Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


16.4.2 Characterization of biopharmaceuticals
Biopharmaceuticals have completely different properties from conven-
tional low molar mass pharmaceuticals. This particularly affects product
characterization, due to the complex production processes and protein
structures. Thus, successful production of biopharmaceuticals relies
mainly on strict protocols, clinical expertise, and follow-up during clinical
application (Crommelin et al., 2003).
For these reasons, the ability to compare products originating from
different processes is more important than characterizing a biopharma-
ceutical product from a well established process. Modifications in the
production process can result in changes in physicochemical properties
and post-translational modifications. These modifications can influence
biological activity, bioavailability, and immunogenicity of the product
(Chirino and Mire-Sluis, 2004). Therefore, as discussed in Chapter 14, the
regulatory agencies worldwide are discussing criteria to be applied to
evaluate comparability of biological products (Food and Drug Adminis-
tration, 2005; European Medicines Agency, 2005).
Among the many tests available for characterizing biopharmaceuticals,
assessment of protein structure, particularly quaternary structure, includ-
ing aggregate formation, seems to be particularly important, since it can
strongly influence immunogenicity. This remains a concern for the char-
acterization of biological products, since preclinical studies, carried out in
animals, are not able to predict the immunogenicity of a biological drug in
the target population (Chirino and Mire-Sluis, 2004). In this context,
product labelling becomes an important issue, since aggregate formation
and continuous administration of a protein drug can lead to antibody
production (Ryff and Schellekens, 2002). Biopharmaceutical labelling
could thus provide information in such a way that the physician would
understand the clinical and biological consequences of antibody produc-
tion and, therefore, could make a decision that could lead to the suspen-
sion of a treatment.
16.4.3 Alternative expression systems
Due to the structural complexity of therapeutic proteins, their recombi-
nant forms are usually produced in mammalian cell culture. When glyco-
sylation is not present or is not essential for biological activity, therapeutic
proteins can be produced in bacteria (e.g. E. coli) or yeast (e.g. Sacchar-
omyces cerevisiae). However, some studies propose the use of these
systems for glycosylated proteins as well.
Baneyx and Mujacic (2004) propose the genetic manipulation of E. coli,
to enable metabolic competency to carry out post-translational modifica-
tions. Gerngross (2004) argues for the use of filamentous fungi and yeast
for the production of therapeutic proteins by the expression of human
glycosylation enzymes. This type of strategy was reported by Chiba et al.
(1998) and Martinet et al. (1998) for S. cerevisiae and Pichia pastoris,
respectively.
Plant cells are another expression system, which is being evaluated for
the production of recombinant therapeutic proteins. According to Hellwig
404 Animal Cell Technology

et al. (2004), recombinant human glycoproteins synthesized in plant cells
show greater similarity to the native proteins than those produced in
recombinant microorganisms. However, there are some limitations, such
as the instability of recombinant proteins in plant cell culture media and
the low protein yields reported so far for plant cells in culture (Hellwig et
al., 2004). Among therapeutic proteins, GM-CSF has been produced in
Nicotiana tabacum (Lee and Kim, 2002) and Lycopersicum esculentum
(Kwon et al., 2003). A concrete example of the application of plant cells is
the first market license issued in January 2006 to a veterinary vaccine
produced in plant cells (Katsnelson et al., 2006). The North American
USDA (United States Department of Agriculture) considered the veter-
inary viral vaccine safe and effective in protecting chickens from New-
castle disease. This subunit vaccine consists of a protein expressed in
recombinant tobacco cells and represents the first vaccine obtained from
plant cells to be approved worldwide.
Another possible system for the production of therapeutic proteins
consists of transgenic animals. Although there is controversy regarding
safety and reproducibility, many reports on this subject have been pub-
lished and some proteins derived from these systems are currently under
clinical trials. Parker et al. (2004) purified and characterized human
fetoprotein Æ secreted in transgenic goat milk. This protein, used in the
treatment of autoimmune diseases, proved to be identical to the native
form by mass spectrometry, circular dichroism, and pharmacokinetic tests.
