Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


A classic way to produce viral vaccines consists of cultivating cells on an
appropriate static support, infecting them with virus, collecting and
purifying the virus produced and formulating the vaccine.
Although many adherent cell lines have already been adapted to cultiva-
tion in suspension, a large number of viral infection agents do not often
allow the usage of those cells as a platform for prompt vaccinal antigen
production. For this reason, overcoming roller bottles scale limits, micro-
carrier systems have been developed as a faithful technological alternative
for simplifying the adherent cell scale-up process.
Nahapetian et al. (1986) obtained high density Vero cells cultures that
reached 3 3 10
7
cells/ml, when using a microcarriers system at a perfusion
rate of eight volumes of medium per day, an amount 10 times higher than
that usually obtained with repeated batch cultures. Similar results have
been described by Mendonc¸a et al. (1993, 1999, 2001) who also obtained
high density Vero cell cultures when using perfusion rates of 3 volumes of
medium per day. According to Nahapetian et al. (1986), endogenous
synthesis of cellular growth factors is probably the growth-limiting factor
of under-fed cultures, while factors related to culture conditions such as
aeration and cell metabolite production are involved in the growth limita-
tion of over-fed cultures.
Table 18.1 compares the relationship between cell culture surface area
and bioreactor volume in many different culture systems usually used with
adherent cells. For microcarriers, this coefficient might reach 60 cm
2
/ml of
medium for culture area prepared with 10 mg of microcarriers per
milliliter. For Roux bottles, this coefficient is around 3 cm
2
/ml. In cultures
initiated with 2 mg of microcarriers per milliliter of medium, high cell
densities of even 3 3 10
6
cells/ml are often reached, compared with smaller
cell densities from 2 to 3 3 10
5
cells/ml usually observed in Roux bottle
systems. Another great advantage of the use of microcarrier culture
systems is the possibility of preparing cell cultures with hundreds or even
thousands of liters (Montagnon et al., 1984).
According to Butler (1987), many materials have been used in the
production of microcarrier particles, and they have specific requirements
that allow appropriate cell adherence and growth:
(i) particle density should be between 1.02 and 1.05 g/cm
3
to facilitate
their maintenance in suspension at low agitation rates (40–150 rpm);
Table 18.1 Relationship between cell culture surface area and bioreactor
volume used with animal adherent cells
Cell culture systems Area/volume (cm
2
/cm
3
)
Roller bottle 0.2–0.7
Cell factories 1.7
Plastic bags 5.6
Fixed-bed 10–15
Hollow-fiber 31
Cytodex
TM
microcarriers (10 g/l) 34
CultiSpher
1
G microcarriers (30 g/l) – LF 120
444 Animal Cell Technology

(ii) particles should preferably be transparent, allowing microscopic eva-
luation;
(iii) rigid materials (polystyrene and glass) are recommended because of
their low porosity;
(iv) surface charge can be positive or negative, but it should not be too
low because of the risk of difficulties of cell adherence, and it should
not be too high because it could inhibit cell growth; the charge should
be equally distributed throughout the surface to insure homogeneous
cell distribution.
Microcarrier particle diameter should ideally be between 100 and 400
m with a size distribution of 25 m to guarantee a homogeneous
culture. According to Butler and Spier (1984), cells tend to adhere
preferably to the smallest particles. However, Hu and Wang (1987)
demonstrated that higher microcarrier diameter tended to promote cell
growth. An increase of diameter from 185 to 265 m resulted in a longer
exponential growth phase, with a final cell concentration four times higher
than that observed with microcarriers with smaller diameter.
Adherent cells attached to microcarriers are particularly susceptible to
damage caused by mechanical shear forces within agitated tanks. This
vulnerability to damage is usually associated with cell immobilization and
with the increase in shear force sensitivity of suspended microcarriers.
Croughan et al. (1987) demonstrated that FS-4 and Vero cells are highly
sensitive to shear forces caused by an increase in the mechanical agitation
of the cell culture. They showed a progressive reduction in cell growth
with higher agitation velocities and also cell lysis at an agitation rate over
180 rpm.
A great variety of mammal, bird, fish, amphibian, and insect cells can be
cultivated in this system. To ensure that cells from so many different
organisms and tissues can be cultivated with success in microcarriers, these
microcarriers should have a physicochemical composition and other
specific characteristics appropriate to the specific cell line. The most
commonly used microcarriers are composed of DEAE-dextran polymers,
polyacrylamide, polystyrene, gelatin, or glass. These present a great variety
of density, size, weight, or electric charge that might have a significant
effect in cell culture (Varani et al., 1983; Reuveny et al., 1985). Some
examples are given in Figure 18.6.
