Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


(Rueda et al., 1999; Maranga et al., 2000b). This type of chimera is
currently under development using HBV VLPs, yeast Ty particles, and
blue tongue virus VLPs, as well as other polioviruses.
A good example of the recent and future evolution of viral vaccines and
their concomitant issues of technology, complexity and competition, is the
rotavirus vaccine. This is of great relevance for the prevention of diarrhea,
which is often deadly in developing countries (half a million deaths per
year) and has high hospitalization costs in rich countries. After successive
failures of monovalent vaccines, multivalent vaccines based on the reshuf-
fling of rotavirus strains comprising the attenuation properties of animal
strains with the external capsid of human serotypes were developed.
The first vaccine (Rotashield
1
, developed by Wyeth Lederle) entered
the market in August 1998, after extensive clinical trials providing efficient
protection (. 90%) and safety. After less than 1 year 15 cases of intussus-
ception were reported, leading to the withdrawal of this vaccine from the
market (Murphy et al., 2003, Fischer et al., 2004). Because this risk was
calculated as being lower than 1 in 5000 cases, the two major candidate
vaccines (pentavalent human-bovine reshuffling, Rotateq
1
Merck & Co.
and monovalent human attenuated Rotarix
1
, Glaxo Smith Kline) are
currently under the most extensive Phase III clinical trial record – over
60 000 patients each! This fact can give an idea of the increasing standard
of what is considered to be an acceptable risk in vaccination programs
(with the obvious exception of anticancer vaccines in initial stages and
therefore without clearly defined acceptance standards). This shows an
evident fact in vaccine praxis: the ‘‘easy’’ vaccines have already been
Table 18.5 Viral vaccines: historical perspective
Year Disease Vaccine type
1796 Rubeola Live/attenuated*
1885 Rabies Killed/inactivated*
1928 Yellow fever Live/attenuated
1936 Flu Killed/inactivated*
1937 Encephalitis killed/inactivated
1945 Japanese encephalitis B Killed/inactivated
1955 Poliomyelitis Killed/inactivated
1958 Poliomyelitis Oral vaccine (live/attenuated)
1963 Measles Live/attenuated
1967 Mumps Live/attenuated
Rubeola Live/attenuated
1981 Hepatitis B Protein
1983 Varicella-zoster Live/attenuated
HB Protein/recombinant DNA subunit
Flu Killed/inactivated
Hepatitis A Subunit, with adjuvant
1997 Flu Subunit, with adjuvant
2000 HA-Typhoid fever Polysaccharide
2003 Flu Live/attenuated
Meningococcal disease Serotypes ACW – polysaccharide
2004 Rotavirus Live/attenuated
*Substituted by a new vaccine.
454 Animal Cell Technology

introduced in the market and the ‘‘difficult’’ vaccines, namely viral
vaccines, are the only ones left.
For this reason, there are several groups in Europe (involving the
authors of this chapter) and in the USA looking for VLPs produced in
insect cells using multicystronic baculovirus presenting different comple-
mentary DNA encoding the critical viral proteins VP2, VP6, VP7, and,
eventually, VP4/VP8 in the same vector under the control of different
promotors (Vieira et al., 2005). Using this technology, after validation of
the humoral and cellular response, it is possible to consider systems
biology strategies to understand the replication, stability, mRNA kinetics,
protein production stoichiometry, and VLP packaging. Such knowledge
will allow the improvement of the molecular biology of the baculovirus
vector and the number of proteins generating appropriate VLPs.
The use of DNA vaccines could also bring additional benefits since they
are based on technological platforms (such as the adenoviral platform
AdVac from Crucell), thus providing great flexibility. It is possible in
these cases to add new antigens (derived from a virus, parasite of bacteria)
to obtain a greater efficacy through the modulation of the immune
response to the mechanism of action of each pathogen. This type of
technology is particularly useful in cases where the production of antigen
is not only difficult but also dangerous (HIV-1, Ebola virus, and West
Nile virus). Nevertheless, there are still important challenges, such as the
definition of formulations that significantly increase the stability of the
vaccine.