Using a different approach, some authors propose post-translational
modification in vitro. Glycosylated proteins could be obtained by in vitro
glycosylation after their production in non-mammalian expression sys-
tems. Synthetic strategies for preparing glycoproteins can be classified into
two main categories: convergent and sequential. In the convergent strat-
egy, the glycan is assembled separately by enzymatic or chemical methods,
or obtained from natural sources, and is then attached to the polypeptide
chain. In the sequential strategy, a mono- or disaccharide unit is intro-
duced into the polypeptide, and then the complete glycan is synthesized
by the sequential attachment of sugars by the action of glycosyltrans-
ferases. However, the development of simple and reliable methods for
obtaining selectively and uniformly glycosylated proteins is still a chal-
lenge and will probably remain a focus of future research (Khmelnitsky,
2004).
Finally, some authors propose the use of enzyme mixtures for the
biosynthesis of complex molecules. A cell free protein synthesis (CFPS)
system is a novel approach that was successfully used for the production
of complex mammalian proteins with multiple disulfide bonds (Bhatta-
charya, 2004). However, even if the technical potential of this method is
proved, its economical feasibility has still to be proven.
16.4.4 Second-generation biopharmaceuticals
Most biopharmaceuticals initially approved, known as first-generation
biopharmaceuticals, consist of simple replacement proteins with an amino
acid sequence identical to the native human protein. Second-generation
biopharmaceuticals are engineered molecules resulting from modifications
Recombinant therapeutic proteins 405

of glycans or of amino acid composition, or from conjugation to other
molecules, such as in the case of pegylated proteins. These alterations in
the protein are carried out with different goals: (i) to improve the
pharmacokinetic profile of the protein; (ii) to increase protein half-life;
and/or (iii) to alter its immunogenic profile.
An example of a second-generation biopharmaceutical obtained by site-
directed mutagenesis is TNKase (Tenecteplase
TM
), which is an altered
form of recombinant tPA produced in CHO cells by Genentech. The
substitution of three amino acids in the native protein results in a molecule
with higher affinity for its receptor (fibrin), a higher resistance to its
natural inhibitor (PAI-1), and an increased half-life (Walsh, 2004).
Second-generation insulins are also available and consist of molecules
with changes in one to two amino acids, aimed at accelerating the onset of
activity (Lispro and Aspart insulin products) or increasing the half-life
(Glargine insulin product).
Humanized antibodies, discussed in more detail in Chapter 17, are also
considered second-generation molecules. By humanization, the high im-
munogenicity of a murine antibody is eliminated, enabling improved
therapeutic use.
Protein engineering can also be used for creating fusion proteins or
hybrid biopharmaceuticals. An example of this type of product is Enbrel
1
(Etanercept), which consists of the extracellular ligand-binding domain of
the 75-kDa receptor for tumor necrosis factor-alpha (TNFÆ) and the Fc
portion of human IgG1. TNF is a proinflammatory cytokine present at
high concentrations in patients suffering from rheumatoid arthritis. En-
brel
1
acts as competitive inhibitor of TNF, as it competes with the
receptor molecules (TNFR) present in the cell. The antibody portion of
the fusion protein increases the half-life in blood (Walsh, 2004).
Another approach relies on modifying the glycan of glycoproteins to
improve pharmacokinetics of biopharmaceuticals. An example was the
introduction of two additional N-glycosylation sites in EPO, resulting in
a hyperglycosylated erythropoietin product (Aranesp
1
), which was ap-
proved for commercialization in 2001. The modification of the carbo-
hydrate structure increased the half-life to 25.3 hours (as compared with
8.5 hours for the first-generation EPO), allowing a decrease in injection
frequency that is attractive for patients.
With the considerable evolution achieved in recent years in the elucida-
tion of structure–function relationship of proteins, as well as in the areas
of protein engineering and bioinformatics, many new developments are
expected in the field of new-generation biopharmaceuticals. These will
have significant advantages for patients, including lower immunogenicity,
lower frequency of injections, and enhanced stability in serum.