Many different culture systems have demonstrated effectiveness for viral
production. As previously described, these systems are based on the
growth of cells in suspension or adherent to microcarriers, which are kept
in suspension by agitation. After achieving high density the cultured cells
can be infected by virus, allowing intracellular viral multiplication until
the viral products are finally collected and processed. After standardiza-
tion and optimization, these systems allow consistent viral particle pro-
duction, and these steps are called the synthesis or upstream phase. Figure
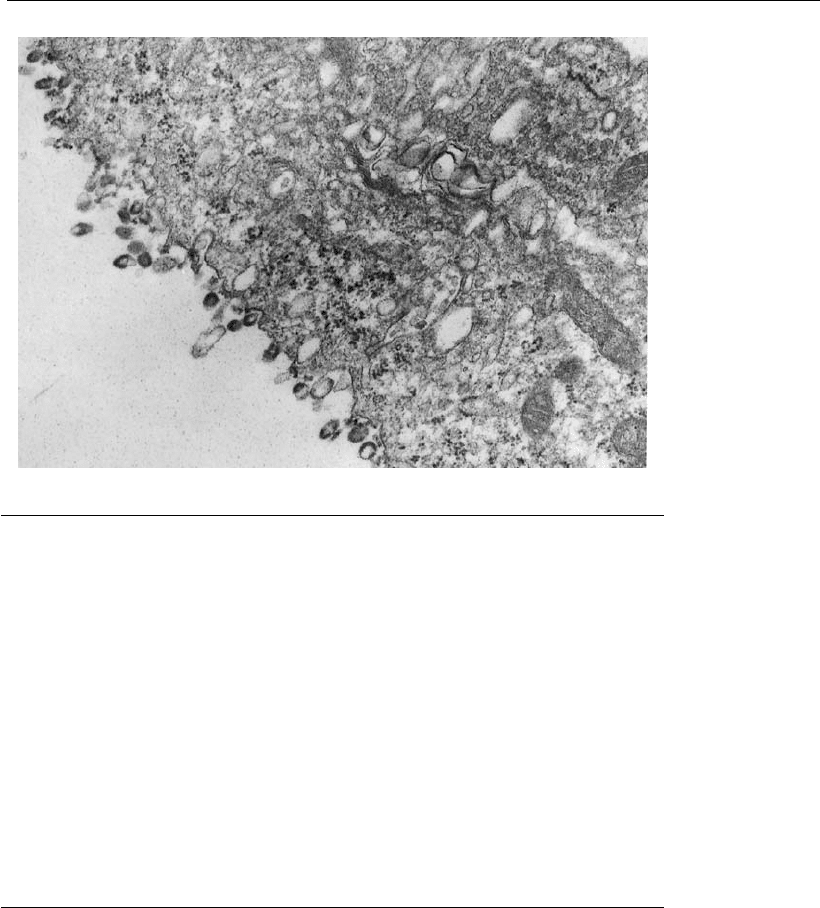
18.7 shows a typical cell membrane structure when rabies viral particles
are leaving the surface of an infected cell.