It is therefore expected that subunit vaccines will proliferate in the
market because they are predominantly safe, as long as their evolution is
supported by the development of new technologies that ensure efficacy.
References
Bansal GP, Hatfield JA, Dunn FE, Kramer AA, Brady F, Riggin CH, Collett MS,
Yoshimoto K, Kajigaya S, Young NS (1993), Candidate recombinant vaccine for
human B19 parvovirus, J. Infect. Dis. 167:1034–1044.
Baumert TF, Ito S, Wong DT, Liang TJ (1998), Hepatitis C virus structural proteins
assemble into virus-like particles in insect cells, J. Virol. 72:3827–3836.
Brown CS, Van Lent JW, Vlak JM, Spaan WJ (1991), Assembly of empty capsids by
using baculovirus recombinants expressing human parvovirus B19 structural
proteins, J. Virol. 65:2702–2706.
Butler M (1987), Growth limitations in microcarrier cultures, Adv. Biochem. Eng.
Biotechnol. 34:57–84.
Butler M, Spier RE (1984), The effects of glutamine utilization and ammonia produc-
tion on the growth of BHK cells in microcarrier cultures, J. Biotechnol. 1:
187–196.
Croughan MS, Hamel JF, Wang DIC (1987), Hydrodynamic effects on animal cells
grown in microcarrier cultures, Biotechnol. Bioeng. 23:130–141.
Cruz PE, Cunha A, Peixoto CC, Clemente J, Moreira JL, Carrondo MJT (1998),
Optimization of the production of virus-like particles in insect cells, Biotechnol.
Bioeng. 60:408–418.
Cruz PE, Maranga L, Carrondo MJT (2002), Integrated process optimization: lessons
from retrovirus and virus-like particle production, J. Biotechnol. 99:199–214.
Viral vaccines: concepts, principles, and bioprocesses 455

Ellis RW (1996), The new generation of recombinant viral subunit vaccines, Curr.
Opin. Biotechnol. 7:646–652.
Ellis RW (1999), New technologies for making vaccines, Vaccine 17:1596–1604.
Fischer TK, Bresee JS, Glass RI (2004), Rotavirus vaccines and the prevention of
hospital acquired diarrhea in children, Vaccine 22( Suppl 1):S49–54.
Frazzatti-Gallina NM, Paoli RL, Moura
˜
o-Fuches RM, Jorge SAC, Pereira CA (2001),
Higher production of rabies virus in serum-free medium cell cultures on micro-
carriers, J. Biotechnol. 92:67–72.
French TJ, Roy P (1990), Synthesis of bluetongue virus (BTV) core-like particles by a
recombinant baculovirus expressing the two major structural core proteins of
BTV, J. Virol. 64:1530–1536.
Fu J, VanDusen W, Kolodin DG, O’Keefe DO, Herber WK, George HA (1996),
Continuous culture study of the expression of hepatitis B surface antigen and its
self-assembly into virus-like particles in Saccharomyces cerevisiae, Biotechnol.
Bioeng. 49:578–586.
Griffiths JB, Cameron DR, Looby D (1987), A comparison of units process systems
for anchorage dependent cells, Dev. Biol. Stand. 66:331–330.
Hernando E, Llamas-Saiz AL, Foces-Foces C, McKenna R, Portman I, Agbandje-
McKenna M, Almendral JM (2000), Biochemical and physical characterization of
parvovirus minute virus of mice virus-like particles, Virology 267:299–309.
Hu YC, Bentley WE (1999), Enhancing yield of infectious Bursal disease virus
structural proteins in baculovirus expression systems: focus on media, protease
inhibitors, and dissolved oxygen, Biotechnol Prog. 15:1065–1071.
Hu WS, Wang DIC (1987), Selection of microcarrier diameter for the cultivation of
mammalian cells on microcarriers, Biotechnol. Bioeng. 30:548-557.
