see Chapter 19).

18.2 Viral replication
The pathological effects of viral diseases are the consequence of various
factors, such as the toxicity of the products of metabolism of infected cells
and host reaction to infected cells expressing viral protein, as well as
changes in the genetic expression of host cells through structural or
functional interactions with genetic material or viral proteins. In some
cases, the symptoms or acute signs of diseases caused by a virus can be
directly related to the elimination of the infected cells.
For a better understanding of the pathological effects caused by viral
infections and of their control by vaccination or antiviral therapy, it is
important to understand how viruses infect cells, express their genes,
multiply, and change the cellular metabolism after the infection. The
genetic characteristics of the host as well as its sensitivity, are factors that
must be considered in evaluating the magnitude of viral replication.
Viruses are exclusively intracellular organisms and therefore depend on
the cells to multiply. A complete viral particle, or virion, consists of one
nucleic acid molecule (RNA or DNA) covered by a protein layer (nucleo-
capsid). Some virions have a lipid cover with a glycoprotein envelope. The
main function of the virion is to transport the viral genome to the interior
of the host cell to be replicated and amplified.
The infectivity of host cells varies considerably between viruses. A
specific virus may have a great diversity of host cells, while another may
be capable of infecting only one type of cell. The sensitivity defines the
capacity of the cell or animal to be infected.
Viral multiplication involves different ways of replication. However,
there are some common characteristics in the replicative cycles of viruses.
Initially, viruses insert their genetic material (RNA or DNA) into the cell,
and the size, composition, and genetic organization of this material vary
significantly between viruses, as well as the proteins that are needed for
replication. After the infection, there is a period called the eclipse phase,
when only few viruses are found in the infected cells. During this phase,
the genome and all the viral machinery is exposed to the host, but the viral
progeny is still small. Afterwards, there is a pause when virions accumulate
inside or outside of the cell at an exponential rate. This pause is called the
maturation phase. After some hours, lytic viruses cause cellular lysis with
the cessation of all metabolic activity and the cells lose their structural
integrity. Cells infected by non-lytic viruses can continue virion synthesis
over a long period of time.
The reproductive cycle of viruses may take hours or days. The infection
of cells does not guarantee the production of viral progeny, which may be
productive, restricted, aborted, or latent. A productive infection occurs in
permissible cells and results in infectious viral particles. An abortive
infection may occur in two circumstances: firstly, although the cell is
sensitive to infection, it is not necessarily permissive, allowing the expres-
sion of only a few viral genes. The second circumstance is when a sensitive
cell, permissive or not, is infected by defective viruses that do not have all
the necessary viral genes for their replication. Furthermore, the cells can
be temporarily permissible. In this case, the viral particles may remain in
the cells until they become permissive or else some viral particles may be
436 Animal Cell Technology

produced for a limited period of time by a fraction of the cell population.
This type of infection is called restricted. In a latent infection, the viral
genome persists in temporarily permissive cells without the destruction of
the infected cells.
The following sections outline the stages of the replication used by
viruses.
18.2.1 Adsorption
This stage consists of the virus binding to the cell. This involves the
specific binding of a glycoprotein that appears on the external structure of
a virus to a host cell receptor.
The sensitivity of a cell to a specific virus is frequently related to the
presence of these receptors in the cell membrane. The concept of sensitiv-
ity is not related to permissiveness. A cell may not be sensitive to a certain
virus due to lack of receptor, but may produce viral progeny if its viral
genome is introduced into its cytoplasm.
18.2.2 Internalizing and unwrapping the viral particle
After the binding, the virus may use various means of penetration: (a)
direct penetration, via the translocation of the entire virus through the
cytoplasmic membrane; (b) endocytosis, which is mediated by receptors,
resulting in the formation of intercytoplasmic vesicles containing many
viral particles; (c) direct fusion of the viral envelope with the cytoplasmic
membrane.