For viral vaccine preparation or downstream processing, the cells and
supernatant of infected cultures should go through concentration and
purifying processes. These are important steps because there could be a
Viral vaccines: concepts, principles, and bioprocesse s 445
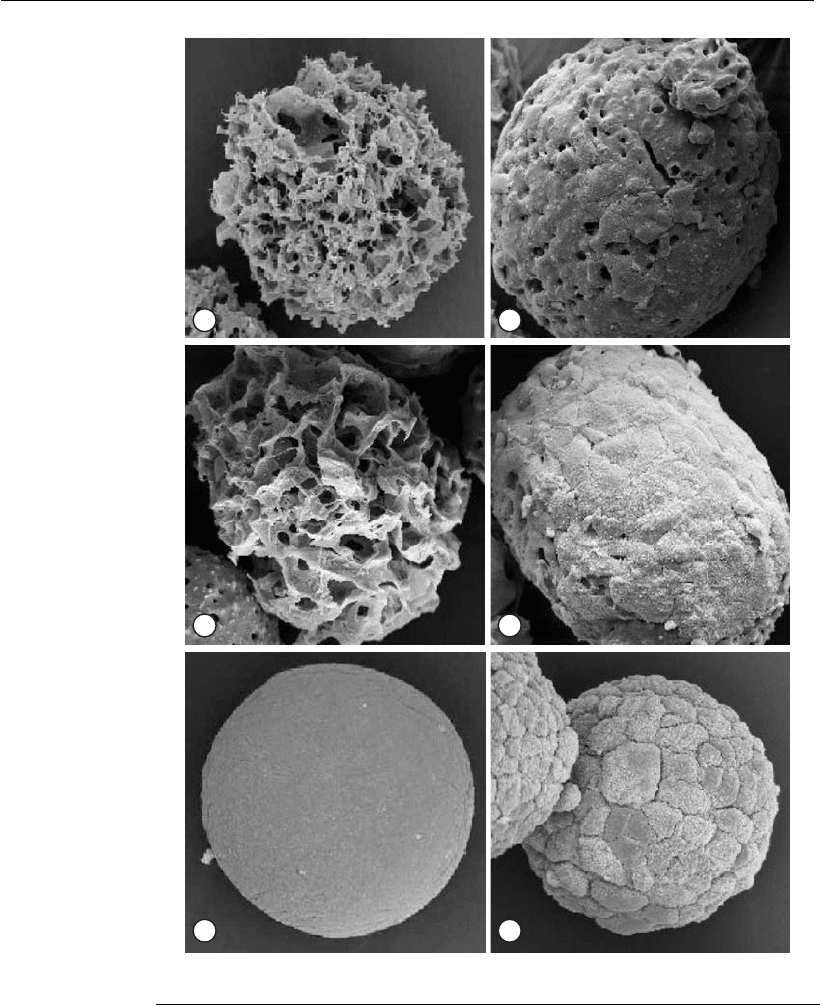
A B
C D
E F
Figure 18.6
Scanning electron microscopy of Vero cells on microcarriers (Yokomizo et al.,
2004). (A) Preparation of a Cytopore
TM
microcarrier without cells (original
magnification 3242). (B) Preparation of a cell-loaded Cytopore
TM
microcarrier at
day 6 (original magnification 3330).(C)PreparationofaCultiSpher
1
G
microcarrier without cells (original magnification 3370). (D) Preparation of a cell-
loaded CultiSpher
1
G microcarrier at day 6 (original magnification 3295). (E)
Preparation of a Cytodex
TM
I microcarrier without cells (original magnification
3520). (F) Preparatio n of a cell-loaded Cytodex
TM
I microcarrier at day 6 (or iginal
magnification 3485).
446 Animal Cell Technology
potential loss of productivity. Therefore, it is not only important to fully
optimize cell growth and the viral infection phase to guarantee a high
product concentration within the synthesis phase, but also to insure that a
highly immunogenic or infective product is fully recovered in the concen-
tration and purifying process. The most relevant optimization parameters
are the multiplicity of infection (MOI) which refers to the virus amount
used in the infection, and the time of infection (TOI), which refers to the
point of infection in relation to the cell multiplication period. MOI and
TOI are parameters that can vary from one type of virus to another or
even from one cell line to another, and their impact is highly significant
for increasing the productivity of the system.
18.4 Strategies for the production of virus-like particles
The main objective of immunization against viruses is the prevention or
modification of the disease. However, most of the existing classical
vaccines are able to prevent the disease but are not so efficient in prevent-
ing infection (Sandhu, 1994; Ellis, 1999). The development of the recombi-
nant DNA technology made possible the creation of vaccines that do not
present the typical side effects of the vaccines of attenuated or inactivated
viruses. Virus-like particles (VLPs) are one of the new vaccine strategies
arising from recombinant DNA technology.
As mentioned above, viruses are generally composed of a number of
different proteins organized in a regular three-dimensional structure
together with DNA or RNA. Viral proteins are in most cases aggregated
in icosahedric membranes on which certain proteins cooperate to form the
Figure 18.7
Transmission electron micr oscopy of Vero cell growth on a microcarrier surface,
7 days after rabies virus infection.
Viral vaccines: concepts, principles, and bioprocesse s 447

external and internal capsids. In several virus species, viral proteins are
able to form three-dimensional aggregates in the absence of nucleic acids,
thus creating VLPs. Similarly, the proteins that normally interact with
nucleic acids can also aggregate in their absence and form core-like
particles – CLPs. Therefore, in contrast to infectious viruses, VLPs consist
of viral proteins aggregated in a three-dimensional structure similar to that
of the native virus but not containing viral DNA or RNA within the
capsid (Cruz et al., 2002).