Hyatt AD, Zhao Y, Roy P (1993), Release of bluetongue virus-like particles from
insect cells is mediated by BTV nonstructural protein NS3/NS3A, Virology
193:592–603.
Jiang B, Barniak V, Smith RP, Sharma R, Corsaro B, Hu B, Madore HP (1998),
Synthesis of rotavirus-like particles in insect cells: comparative and quantitative
analysis, Biotechnol. Bioeng. 60:369–374.
Kent BL, Mutharasani R (1992), Cultivation of animal cells in a reticulated vitreous
carbon foam, J. Biotechnol. 22:311–328.
Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT (1992), Papillomavirus L1
major capsid protein self-assembles into virus-like particles that are highly
immunogenic, Proc. Natl Acad. Sci. U S A 89:12180–12184.
Kosukegawa A, Arisaka F, Takayama M, Yajima H, Kaidow A, Handa H (1996),
Purification and characterization of virus-like particles and pentamers produced
by the expression of SV40 capsid proteins in insect cells, Biochim. Biophys. Acta
1290:37–45.
Lanford RE, Luckow V, Kennedy RC, Dreesman GR, Notvall L, Summers MD
(1989), Expression and characterization of hepatitis B virus surface antigen
polypeptides in insect cells with a baculovirus expression system, J. Virol.
63:1549–1557.
Le Blois H, Fayard B, Urakawa T, Roy P (1991), Synthesis and characterization of
chimeric particles between epizootic hemorrhagic disease virus and bluetongue
virus: functional domains are conserved on the VP3 protein, J. Virol. 65:
4821–4831.
Maranga L, Cruz PE, Aunins JG, Carrondo MJT (2002a), Production of core and
virus-like particles with baculovirus infected insect cells, Adv. Biochem. Eng.
Biotechnol. 74:183–206.
Maranga L, Rueda P, Antonis AF, Vela C, Langeveld JP, Casal JI, Carrondo MJT
(2002b), Large scale production and downstream processing of a recombinant
porcine parvovirus vaccine, Appl. Microbiol. Biotechnol. 59:45–50.
456 Animal Cell Technology

Maranga L, Cunha A, Clemente J, Cruz PE, Carrondo MJT (2004), Scale-up of virus-
like particles production: effects of sparging, agitation and bioreactor scale on cell
growth, infection kinetics and productivity, J. Biotechnol. 107:55–64.
Martinez C, Dalsgaard K, Lopez de Turiso JA, Cortes E, Vela C, Casal JI (1992),
Production of porcine parvovirus empty capsids with high immunogenic activity,
Vaccine 10:684–690.
Mendonc¸a RZ, Ioshimoto LM, Mendonc¸a RMZ, De Franco M, Valentini EJG, Bec¸ak
W, Pereira CA (1993), Preparation of human rabies vaccine in VERO cell culture
using a microcarrier system, Braz. J. Med. Biol. Res. 26:1305–1317.
Mendonc¸a RZ, Prado JCM, Pereira CA (1999), Attachment, spreading and growth of
VERO cells on microcarriers for the optimization of large scale cultures, Biopro-
cess Eng. 20:565–571.
Mendonc¸a RZ, Arro´zio SJ, Antoniazzi MM, Ferreira JMC Jr, Pereira CA (2001),
Metabolic active-high density VERO cell cultures on microcarriers following
apoptosis prevention by galactose/glutamine feeding, J. Biotechnol. 97:13–22.
Montagnon B, Vincent-Falquet JC, Fanget B (1984), Thousand litre scale microcarrier
culture of VERO cells, Dev. Biol. Stand. 55:37–42.
Montross L, Watkins S, Moreland RB, Mamon H, Caspar DL, Garcea RL (1991),
Nuclear assembly of polyomavirus capsids in insect cells expressing the major
capsid protein VP1, J. Virol. 65:4991–4998.
Murphy TV, Smith PJ, Gargiullo PM, Schwartz B (2003), The first rotavirus vaccine
and intussusception: epidemiological studies and policy decisions, J. Infect. Dis.