Non-enveloped viruses generally use the first two penetration mechan-
isms, while the enveloped viruses enter a cell by endocytosis followed by
binding with the membrane of an endosome. In addition to this mechan-
ism, the enveloped viruses fuse directly with the cell membrane. The
fusion of the viral envelope with the cell membrane requires the inter-
action of the glycoproteins of the virus with a cell receptor. After the
internalization of the viral particle, the genome is freed for later expres-
sion. This process is known as unwrapping and it involves both cellular
and viral enzymes.
18.2.3 Structure and organization of viral genomes
Viral genomes consist of RNA or DNA, which can be single- or double-
stranded, and may consist of one or more fragments. During viral replica-
tion, both DNA and RNA viruses synthesize protein by translation of
messenger RNA. The mRNA is then translated by the cell into the viral
proteins that will constitute the viral particles.
Virus with single-strand RNA
There are three groups of virus with a single-stranded RNA genome
(ssRNA). The first group comprises viruses with RNA that functions both
as genomic and messenger (for example, picornovirus and flavovirus). The
ssRNA in these viruses is conventionally called positive-strand virus
Viral vaccines: concepts, principles, and bioprocesses 437
(+sRNA). After it enters the cell, the +sRNA functions as mRNA, binding
with cellular ribosomes to complete its translation into proteins. The
product of this translation is a polyprotein that will later be cleaved.
The second function of this RNA is genomic, to be used as a precursor for
the synthesis of a complementary negative RNA strand (RNA–) catalyzed