18.4.1 Advantages of VLPs
The major advantage of VLPs over individual purified antigens is the more
efficient antigen presentation to the immune system, since the epitopes
resulting from the three-dimensional structure of the capsid will be avail-
able for recognition by the immune system. Also, many of the viruses for
which immunogenic VLPs were developed are difficult to replicate in vitro
(e.g. HBV, parvovirus B19, Norwalk calcivirus, and human papilloma-
virus). Finally, it should be noted that VLPs are not infectious, thus
eliminating the need for inactivation and subsequent epitope modification
due to the inactivation agent, as in the case of inactivated viruses (Cruz
et al., 2002).
18.4.2 VLP production technology
Due to the availability of the viral coding sequences, it has been possible to
develop methods for the production of the viral proteins using appropriate
expression vectors. The fact that VLPs are multimeric structures makes the
use of complex expression technologies mandatory. In addition, viruses
typically infect eukaryotic cells and thus the use of such cells is required for
correct viral protein production. Although this is not necessarily true for
the production of a single viral protein that does not have complex post-
translational modifications, such as glycosylation and phosphorylation, for
production of complete VLPs, the use of complex systems cannot be
avoided. This is due to the fact that the processing of viral proteins within
the cells and capsid assembly, require the mediation of host proteins – the
chaperones. In recent years several systems were developed with the goal of
producing viral proteins in yeast, insect, and mammalian cells. The use of
yeast was first demonstrated in the production of the HBV surface antigen
(HBsAg) used as a vaccine against hepatitis B (Fu et al., 1996). The expres-
sion of this specific protein had been previously shown using Escherichia
coli; however, the molecules did not form VLPs and their immunogenicity
was low. The vaccine against hepatitis B is an important milestone in the use
of VLPs as a vaccination strategy, leading to replacement of the previously
existing inactivated virus vaccine.
A second milestone in the production of viral antigens was the develop-
ment of the technology associated with insect cells – the baculovirus
expression system. The Autographa californica baculovirus produces viral
particles as a part of its life cycle. These particles accumulate in the
polyhedrin protein matrix, which confers protection from inactivation due
to environmental factors. The polyhedrin is produced in large amounts
448 Animal Cell Technology

(1 mg/10
6
cells) under the control of a very strong promoter. The use of
this promoter to drive the expression of foreign proteins became the
obvious next step in producing recombinant baculoviruses. The cells
derived from the ovary of the caterpillar Spodoptera frugiperda (Sf-9 and
Sf-21) are the most widely used for the production of heterologous
proteins using baculoviruses (Table 18.2). Nevertheless, other insect cells
have also been used for this purpose, including Trichoplusia ni (High-
Five
TM
) (Jiang et al., 1998; Wang et al., 2000).
18.4.3 VLP composition
The proteins to be included in VLPs should be those necessary to confer
the desired degree of immunogenicity. As a consequence, VLPs are often
composed of more than one protein. In order to use baculovirus-infected
insect cells to produce VLPs it is necessary to predefine the proteins to be
included, since the expression of these proteins could determine the
stability (Hyatt et al., 1993) and location – intra- or extracellular – of the
particle (French and Roy, 1990).
The antigenicity, rather than the immunogenicity, should drive the
research and development of new VLP-based vaccines and thus antibody
reactivity tests should be performed as early in the process as possible. For
example, a porcine parvovirus vaccine is composed of a single viral protein
(VP2), which represents 95% of the native virus total protein and is able
do induce antibody production in immunized animals (Rueda et al., 1999).