187:1309–1313.
Nahapetian AT, Thomas JN, Thilly WG (1986), Optimization of environment for high
density VERO cell culture: effect of dissolved oxygen and nutrient supply on cell
growth and changes in metabolites, J. Cell Sci. 81:65–103.
Papaevangelou G (1998), Current combined vaccines with hepatitis B, Vaccine 16:
S69–72.
Powell K (2004) DNA vaccines – back in the saddle again?, Nat. Biotechnol. 22:
799–801.
Reuveny S, Coret R, Freeman A, Kotler M (1985), Newly developed microcarrier
culturing systems an overview, Dev. Biol. Stand. 60:243–253.
Roy P (1990), Use of baculovirus expression vectors: development of diagnostic
reagents, vaccines and morphological counterparts of bluetongue virus, FEMS
Microbiol. Immunol. 2:223–234.
Rueda P, Martinez-Torrecuadrada JL, Sarraseca J, Sedlik C, del Barrio M, Hurtado A,
Leclerc C, Casal JI (1999), Engineering parvovirus-like particles for the induction
of B-cell, CD4(+) and CTL responses, Vaccine 18:325–332.
Sandhu JS (1994), Engineered human vaccines, Crit. Rev. Biotechnol., 14:1–27.
Tsao EI, Mason MR, Cacciuttolo MA, Bowen SH, Folena-Wasserman G (1996),
Production of parvovirus B19 vaccine in insect cells co-infected with double
baculoviruses, Biotechnol. Bioeng. 49:130–138.
Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu
MA, Donnelly JJ, Caulfield MJ (1998), Protective CD4+ and CD8+ T cells against
influenza virus induced by vaccination with nucleoprotein DNA, J. Virol.
72:5648–5653.
Van Wezel AL (1967), Growth of strains and primary cells on microcarriers in
homogeneous culture, Nature 216:64–65.
Varani J, Dame M, Beals TF, Wass JA (1983), Growth of three established cell lines on
glass microcarriers, Biotechnol. Bioeng. 25:1359–1372.
Vieira HLA, Esteva
˜
o C, Rolda
˜
o A, Peixoto C, Sousa M, Cruz PE, Carrondo MJT,
Alves PM (2005), Triple layered rotavirus VLP assembly: kinetics of vector
replication, mRNA stability and recombinant protein production, J. Biotechnol.
120:72–82.
Viral vaccines: concepts, principles, and bioprocesses 457

Wang MY, Kuo YY, Lee MS, Doong SR, Ho JY, Lee LH (2000), Self-assembly of the
infectious bursal disease virus capsid protein, rVP2, expressed in insect cells and
purification of immunogenic chimeric rVP2H particles by immobilized metal-ion
affinity chromatography, Biotechnol. Bioeng. 67:104–111.
White LJ, Hardy ME, Estes MK (1997), Biochemical characterization of a smaller form
of recombinant Norwalk virus capsids assembled in insect cells, J. Virol. 71:
8066–8072.
Yang TC, Dayball K, Wan YH, Bramson J (2003), Detailed analysis of the CD8+
T-cell response following adenovirus vaccination, J. Virol. 77:13407–13411.
Yokomizo AY, Antoniazzi MM, Galdino PL, Azambuja NJ, Jorge SAC, Pereira CA
(2004), Rabies virus production in high Vero cell density cultures on macroporous
microcarriers, Biotechnol. Bioeng. 85:506–515.
458 Animal Cell Technology

19
Bioinsecticides
Ma
´
rcia Regina da Silva Pedrini and
Ronaldo Zucatelli Mendonc¸a
19.1 Introduction
This chapter describes the application of animal cells, particularly insect
cells, for baculovirus production and its use as a bioinsecticide. The use of
baculovirus in the control of insect pests and its production in vitro are
discussed.