by a polymerase derived from the polyprotein cleavage. The negative
strand will be transcribed once again as genomic RNA (RNA+) through a
viral polymerase. During this process, several strands of genomic RNA
will be produced, as well as proteins that will be used to produce the viral
particles. One characteristic of the RNA+ virus replication is the capacity
of its genomic RNA to function as mRNA after the infection, thus leading
to the synthesis of the enzymes responsible for the viral genome replica-
tion, without need for the complete viral particle to carry the enzymes
(Figure 18.1). This allows the RNA extracted from the virus to become
infectious, although less than the complete viral particle.
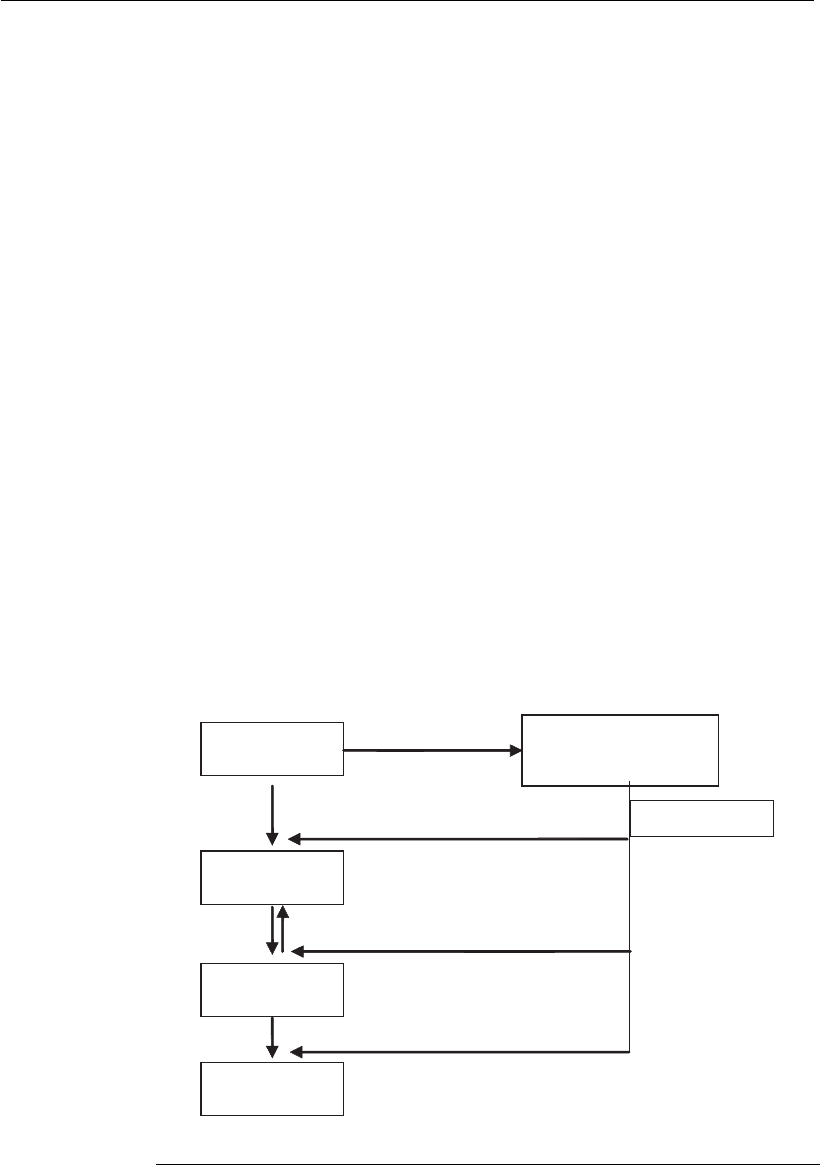
Another group of ssRNA viruses consists of those that have negative
single-strand RNA (–sRNA). This group consists of orthomyxovirus,
paramyxovirus, and rhabdovirus, among others. The genomic RNA of
these viruses has two functions: to serve as a template for transcription and
for replication. The viral genome must translate its own mRNA in order
to synthesize viral proteins because the host cell does not have the
appropriate enzymes. Therefore the –sRNA virus will have a transcriptase
along with its genome. The isolated viral genome is not infectious, because
there must be a viral transcriptase to translate the genomic RNA into
several mRNA strands that will later be translated into viral proteins.
These are used to make a copy of all its genome, producing a large strand
Viral progeny
Viral RNA
strand )(⫹
Polyprotein coded by
viral genome
RNA
strand ( )⫺
Viral proteases
RNA
strand ( )⫹
Figure 18.1