In contrast, the human parvovirus B19 contains the exact same proportion
of VP2 in the native virus but VLPs made solely of VP2 are unable to
induce neutralizing antibodies (Brown et al., 1991; Tsao et al., 1996). In
this case even a VLP containing VP1 and VP2 at a ratio of 1:24,
respectively, which is very similar to that of the native virus, was not
Table 18.2 Virus-like particles (VLPs) produced in baculovirus-infected insect
cells
VLPs Cell line Reference
Human parvovirus B19 Sf-9 Bansal et al., 1993
Blue tongue virus Sf-9, Sf-21 Roy, 1990
Epizootic hemorrhagic disease Sf-9 Le Blois et al., 1991
Hepatitis B Sf-9 Lanford et al., 1989
Hepatitis C Sf-9 Baumert et al. 1998
HIV Sf-9 Cruz et al., 2002
Infectious bursal disease Sf-9, HighFive Wang et al., 2000;
Hu and Bentley, 1999
Norwalk virus Sf-9 White et al., 1997
Papillomavirus Sf-9 Kirnbauer et al., 1992
Minute virus of mice Sf-9 Hernando et al., 2000
Polyomavirus Sf-9 Montross et al., 1991
Porcine parvovirus Sf-9, Sf-21 Maranga et al., 2004;
Martinez et al., 1992
Rotavirus Sf-9, HighFive Vieira et al., 2005;
Jiang et al., 1998
SV40 Sf-9 Kosukegawa et al., 1996
Viral vaccines: concepts, principles, and bioprocesses 449

efficient in inducing an immune response. The final composition of the
antigenic VLP included VP1 at a percentage between 25 and 40%, that is,
6–10-fold higher than in the native viral particle (Tsao et al., 1996). This
clearly shows that there is still a need for a better understanding of the
immunization phenomenon.
Therefore, to ensure antigenicity, the particle composition has to be
studied and will probably lead to different baculovirus infection strategies,
using a different number of proteins per virus. For instance, the blue
tongue virus (BTV), an Orbivirus from the Reoviridae family, is a non-
enveloped virus that contains seven different structural proteins (VP1–
VP7) (Hyatt et al., 1993). The outer capsid consists of two proteins, VP2
and VP5, while the core is composed of the remaining five proteins, two
major (VP3 and VP7) and three minor (VP1, VP4, and VP6), classified
according to their abundance. The production of BTV VLPs can be
performed by co-infecting the cells with two dual-gene vectors expressing
VP3 + VP5 and VP2 + VP7 (Brown et al., 1991) or through the infection
of a multigenic vector containing all four proteins (VP2, VP3, VP5, and
VP7) (Martinez et al., 1992). The factors related to the VLP composition,
including the use of multigenic vectors, production technology, and large-
scale production VLPs, have been analyzed by Maranga et al. (2000a).
18.4.4 VLP production processes
Hepatitis B vaccine
HBV is transmitted among humans and is manifested as a chronic
infection that debilitates infected individuals and may cause severe liver
damage, primary carcinoma, and ultimately death. Although in most cases
the patients recover, there are large segments of the population that have
chronic hepatitis B (especially in African and Asian countries) and can
transmit the disease as a pandemic.
HBV is an enveloped virus whose nucleocapsid involves one single
DNA molecule. Several surface antibodies have been found against the
surface antigens (HBsAg) in the serum of HBV-infected individuals. The
HBsAg is present in the blood of infected individuals in the form of
spherical particles with a diameter of 22 nm. Since these particles are able
to induce immunization in humans, a production process based on yeast
cloned with the gene encoding the HBsAg monomer has been developed.
However, since yeasts do not secrete the VLPs, sonication is used to
extract the particles, which are subsequently purified.
The VLPs produced by yeast consist of about 100 HBsAg monomers
and confer immunization when injected into humans in spite of the
different glycosylation as compared with the native virus (Fu et al., 1996).
Nevertheless, the vaccine only confers immunity after three doses and
requires revaccination after 5 years.