Brazil is one of the largest agricultural producers in the world. Accord-
ing to data from the Institute of Agriculture (IEA), in 2003 the economic
balance of the Brazilian agro business was US$ 17.61 billion (Vicente et
al., 2003). In 2005, the Brazilian production was about 113 million tons of
grains, with soy accounting for about 48% of this production (IBGE,
2005).
Pesticides have an important role in the development of agricultural
production, in controlling pests and insuring a sustainable agriculture.
However, the constant use of chemical pesticides, in an indiscriminate
way, causes a reduction of the population of beneficial organisms and
induces resistance of the targeted insect pests, which creates even more
dependence on chemical products. Therefore, the reduction of the usage of
these pesticides in agriculture is of great importance for commercial and
environmental reasons.
The biological control of insect pests in agriculture is an old practice.
More than 120 years ago, an infestation of citrus fruit production by
Icerya purchasi in Florida State (USA) was treated using a ladybug from
Australia, a procedure that was later used in other countries, including
Brazil (Carvalho et al., 1999). Currently, several bioinsecticides are used
for biological control, the most common being insects (wasps, acarids,
etc.), bacteria (Bacillus thuringiensis), fungi, or virus (baculovirus).
Bioinsecticides based on baculovirus have had a strong impact on the
production of grain. Brazilian research on the use of baculovirus was
initiated by EMBRAPA (Brazilian Agricultural Research Corporation)
during the 1970s (Moscardi, 1993). Because of this research, currently, soy
farming is maintained entirely without the use of chemical pesticides. The
biologically treated area exceeds two million hectares, distributed among
several different states of Brazil. This is the best worldwide example of
large-scale viral biopesticide usage (Ageˆncia Brasil, 2004).
In such a system, a species-specific virus is used. In the case of soy
cultivation, this virus is Baculovirus anticarsia, which specifically kills a
caterpillar found on the soy – Anticarsia gemmatalis – a defoliating pest
that substantially reduces farming productivity. With this bioinsecticide,

the use of millions of liters of chemical insecticide per harvest is avoided,
thus generating an enormous environmental and economical benefit to the
country (EMBRAPA, 2002).
In addition to the economic and environmental gains, other advantages
of this product are the facts that the manipulation of chemicals can be
avoided and that the virus is species-specific. It only affects invertebrates
and causes no harm to other insects that could be natural bioinsecticides to
other pests. Moreover, other factors that contribute to its success are: the
high virulence of the virus and its infection efficiency, the low cost of the
product compared with chemical insecticides, and the fact that this is a
unique pest in most producing areas. The sum of these advantages makes
the baculovirus very attractive as a bioinsecticide (Moscardi, 1999;
Moscardi and Souza, 2002; Szewczyk et al., 2006).
Nevertheless, the bioinsecticide has some limitations. Although the high
specificity is very important in ecological terms, this restricts its market.
The bioinsecticide has low activity. However, this can be improved
through modification of the baculovirus genome, suppressing or incorpor-
ating genes from other organisms, such as those that express hormones or
toxins. According to Szewczyk et al. (2006), genetically modified baculo-
virus will be gradually introduced into countries that have a general
acceptance of genetically modified organisms (GMOs).
Another aspect to be considered is the need for increased production.
At the moment, the production of the bioinsecticide is accomplished by
harvesting infected caterpillars in the areas infected or by growing the
caterpillars in a laboratory. In the first case, there is great variability in
productivity from year to year, since the production depends on the insect
abundance during each harvest, which varies with multiple factors. How-
ever, there has been considerable progress in the production of the virus
under laboratory-controlled conditions (Moscardi and Santos, 2005). In
March of 2005 EMBRAPA finished the construction of a pilot plant with
the capacity to inoculate about 30 000 caterpillars per day, and another
institution, the COODETEC (Cascavel, PR), is enlarging its production
capacity to 600 000 caterpillars per day. However, the caterpillars have to
be fed with artificial diets and the cost of production of a dose of the
biopesticide, based on Baculovirus anticarsia, using raw material produced
locally, is approximately 90% higher than that obtained by direct harvest-
ing from the field. The use of alternative formulations, such as substituting
agar for carrageen as a gelling agent, has reduced production cost, making
it more economically feasible to produce the bioinsecticide in the labora-
tory (Santos, 2003). However, this type of process has high labor demands.