References
Anderson PM, Markovic SN, Sloan JA, Clawson ML, Wylam M, Arndt CAS,
Smithson WA, Burch P, Gornet M, Rahman E (1999), Aerosol granulocyte
macrophage-colony stimulating factor: a low toxicity, lung-specific biological
therapy in patients with lung metastases, Clin. Cancer Res. 5:2316–2323.
406 Animal Cell Technology

Baneyx F, Mujacic M (2004), Recombinant protein folding and misfolding in Escher-
ichia coli, Nat. Biotechnol. 22:1399–1408.
Bhattacharya SK (2004), The use of enzyme mixtures for complex biosyntheses, Curr.
Opin. Biotechnol. 15:449–455.
Bhattacharyya MS, Singh J, Soni P, Banerjee UC (2003), Recombinant factor VIII for
haemophilia: an overview of production technologies, CRIPS 4:2–8.
Boedeker BGD (1992), The manufacturing of the recombinant factor VIII Kogenate
1
,
Transf. Med. Rev. 6:256–260.
Castilho LR, Medronho RA (2002), Cell retention devices for suspended-cell perfusion
cultures, Adv. Biochem. Eng. Biotechnol. 74:129–169.
Chiba Y, Suzuki M, Yoshida S, Yoshida A, Ikenaga H, Takeuchi M, Jigami Y,
Ichischimak K (1998), Production of human compatible high mannose-type
(Man
5
GlcNAc
2
) sugar chains in Saccharomyces cerevisiae, J. Biol. Chem.
273:26298–26304.
Chirino AJ, Mire-Sluis A (2004), Characterizing biological products and assessing
comparability following manufacturing changes, Nat. Biotechnol. 22:1383–1391.
Crommelin DJA, Storm G, Verrijk R, Leede L, Jiskoot W, Hennink WE (2003),
Shifting paradigms: biopharmaceuticals versus low molecular weight drugs, Int. J.
Pharm. 266:3–16.
Dudzinski DM (2004), Reflections on historical, scientific, and legal issues relevant to
designing approval pathways for generic versions of recombinant protein-based
therapeutics and monoclonal antibodies, Harvard Law School, Thesis. Available
at: http://leda.law.harvard.edu/leda/data/630/(accessed February 2006).
Edwards J, Kirby N (1999), Recombinant coagulation factor IX (BeneFix
1
). In: Walsh
G, Murphy B (Eds), Biopharmaceuticals: An Industrial Perspective, Springer
Verlag, Berlin, pp. 73–108.
European Medicines Agency (2005), Guidance documents on biosimilar products.
Available at: http://www.emea.eu.int/htms/human/biosimilar/biosimilarfin.htm
(accessed October 2005).
European Medicines Agency (2006), EPARs for authorised medicinal products for
human use. Available at: http://www.emea.europa.eu/htms/human/epar/a.htm
(accessed December 2006).
Food and Drug Administration (2005), Scientific considerations related to developing
follow-on protein products. Available at: http://www.fda.gov/cder/meeting/
followOn/followOnPresentations2_2005.htm and http://www.fda.gov/cder/
meeting/followOn/followOnPresentations.htm (accessed May 2005).
Food and Drug Administration (2006), FDA approved products. Available at: http://
www.accessdata.fda.gov/scripts/cder/drugsatfda/(accessed December 2006).
Gerngross TU (2004), Advances in the production of human therapeutic proteins in
yeast and filamentous fungi, Nat. Biotechnol. 22:1409–1414.
Hellwig S, Drossard J, Twyman RM, Fischer R (2004), Plant cell cultures for the
production of recombinant proteins, Nat. Biotechnol. 22:1415–1422.
Katsnelson A, Ransom J, Vermij P, Waltz E (2006), News in brief: USDA approves
the first plant-based vaccine, Nat. Biotechnol. 24:233–234.
Khmelnitsky YL (2004), Current strategies for in vitro protein glycosylation, J. Mol.
Catal. B Enzym. 31:73–81.