Flow of events during the replication of a positive-strand RNA virus.
438 Animal Cell Technology
of +mRNA that will serve as a template for the synthesis of genomic –
RNAs (Figure 18.2).
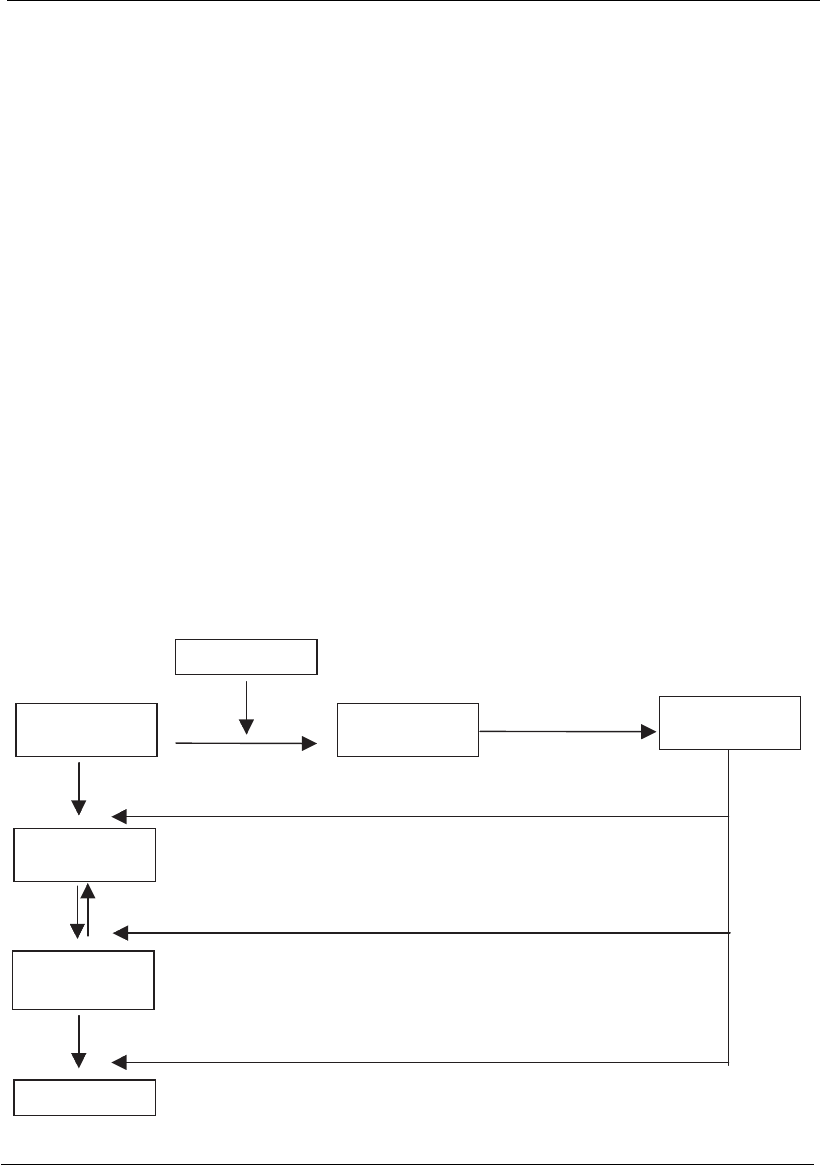
The retroviruses are the third group of single-strand RNA virus. These
viruses have a more complex strategy for the production of mRNA. The
retroviruses have a diploid genome associated with a DNA-dependent
RNA polymerase (reverse transcriptase) that transcribes RNA into a
hybrid DNA–RNA. The RNA strand is digested by the viral ribonuclease
H and a complementary DNA is synthesized, thus producing a double-
strand DNA (dsDNA) that will be inserted into the cellular genome with
the participation of the viral integrase enzyme, producing a provirus
(Figure 18.3). After the integration, the cellular-dependent polymerase
RNA–DNA will initiate the synthesis of the proteins that are essential to
replicate the viral genome followed by the synthesis of proteins to produce
the virus.
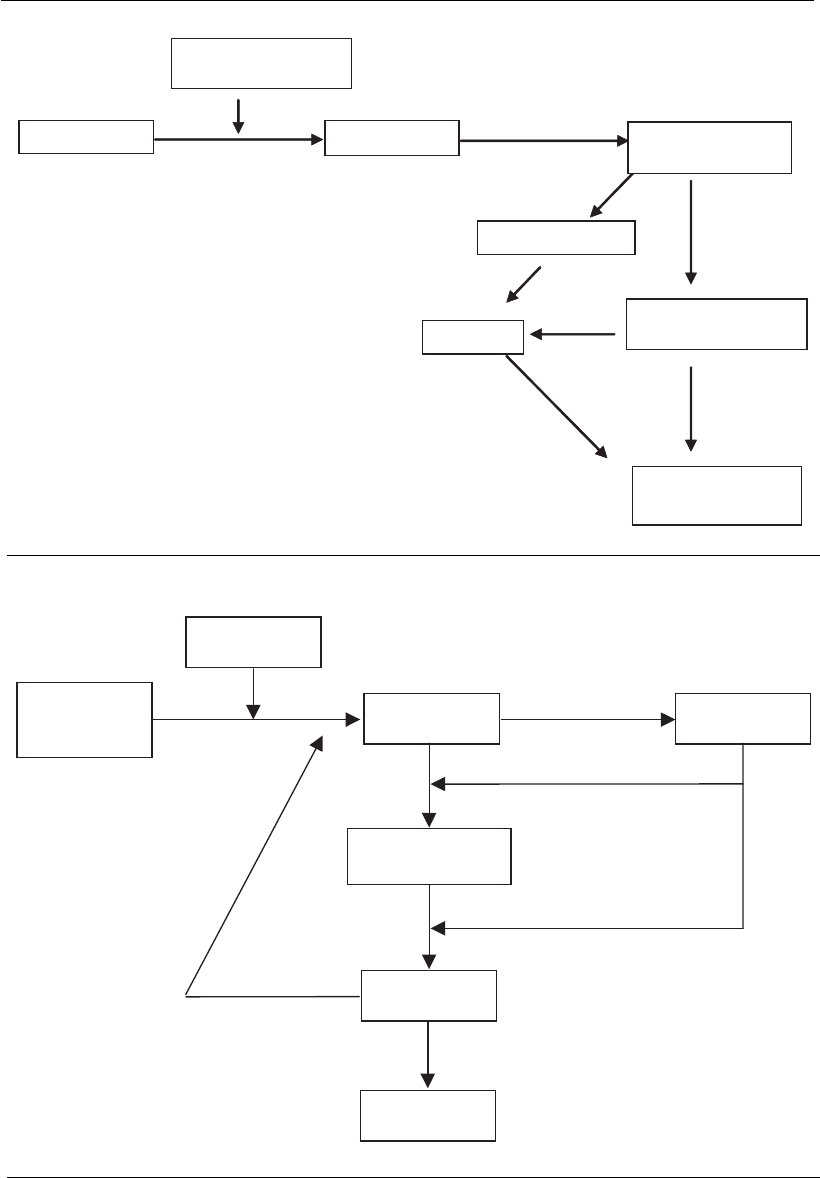
Virus with double-strand RNA
The genome of reoviruses is formed by double-strand RNA (dsRNA)
composed of several dsRNA fragments (10–12). The RNA fragments are
transcribed inside a capsid by a polymerase, giving rise to several mRNAs.
Here, the mRNA molecules have two functions: (a) they are translated as
monocistronic messengers, forming viral proteins; (b) they serve as a
template for the synthesis of a complementary RNA strand, giving rise to
double-strand RNA genomic fragments (Figure 18.4).
Viral RNA
strand ( )⫺
⫹mRNA
Proteins
RNA
strand ( )⫹
Progeny RNA
strand ( )⫺
Viral progeny
Viral enzymes
Figure 18.2