Large-scale virus production in cell culture in bioreactors is a desirable
development, since it allows virus multiplication in a smaller area and it
reduces labor requirement, in comparison with in vivo production in the
laboratory.
19.2 Baculovirus as a bioinsecticide: mechanism of action
Baculovirus belongs to the Baculoviridae family, which has two different
genera: Nucleopolyhedrovirus (NPV) and Granulovirus (GV). Baculo-
460 Animal Cell Technology
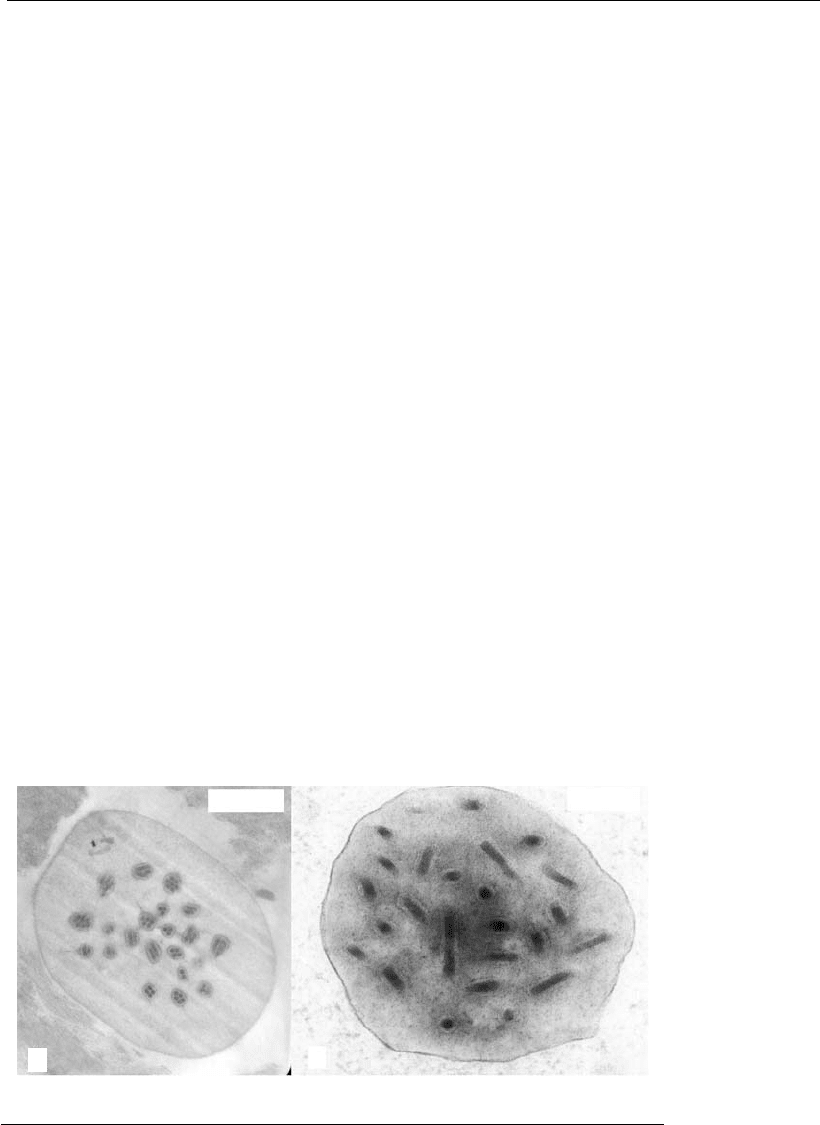
virus exists as two phenotypes designated extracellular virus or budded
virus (BV) and the occlusion bodies, also known as OBs, COPs, GDP, or
polyhedra (Figure 19.1). The occlusion bodies are structures that are
formed inside the nucleus of the infected insect cells by baculovirus
(Friesen and Miller, 2001). These are highly resistant and able to stay
viable in the environment for several years under different climatic condi-
tions. The protected viral particles (virions) are known as ODVs (occlu-
sion-derived virus) (Harrap, 1972).