Kwon TH, Kim YS, Lee JH, Yang MS (2003), Production and secretion of biologically
active human granulocyte macrophage-colony stimulation factor in transgenic
tomato suspension cultures, Biotechnol. Lett. 25:1571–1574.
Lee SY, Kim DI (2002), Stimulation of murine granulocyte macrophage-colony
stimulation factor production by pluronic F-68 and polyethylene glycol in
transgenic Nicotiana tabacum cell culture, Biotechnol. Lett. 24:1779–1783.
Martinet W, Maras M, Saelens X, Jou WM, Contreras R (1998), Modification of the
Recombinant therapeutic proteins 407

protein glycosylation pathway in the methylotrophic yeast Pichia pastoris, Bio-
technol. Lett. 20:1171–1177.
Muller RH, Keck CM (2004), Challenges and solutions for the delivery of biotech
drugs – a review of drug nanocrystal technology and lipid nanoparticles,
J. Biotechnol. 113:151–170.
Parker MH, Birck-Wilson E, Allard G, Masiello N, Day M, Murphy KP, Paragas V,
Silver S, Moody MD (2004), Purification and characterization of a recombinant
version of human Æ-fetoprotein expressed in the milk of transgenic milk, Prot.
Express. Purif. 38:177–183.
Pavlou AK, Belsey MJ (2005), The therapeutic antibodies market to 2008, Eur. J.
Pharm. Biopharm. 59:389–396.
PhRMA (2004), 324 Biotechnology medicines in testing promise to bolster the arsenal
against disease. Available at: http://www.phrma.org/files/Biotech%20Survey.pdf
(accessed February 2006).
Reichert J, Pavlou A (2004), Monoclonal antibodies market, Nat. Rev. Drug Discov.
3:383–384.
Research and Markets (2005a), Biopharmaceuticals – current market dynamics &
future outlook. Available at: http://www.researchandmarkets.com/reports/39083
(accessed May 2005).
Research and Markets (2005b), Erythropoietin – current market dynamics & future
outlook. Available at: http://www.researchandmarkets.com/reportinfo.asp?
report_id¼298258 (accessed May 2005).
Research and Markets (2005c), Colony stimulating factors – current market dynamics
& future outlook. Available at: http://www.researchandmarkets.com/reportinfo.
asp?report_id¼235022 (accessed May 2005).
Research and Markets (2005d), Monoclonal antibody therapeutics: current market
dynamics & future outlook. Available at: http://www.researchandmarkets.com/
reportinfo.asp?report_id¼75046 (accessed May 2005).
Ryff JC, Schellekens H (2002), Immunogenicity of rDNA-derived pharmaceuticals,
Trends Pharmacol. Sci. 23:254–256.
Walsh G (2002), Biopharmaceuticals and biotechnology medicines: an issue of nomen-
clature, Eur. J. Pharm. Sci. 15:135–138.
Walsh G (2003), Biopharmaceuticals – Biochemistry and Biotechnology, 2nd Ed., John
Wiley and Sons, Chichester.
Walsh G (2004), Second-generation biopharmaceuticals, Eur. J. Pharm. Biopharm.
58:185–196.
Werner RG (2004), Economic aspects of commercial manufacture of biopharmaceuti-
cals, J. Biotechnol. 113:171–182.
408 Animal Cell Technology
17
Monoclonal antibodies
Wirla M.S.C. Tamashiro and Elisabeth F.P. Augusto
17.1 Introduction
Antibodies are molecules secreted by plasmocytes; these are differentiated
from B lymphocytes following previous contact with a specific antigen.
The number of different antibodies produced by humans is extremely
large, incorporating between 10
7
and 10
8
distinct molecules, but any one B
lymphocyte only produces antibodies against a single specific antigen.
After immunization or contact with the antigen, the B cells which
recognize each antigenic determinant in an antigen molecule will produce
clones, thus increasing the number of lymphocytes specific for that
antigen. Although the antibodies produced by all of these lymphocyte
clones will be specific for the same antigen, these antibodies will not
necessarily be structurally identical, that is, they are polyclonal antibodies.