Flow chart of events during the replication of negative single-strand RNA virus.
Viral vaccines: concepts, principles, and bioprocesse s 439
Viral progeny
tRNA
viral enzymes
RNA progeny
mRNA
Linear DNA
Viral RNA
Integrate in
DNA
Proteins
Figure 18.3
Flow chart of event s during the replication of retroviruses.
Double-strand
RNA progeny
Viral progeny
Partial production
Double-strand
viral RNA
Proteins
Viral enzymes
sRNA
( ) strand⫹
Figure 18.4
Flow chart of events during the replication of reovirus.
440 Animal Cell Technology
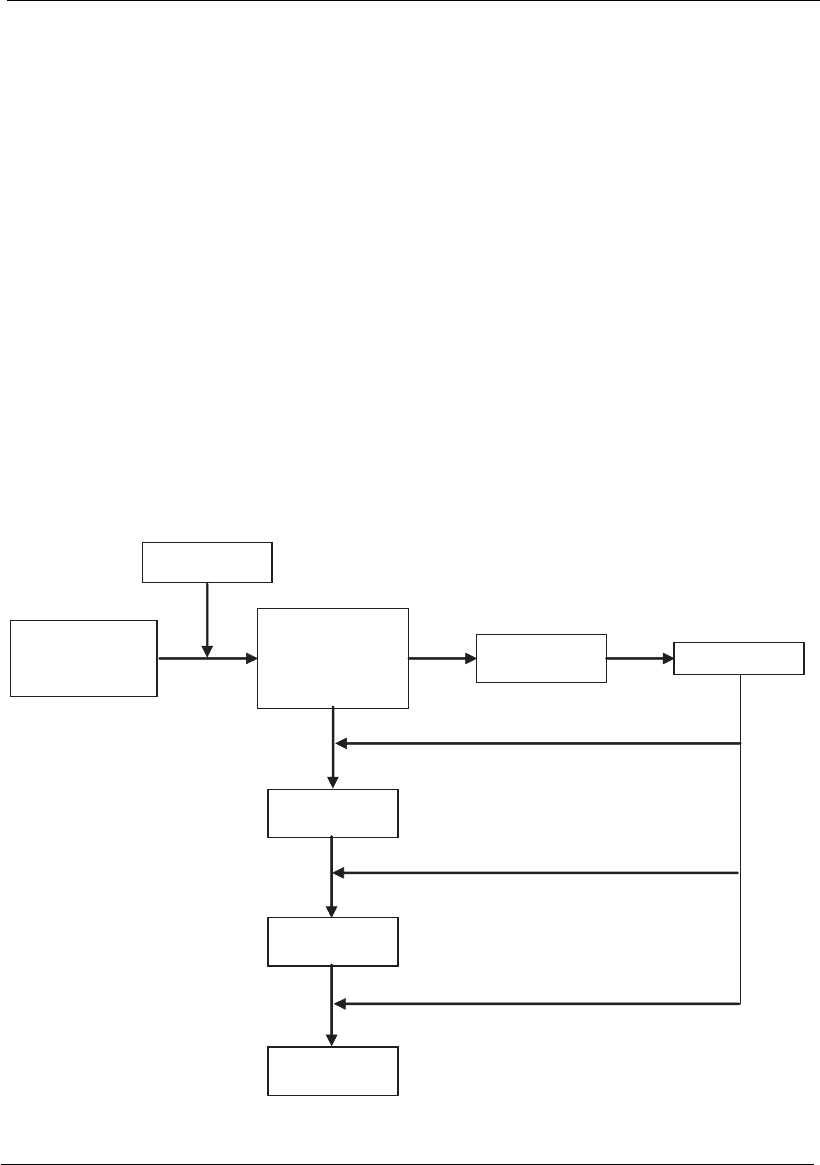
Virus with DNA
The double-strand DNA viruses are transported into the nucleus where
they translate and replicate their genome using cellular enzymes to
produce mRNA. These mRNA strands are later translated into proteins
that direct the synthesis of the proteins and genomes of the virus. The
isolated DNA of these viruses is considered infectious (Figure 18.5). There
are some double-strand DNA viruses, such HBV, which have a different
replication strategy that includes an intermediary RNA and a reverse
transcription phase. In this case, after the viral DNA penetrates the
nucleus of the cell, it is converted into a covalently closed circular DNA
molecule (cccDNA). Before the viral genome replication occurs, a cellular
enzyme (DNA-dependent RNA II polymerase) translates several RNAs
from cccDNA and genomic and subgenomic RNAs (Figure 18.5). Most
DNA viruses replicate in the nucleus of the cell, except for poxviruses,
which replicate in the cytoplasm. These viruses are practically autonomous
in terms of translation factors. Parvoviruses have a single-strand DNA
genome and replicate inside the cellular nucleus using replicative-cycle