The protein contained in the GV OBs is called granulin and that in the
NPV is called polyhedrin (Rohrmann, 1999; Winstanley and O’Reilly,
1999). GVs are smaller than NPVs and possess rounded OBs with just one
virion occluded (Winstanley and O’Reilly, 1999). NPVs present a poly-
hedral form and they can be multiple type (MNPV) (Figure 19.1A)or
simple type (SNPV) (Figure 19.1B ), depending on the number of capsids
per virion (Rohrmann, 1999). Since most of the baculovirus used as
biopesticidas are NPVs, in this chapter the OBs will be referred as
polyhedra.
The names of different baculovirus species come from the host name in
which the virus was initially identified in nature. In this way, each
baculovirus species receives the name of this host and the name of the
family to which the virus belongs.
The infection cycle is shown in Figure 19.2. When the polyhedra are
dispersed in the environment, the viral particles inside them (ODVs) are
deposited on the plant leaves. When the caterpillars feed on the virus-
contaminated foliage, they ingest the polyhedra. The alkaline environment
in the caterpillar medium intestine causes breakdown of the polyhedra and
the viral particles are released from the polyhedra. The infection of the
cells occurs via a receptor-mediated fusion process. Once in the cytoplasm,
the nucleocapsids without membrane are transported to the nucleus of the
cell, where gene expression and genome replication begins.
A
B
MNPV
SNPV
Figure 19.1
Electron micrographs illustrating polyhedra produced by nucleopolyhedrovirus in
insect cell culture with multiple nucleocapsids per envelope (A), or with single
nucleocapsids per envelope (B).
Bioinsecticides 461
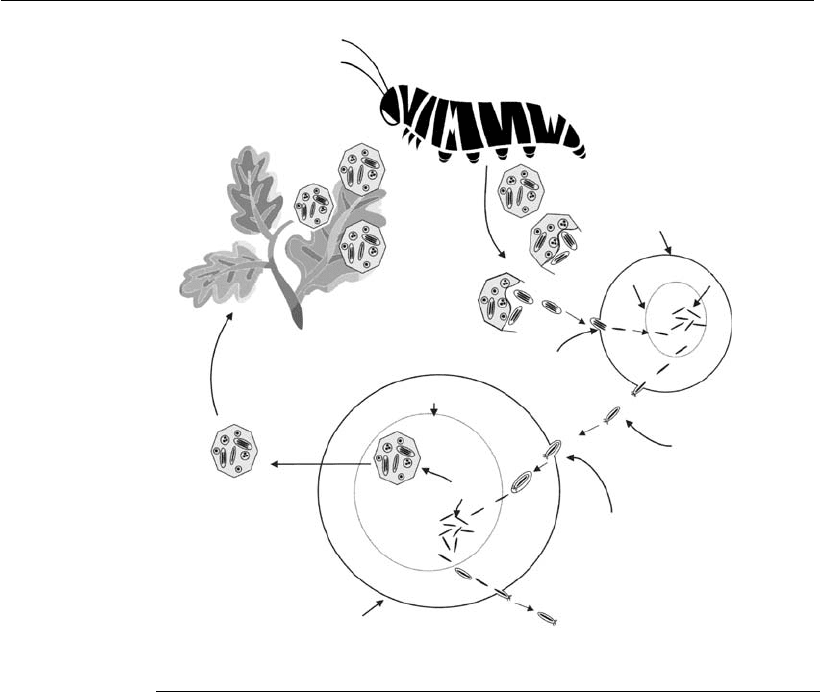
The nucleocapsids are envelopes that contain a nucleoprotein in the
center, which transports the viral DNA to the cell nucleus. After replica-
tion, new copies of the viral genome are encapsulated in the nucleus,
creating new nucleocapsids. The nucleocapsids then migrate to the cyto-
plasm and leave the cell by a budding process. In this process, the
nucleocapsids become enveloped by the acquisition of the host cell plasma
membrane. The resulting virions are extracellular (budded virus, BV) and
these constitute the main products of the first stage of the infection process
in the caterpillar, known as primary infection.