The result of this process is an increase in the level of specific antibodies in
the serum or body secretions of the immunized individual. Even immuni-
zation with highly purified antigens results in the production of polyclonal
antibodies, so that the composition and properties of immune sera vary
with each preparation (Abbas et al., 2000).
Attempts to produce homogeneous antibodies in vitro arose almost as
soon as lymphocytes and their biological properties were discovered.
However, B lymphocytes normally do not survive in cell cultures. For
survival, they must undergo a malign transformation, such as that induced
by the Epstein–Barr virus (Steinitz et al., 1977). Some tumors of B
lymphocytes, the multiple myelomas, can arise spontaneously and others
can be induced by the administration of mineral oil. These tumors,
whether spontaneous or induced, can be adapted to in vitro cultivation,
and they will secrete highly homogeneous antibodies (monoclonal anti-
bodies, mAbs) since they originate from a single tumor cell (Horibata and
Harris, 1977). However, even if all antibodies are homogeneous in a
myeloma culture, it is impossible to predict what this specificity will be.
The production in vitro of mAbs with a predetermined specificity has
only been possible since the advent of the technology of hybridomas,
which was introduced by Kohler and Milstein in 1975. These hybridomas
are the products of in vitro fusion of myelomas with normal B lympho-
cytes. The fusion products preserve the capacity for self-propagation in a
culture, as well as the secretion of the antibodies of interest, characteristics
inherited from the parent myeloma and the normal B lymphocyte,
respectively. The myelomas used in such fusions generally involve cell
lines from B-lymphocyte tumors developed in mice or rats (Cotton and
Milstein, 1973; Ko
¨
hler and Milstein, 1975b; Shulman et al., 1978), while

the B lymphocytes come from mice or rats previously immunized with the
antigen of interest. After the fusion of the myeloma with the normal B
lymphocyte, the hybridomas of interest can be selected and cloned to
obtain an unlimited quantity of homogeneous antibodies that are highly
specific (Ko
¨
hler and Milstein, 1975a).
The production of such mAbs using hybridoma technology has made it
possible to detect and quantify a wide variety of molecules produced by
live organisms in highly specific and sensitive assays, thus making possible
enormous advances in various areas of biological research. The specificity
of these mAbs has also facilitated the improvement of clinical diagnoses, as
well as creating expectations about their use as a therapeutic agent for
human diseases.
One problem with the use of these antibodies in humans, however, is
the immunogenicity (the ability to induce an immune response) of murine
mAbs, and various associated adverse reactions. Some mouse mAbs have,
however, been licensed for use in human patients (Lin et al., 2005). The
adverse reactions can be controlled to a certain extent by ‘‘humanization’’
of the murine reagents (produced by cells transfected with antibody
genes), as well as by the use of recombinant DNA techniques to produce
antibody fragments preserving the antigen-binding capacity of the original
antibody molecules (Huhalov and Chester, 2004). Only recently has the
establishment of a line of human myeloma cells (called Karpas 707H) been
reported, and this is proving useful in the production of hybridomas via
fusion with B lymphocytes obtained from immunized or infected humans
(Karpas et al., 2001; Vaisbourd et al., 2001). Obtaining all these highly
specific mAbs, whether murine, humanized, or antibody fragments, is
dependent upon the production of a hybridoma.
Both hybridomas and humanized antibody producer cells can be culti-
vated indefinitely in conventional cultures, usually containing fetal bovine
serum in the medium. In the supernatants of these cultures, the quantity of
mAbs varies from 20 to 100 g/ml of protein, depending on the cell and
the system of cultivation.
There are, however, in vivo methods of obtaining mAbs, and these
produce much larger quantities. One of these is intraperitoneal administra-
tion of the hybridomas into histocompatible animals (of the same cell line
as the parents of the hybridoma) or immunodeficient animals (individuals
with no functional immune system). These receptor animals will develop
ascitic tumors containing from 1 to 40 mg/ml of the mAb secreted by the
hybridoma (Kretzmer, 2002). However, despite the fact that this method
is well documented and has been used widely in the past, the procedure is
presently being rejected because of ethical concerns over the use of
laboratory animals.