cellular polymerases.
Circular genomic
DNA
Covalently closed
DNA
mRNA
strand ( )⫹
Protein
Genomic DNA
Viral enzymes
Viral progeny
Genomic RNA
Figure 18.5
Flow chart of events during the replication of the hepatitis B virus.
Viral vaccines: concepts, principles, and bioprocesses 441

18.2.4 Production and maturation of viral particles
Viruses use three strategies to assemble, mature, and release from infected
cells. With the first strategy, represented by picornavirus and adenovirus,
the assembly and maturation are completed inside the cell, either in the
cytoplasm or in the nucleus. As a rule, all non-enveloped viruses are
produced and become infectious inside the cell and depend on the cellular
lysis for release.
The second strategy is used by enveloped viruses, such as togavirus and
retrovirus, which are enveloped in the cytoplasmic membrane. They are
generally released by the budding of cellular membranes or exocytose
through a vacuole, usually without cellular lysis.
The third strategy is used by viruses whose nucleocapsids are produced
in the nucleus of the cell, with assembly and maturation involving the
nuclear membrane. The enveloped viruses accumulate in the endoplasmic
reticulum and are carried to the cell surface via vacuoles. Some viruses that
use this process, such as herpesvirus, are cytolytic (cause cell lysis).
Knowledge of viral replication strategies has helped to optimize the
approaches for the genetic modification of the viruses for better expression
of the heterologous genes. It has also led to the introduction of biopro-
cesses for the production of infectious viral particles or viral-like particles,
as will be discussed next.