Production of HIV-1 and porcine parvovirus VLPs
The production processes for HIV-1 and porcine parvovirus (PPV) VLPs
are presented in Figure 18.8. In both cases, the insect cell–baculovirus
450 Animal Cell Technology
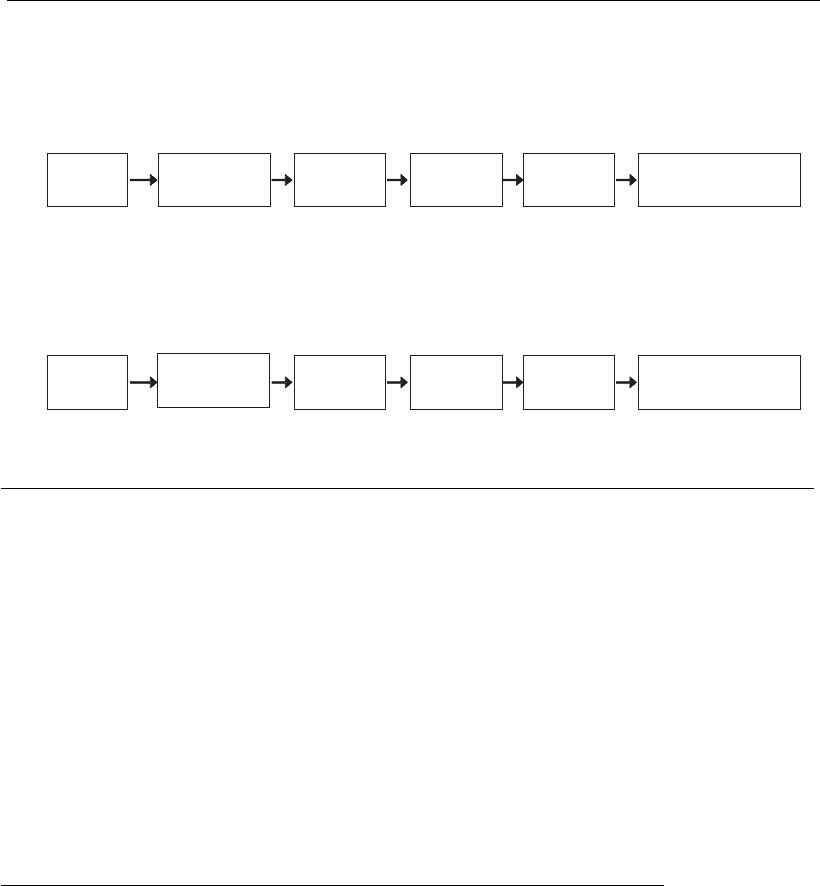
system was used but the production strategy has some significant differ-
ences. In the case of HIV-1 VLPs, Sf-9 cells were infected with a high
MOI (Cruz et al., 1998), while in the case of PPV VLPs, Sf-21 cells were
preferred and a low MOI was used for economic reasons, as these VLPs
are developed as a veterinary vaccine (Maranga et al., 2004). Also, at the
purification level there are some differences, since the HIV-1 VLPs are
secreted and PPV VLPs are intracellular, thus requiring a cell disruption
step. However, it should be noted that both downstream processing
strategies are based on the relatively larger size of the VLP in comparison
with other contaminants, although chromatographic steps are used only
for HIV-1 VLPs (Cruz et al., 2002).
18.5 Development of viruses for DNA vaccines
Free DNA-mediated immunization, also referred to as genetic immuniza-
tion, consists of the administration of genetic material (DNA in the form
of a plasmid), which will lead to the in vivo expression of proteins that
induce an immune response. This vaccination method is simple and
constitutes a relevant alternative to the classical vaccination, mainly due to
two important advantages. First, the long-term expression of small
amounts of antigens is possible, avoiding the need for re-administration, as
long as no allergies, tolerance, or autoimmunity occur. Second, the
antigens are synthesized in vivo, no infection exists and therefore inad-
vertent reactions are avoided and the treatment of infected individuals
becomes possible. The first animal studies using this type of vaccines
demonstrated their potential in the protection against the flu virus. DNA
vaccines also have other advantages, namely the higher thermal, chemical,
A
B
Bioreaction
.
Infection (high MOI)
Cell
growth
Production
Centri-
fugation
Micro-
filtration
Ultra-
filtration
Gel exclusion
chromatography
Baculovirus
inactivation
.
Cell
growth
Centri-
fugation
Cell
disruption
Preci-
pitation
Ultra-
filtration
Production
(batch)
Purification
(batch) (0.45 m)µ (300 kDa)
Figure 18.8
Production and downstream processing of HIV-1(A) and PPV (B) VLPs (adapted from Cruz et al.,
2002).
Viral vaccines: concepts, principles, and bioprocesses 451

and biological stability of DNA in comparison with classical vaccines.
This lowers the requirements for storage at low temperature, thus reducing
the costs, especially for developing countries.
Nevertheless, the large-scale application in healthy individuals still
involves delicate safety issues, although clinical trials are expected for
cancer and HIV infection.
The spectrum of target diseases can be expanded through the use of a
mix of different plasmids, a strategy that is difficult to implement when
using proteins due to interference. Given the ease of modification of DNA
sequences, a single gene may include several epitopes of more than one
antigen or different pathogenic strains.