This has led to an increased interest in methods for the in vitro
production of large quantities of mAbs. Submersed cultivation in bioreac-
tors is one possibility. In contrast with traditional methods, this procedure
uses large quantities of culture medium, increasing the scale of the process
and making possible the production of virtually unlimited quantities of
mAbs by a given hybridoma.
In this chapter, the fundamentals of hybridoma technology are dis-
cussed and the most widely used protocols and procedures are presented.
410 Animal Cell Technology
The main advances in large-scale in vitro production of mAbs are also
presented. This topic is of extreme importance in the biotechnology
industry, as can be seen by the extent of the use of mAbs in diagnostic and
therapeutic applications.
17.2 Antibodies
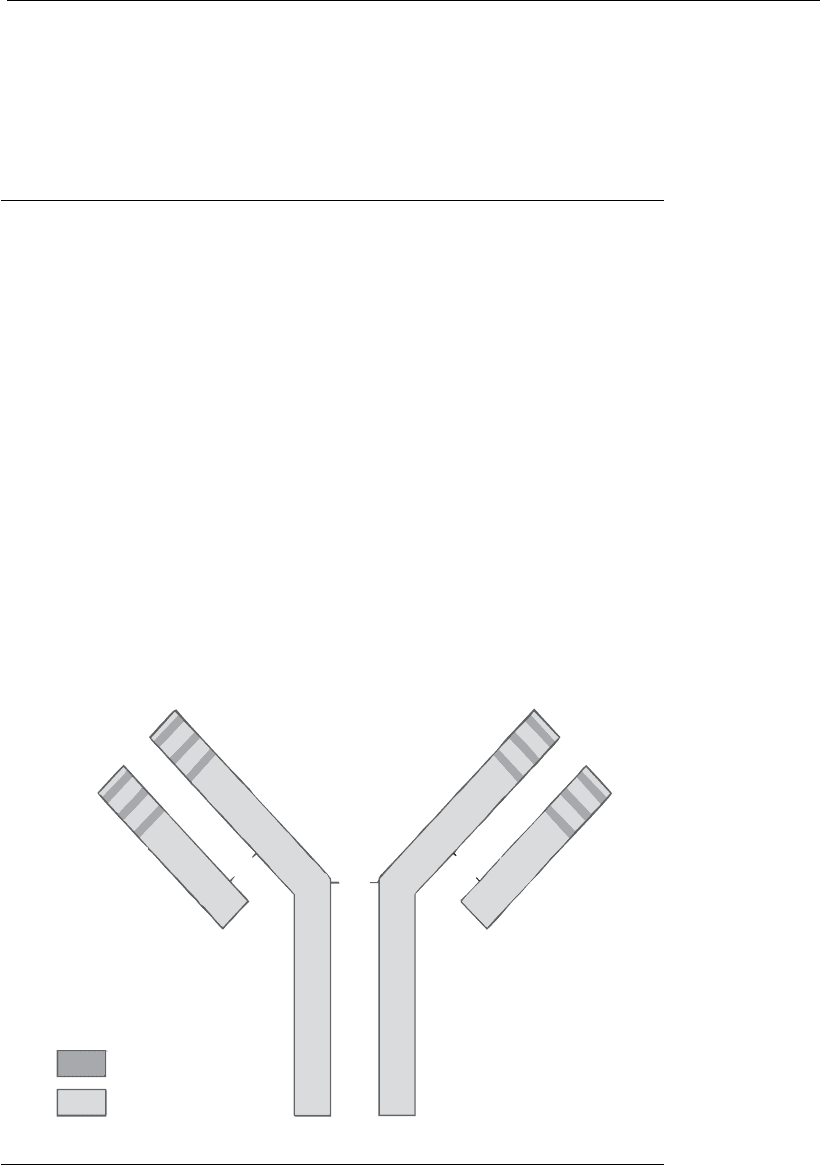
Antibodies constitute the prototype of the superfamily of immunoglobins
(Ig), molecules, which have globular domains resulting from the existence
of cysteine residues along their polypeptide chains. These residues form
disulfide bridges (S-S) within and between chains. The functional units of
the antibody molecules are generally monomers, each formed by a pair of
light polypeptide chains of approximately 25 kDa each, and another pair
of heavy ones of about 50 kDa (Figure 17.1). The chains of each pair are
identical, with all four chains linked by inter-chain disulfide bridges. Each
of the antibody chains in this monomer has an N-terminal domain,
identical for the two members of each pair, although these domains vary
from one molecule to another. The heavy chains also have three or more
constant domains (C-terminals), whereas the light chains have only a
single one. The variable N-terminal domains of the Ig molecule constitute
the antigen combining sites, with each of the two sets of heavy and light
chains of the monomer constituting a single site for antigen binding.