The BVs are taken to other tissues of the larva by the tracheal system,
where they enter the cells by endocytosis, starting with the secondary
infection process in which the infection is spread to all cells of the host
organism. Two virion types are produced in this stage of the infection.
Some of the newly synthesized nucleocapsids migrate to the cytoplasm
and are released from the cytoplasmic membrane, forming new BVs.
Other nucleocapsids are enveloped again in the nucleus. The virions that
are occluded (ODVs), are surrounded by the protein crystal structure,
forming the polyhedra. In the final phase of infection, the larva becomes
cream in color and the outer covering (tegument) becomes fragile. The
Insect feeding on
virus-contaminated
foliage
Polyhedra
Polyhedra are
released upon cell
lysis and death
of the host
Lysis
Insect cell
Replication
Replication
Nucleus
Nucleus
Budding
BV
Secondary
infection
endocytosis
Budded virus
BV
Primary
infection
Fusion
Cell
Dissolution of polyhedra
in the midgut releasing the
virions (occlusion-derived
virus)
Figure 19.2
Diagram of the life cycle of a nucleopolyhedrovirus.
462 Animal Cell Technology

rupture of the tegument causes the release of the polyhedra, which are
then available for the infection of other caterpillars (Federici, 1986;
Granados and Federici, 1986; Adams and Bonami, 1991; Rohrmann, 1999;
Winstanley and O’Reilly, 1999; Friesen and Miller, 2001).
19.3 Animal cell cultur es for baculovirus production
The insect baculovirus–cell system has been widely used, mainly for the
production of recombinant proteins (Maiorella et al., 1988; Jarvis, 1997).
This system has several advantages, including the ability to produce
functional recombinant proteins that are immunogenically active, the
ability to make post-translation modifications, and the fact that it contains
a powerful promoter (polyhedrin), as well as the fact that the virus is not
pathogenic to plants and vertebrates (Caron et al., 1990; Nguyen et al.,
1993; Godwin et al., 1996).
However, for bioinsecticide production there are specific factors to be
taken into account:
(i) The large-scale cell production to be used in virus production, since
large volumes of cells are necessary, at a competitive cost.
(ii) Economic production processes and cheap culture media are needed
to make production viable.
(iii) It is necessary to establish the most effective cell line for viral
production with high virus per cell productivity. The productivity
depends on both the cell type and virus.
(iv) Mutant generation must be monitored and avoided. The risk increases
with the number of passages of the virus in cells.
(v) It is necessary to maintain viral virulence when cultured in vitro.It
has been shown that there is a tendency for a loss of virulence with
viral passage in cell culture.
(vi) The activity of the polyhedra produced in vitro should be compared
with those obtained in caterpillars.
19.4 Effect of culture medium, cell line, and virus isolate on
biopesticide production
Lepidoptera-derived insect cell lines are used for biopesticide production.
Sf21 cells, originated from pupal ovarian tissue of the fall armyworm
Spodoptera frugiperda, and Sf9 cells, a subclone of the Sf21 cells, are the
most widely used cell lines for biopesticide production (King and Possee,
1992) (Figure 19.3). The availability of insect cell lines from Invitrogen (La
Jolla, EUA) was an important step for increasing the use of these cell lines
worldwide.
The establishment of insect cell line cultures allowed a detailed study of
the infection cycle of many baculoviruses. These cell lines are easily
cultured in vitro and their maintenance is relatively simple. The majority
of the cell lines can be cultivated using a temperature of 25–308C. How-
ever, the best temperature for the growth and infection of Sf9 cells is
around 27–288C (King and Possee, 1992).
Bioinsecticides 463