There are several methods of administration. Free DNA can be directly
injected into muscle cells (currently the most efficient method), linked to
gold particles which are then bombarded onto the tissue (difficult to
prepare), pulverized without injection or administered orally. In spite of
the low efficiency (in terms of modified cells), this new vaccination
method frequently leads to surprisingly high immune responses at both
the humoral and cellular level. The technology used in this type of
vaccination has the additional advantage of allowing research in the area of
molecular immunology, as well as in the study of the immune system and
new vaccine development due to the ease of producing plasmids encoding
different proteins. This can be performed within a few weeks as compared
with the months necessary for new viral vectors. Nevertheless, viral
vectors, especially adenoviruses, are currently being used in DNA vaccina-
tion. The ability of adenoviruses to very efficiently induce an immune
response has several advantages: (a) to provide high amounts of antigen to
lymphoid tissue; (b) to induce a rapid T-cell expansion and migration in
the lymphatic system; and (c) to promote and prolong T-cell response
(Yang et al., 2003). In addition, adenoviruses are non-integrating vectors
and their biology is well understood. In parallel, viral vectors based on
other virus types have been developed, for example influenza virus (Ulmer
et al., 1998). Currently, Crucell is developing two DNA vaccines against
malaria and Ebola virus based on adenovirus Ad35 and Ad11, two
serotypes that are not common, thus eliminating the problem of existing
immunity against Ad5. In this case it is possible to obtain a good immune
response and an effective protection against infection. There are several
DNA vaccines under development against a number of infectious diseases
(Table 18.3).
The application of the DNA vaccine technology is not limited to
infectious diseases and is currently being used in therapeutic vaccines
against cancer. Table 18.4 presents the therapeutic DNA vaccines under
development (Powell, 2004).
Further detail on gene therapy is provided in Chapter 21, which focuses
on the use of viruses for therapeutic applications instead of prophylaxis.
18.6 Perspectives for the evolution of viral vaccine production
Vaccination has prevented, in a safe and efficient way, more diseases and
deaths due to infectious agents than any other public health policy except
452 Animal Cell Technology
for clean water supply. Among the several international programs
launched in the 20th century to eradicate diseases, the only successful one
to date was based on vaccines – the eradication of smallpox. Poliomyelitis
may be the next success within the next decade. Table 18.5 shows the
evolution of the viral vaccines available on the market from live/attenuated
vaccines to the more recent subunit and recombinant DNA vaccines.
The major problem associated with recombinant vaccines is related to
the immune response that is often only humoral (antibody production)
and not cellular (e.g. cytotoxic T-lymphocytes, CTL) (Ellis, 1999). Nor-
mally, viral vaccines are able to stimulate both types of immune response,
thus avoiding the need for revaccination (Ellis, 1996).
Another important issue concerns the number of vaccines used for
children under 2 years of age; the vaccination program includes around 15
injections causing some discomfort not only to the children but also to
their parents (Papaevangelou, 1998). It is therefore necessary to develop
combined vaccines – live, inactivated or mixed with VLPs or other
subunits – for which the allergic reactivity is minimal.
To circumvent these two problems and taking advantage of the VLP
stability, some methods have been developed to introduce multiple epi-
topes in the same VLP. One example is the use of porcine parvovirus VLP
containing epitopes to induce cellular immune response (B cells, CD4+,
and CTL). This strategy should allow the production of cheaper and more
potent vaccines that will in turn induce a more effective immune response
Table 18.3 DNA vaccines under development
Vector type Target Company Development stage
Adenovirus Ebola Crucell/NIH Preclinical
Hepatitis C Merck & Co. Preclinical
HIV Merck & Co. Phase I
Malaria Crucell/GlaxoSmithKline Preclinical
Rabies Vaxin Preclinical
West Nile virus Pfizer/Kimron Vet. Inst Preclinical
Plasmids Ebola NIH Vaccine Research Center Phase I
HIV Wyeth Lederle Phase I complete
HIV Wyeth Lederle Phase I
HIV Chiron Phase I
HIV Epimmune Phase I
Table 18.4 Therapeutic DNA vaccines under development
Vaccine Company Development stage
HIV-1 Corixa Phase I
Melanoma ImClone Systems Phase I
Lung cancer ImClone Systems Phase III
Prostate cancer Inovio Phase I/II
Melanoma M.D. Anderson Cancer Center Phase I/II
Melanoma Vical Phase III
Viral vaccines: concepts, principles, and bioprocesses 453