Hence, each monomer of the immunoglobulin has duplicate sites for
combining with a specific antigen. The rest of the molecule
(the C-domains) effects the other functions of the antibody: interaction
with the cells of the immune system (via Ig receptors), linking to the
molecules of the complement system, and crossing the placenta or epithe-
Mouse
Human
CDR1
CDR2
CDR3
CDR1
CDR2
CDR3
S-S
S-S
S-S
Figure 17.1
Structure of an antibody molecule.
Monoclonal antibodies 411

lial mucosa. The Ig classes (IgD, IgM, IgG, IgE, and IgA) are determined
by the primary sequence of the constant domains of the heavy chain pair.
IgD and IgM monomers are expressed on the surface of the naı
¨
ve B
lymphocyte, which constitute B-cell receptors (BCRs) for antigens. Except
for IgD, all of the Ig classes can be secreted by plasmocytes upon contact
with a specific antigen, whether a foreign substance or an altered native
constituent. Although IgG and IgE are always monomers, IgA molecules
can be secreted as either monomers or dimers. IgM is always secreted as a
pentamer. In humans, there are four IgG subclasses: IgG1, IgG2, IgG3,
and IgG4, whereas there are two IgA subclasses: IgA1 and IgA2 (Abbas et
al., 2000).
There are, however, enormous numbers of antigens that may be encoun-
tered by the immune system. To deal with them, B lymphocytes undergo
a unique developmental process involving genetic recombination of gene
segments to form a wide variety of antibodies. This process, which takes
place in the bone marrow of mammals and the Fabricius bursa of birds,
was determined some 30 years ago by Tonegawa et al. (1978). The critical
aspect of this process is the occurrence of cuts in the parent DNA chain
of the developing lymphocyte by special enzymes (RAG1 and RAG2),
that are only found in these cells. This cutting and the subsequent re-
arrangement of gene segments allows great variety in the gene, which is
responsible for the formation of the N-terminal (variable) regions of the Ig
chains. This enables coupling with the wide variety of antigen determi-
nants encountered by the organism.