18.3 Production of viral particles by cell culture
Viral particle production from cell cultures has several differences from
other bioprocesses. The production of molecules like enzymes, toxins, or
other proteins synthesized by bacteria, fungi or animals, depend upon
culture parameters, such as pH, temperature, dissolved oxygen, or nutri-
ents. Product formation may occur through secondary metabolic path-
ways, which are not related to the development or growth of the cell. In
these situations, research and technological development must be directed
to the specific cell and this involves the improvement of the cell as a better
molecular production unit. So, there is a direct relation between nutrient
conversion, cell growth, and the expected improvement of the final
productivity.
Viral particle production processes by cell culture infection, cannot be
characterized in such a simple way, since the final product – ‘‘virus’’ –
does not result from a secondary metabolic pathway. However, it can be
better described as a process redirecting the cell machinery towards viral
particle production, which only happens after viral infection. The virus
production process can be divided into two different steps. The first
involves cell multiplication, which results from the conversion of culture
medium substrates into cell mass. At the instant of viral infection, the
cellular production unit no longer exists, since the viral genetic material
forms a new production unit, initiating the second step of the virus
production process. This production unit is the infected cell and is the
producer of new viral particles. This production phase requires nutritional
and metabolic conditions that are not observed during cell growth. These
conditions are normally studied separately. Nevertheless, virus production
442 Animal Cell Technology

essentially requires the development of the cell that is ‘‘consumed’’ during
the virus production phase, and the metabolic status of the cell at the
moment of infection is a key factor for the success of the viral particle
production process.
Industrial production of viral vaccines preferably requires the use of
continuous or immortalized cell lines as the basis for viral multiplication.
BHK-21, Vero, MDCK, MDBK, CHO, or HeLa cells are the main
platforms used in the production of a huge variety of viral vaccines, since
they are considered stable, do not suffer significant genetic modifications
even after numerous generations of growth, and have a great capacity for
in vitro growth. In addition, these cell lines are susceptible to infection by
several different viruses, irrespective of their origin. As an example, the
Vero cell line has been obtained from an African green monkey kidney.
Although this cell immortalization process results in metabolic and func-
tional changes not observed in normal cells, Vero cells still preserve some
typical kidney cell characteristics. Despite this, certain viruses, like rabies
or poliovirus, which naturally infect nervous tissues, are easily adapted to
infect Vero cells (Frazzatti-Galina et al., 2001). The multifunctional
property of continuous cell lines is important for establishing the best
platforms for an industrial production process.
Even though there are many cell lines capable of cultivation in suspen-
sion, the majority of cells isolated from animal tissues retain their adherent
physiological characteristics and must be grown on a solid surface. These
are named adherent cells.
The basic need for a solid support guides all production choices invol-
ving industrial processes for adherent cells. A large variety of vessels has
been developed for adherent cell cultures. Petri dishes, Roux bottles,
T-flasks, and roller bottles are examples of cell culture vessels with a glass
or polystyrene surface. The system of choice is dependent on the scal-
ability of multiple steps, as well as the cost of equipment and qualified
operators.
As mentioned in Chapter 9, since production scale-up is related to the
increase of cell culture surface for adherent cells, consideration must be
given to the relationship between the surface area available for cell growth
and the bioreactor volume (Kent and Mutharasani, 1992).
The first adherent cell culture industrial process was conducted in roller
bottles, a system with limited scale-up possibilities. However, a great
advance in scale-up of high density cultures was achieved by Van Wezel in
1967. This allowed the culture of adherent cell lines on the surface of
microspheres, called microcarriers. Even under low agitation conditions,
microcarriers remain suspended in medium, which allows the culture to be
homogeneous and to be controlled easily to maintain optimal physiologi-
cal conditions, such as pH, temperature, and aeration. Besides, this is the
only system for adherent cells that allows constant monitoring of cell
growth. This enables cell morphology to be monitored and combines all
the advantages of suspension culture systems with the requirements of
adherent cells (Griffiths et al., 1987). So, the process can be well monitored
and controlled with high density cell cultures obtained in small bioreactor
volumes.
Vaccine production based on cells can use different methods of culture.
Viral vaccines: concepts, principles, and bioprocesses 443