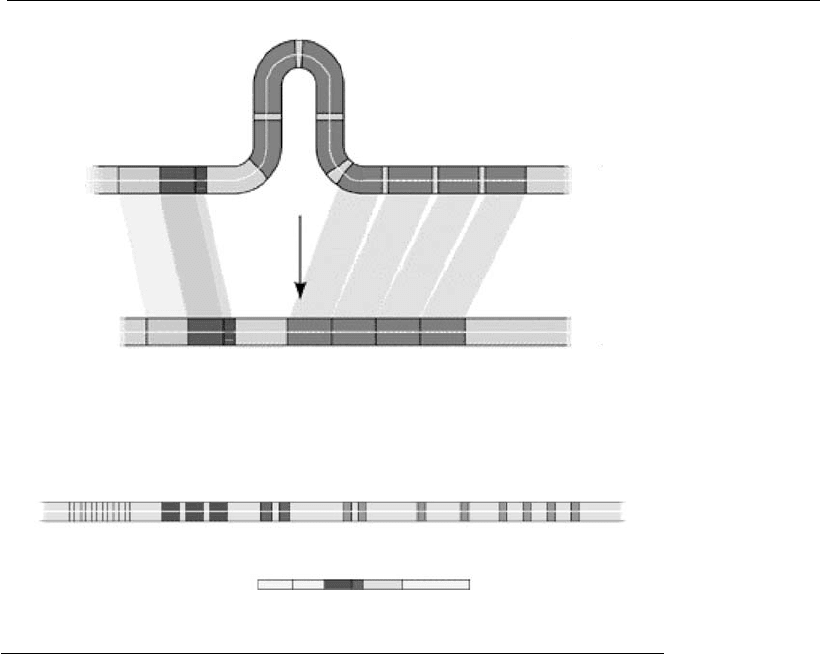
Three regions of the parent DNA heavy chain are affected by these cuts:
V, D, and J (for variable, diversity, and junction, respectively). Initially,
simultaneous cuts occur in a D and a J region of the chain; the segment
between the two cuts is eliminated, and the two loose ends are joined in a
DJ sequence. Then, two additional cuts occur, one in a V region, and the
second immediately prior to the new DJ sequence. The intervening
segment is eliminated, and the V region is attached to the DJ sequence to
form the final VDJ gene sequence, which codifies the variable portion of
what will be the heavy Ig chain. Figure 17.2 shows this gene recombination
in the formation of the heavy chain variable region.
The second step in the formation of the heavy chain involves transcrip-
tion of the DNA to form the primary transcript of the RNA of the
developing lymphocyte, which includes the new VDJ sequence. This
primary transcript (tRNA), however, still includes non-codifying seg-
ments (introns). In the third step, these introns are eliminated, and the
final RNA includes only the variable N-terminal gene and the genes for all
of the constant regions.
This mRNA is then translated to heavy polypeptide chains in the
cytoplasm. The first set formed is the heavy chain of IgM, consisting of a
VDJ region and a C region (the first of the sequence of constant genes)
(Maki et al., 1980). Each pair of these heavy chains is joined with a pair of
surrogate light chains (formed without genetic recombination) and mi-
grates to the surface of the developing lymphocyte. This provisional
antibody probably attaches to a self-antigen, triggering the rearrangement
of the genes for the variable region of the light chains. This rearrangement
is similar to that undergone in the formation of the heavy chain, except
412 Animal Cell Technology
that only a single pair of cuts in the V and J regions is involved, because
there is no D region in light chains.
The first or primary transcript of this light chain gene segment includes
both the VJ gene sequence and the two genes for the constant region
(kappa and lambda). The formation of the mRNA for the light chains is
similar to that of the heavy chains. Kappa chains are the first light chains
to be formed and lambda chains will only be formed if the molecules are
non-functional.
Once the definitive light chains have been produced, they replace the
surrogate chains in the constitution of the antigen receptor of developing
B lymphocyte, and then the immature lymphocyte migrates to the periph-
eral lymphoid organs (spleen, lymph nodes, etc.). Once definitive chains
have been produced, their presence inhibits rearrangement of the genes of
the corresponding allele. This process, called allele exclusion, leads to the
production of surface immunoglobulins capable of recognizing only one
specific antigenic determinant. The set of genetic events described here is
responsible for the generation of the enormous repertoire of antibodies in
organisms with an immune system (Sakano et al., 1980).
During the mid-1970s, this process of antibody generation was not
understood, and the laboratory of Cesar Milstein was one of those trying
to decipher the mechanisms involved. The experimental approach adopted
DNA
DNA
IgM gene
IgG gene
DNA re-arrangement
V
V D
D
I
I
µ
δ
γ
3
γ
3
γ
1
γ
1
γ
2b
γ
2b
γ
2a
γ
2a
ε
ε
α
α
VV V
1 2 100
, ...
(variable)
segments
⫺
DD D
12 30
, ...
(diversity)
segments
⫺
J1, J2...J6
(joining)
segments
1234...100 1 2...30 1...6
µ
δ
mRNA
VDJ
µ
Figure 17.2
Genes for the expression of immunoglobulins heavy chain.
Monoclonal antibodies 413
