Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


Table 16.1 Examples of biophar maceuticals produced in mammalian cells approved for
commercialization
Classification Product name Protein Indication
Cytokines Avonex
1
Interferon 1a Multiple sclerosis
Rebif
1
Interferon 1a Multiple sclerosis
Hematopoietic Epogen
1
/Procrit Epoetin Æ Anemia
growth factors Epogin/Recormon Epoetin Anemia
Neorecormon
1
Epoetin Anemia
Nespo/Aranesp
1
Darbepoetin Æ Anemia
Neutrogin
1
/Granocyte G-CSF (lenograstim) Neutropenia
Hormones Gonal-f
1
FSH Female infertility
Luveris
1
/Lutropin alfa Luteinizing hormone Female infertility
Ovitrelle
1
/Ovidrel/
Choriogonadotropin Æ
Gonadotropin Female infertility
Puregon
1
/Follistim
AQ/Follitropin
FSH Female infertility
Thyrogen
1
Thyrotropin Æ Thyroid function (in vivo
diagnostics)
Growth factors InductOS
1
/Dibotermin Æ Bone morphogenetic
protein-2
Tibia fracture, spinal surgery
Osigraft
1
/Eptotermin Æ Bone morphogenetic
protein
Tibia fracture
Blood Advate Factor VIII Hemophilia A
coagulation BeneFIX Factor IX Hemophilia B
factors Helixate
1
NexGen/
Kogenate
1
FS
Factor VIII Hemophilia A
Kogenate
1
Factor VIII Hemophilia A
NovoSeven
1
Factor VIIa Hemophilia A and B
Recombinate Factor VIII Hemophilia A
Refacto
1
B-domain deleted factor
VIII
Hemophilia A
Therapeutic Activase
1
/Alteplase tPA Acute myocardial infarction
enzymes Cerezyme
1
Glucocerebrosidase Gaucher’s disease
Fabrazyme
1
/Agalsidase Æ-Galactosidase A Fabry disease
Hylenex
TM
Hyaluronidase Increase absorption and
dispersion of other injected
drugs
Myozyme
1
/Alglucosidase
Æ
Acid Æ-glucosidase Pompe disease
Naglazyme
TM
/Galsulfase N-Acetylgalactosamine
4-sulfatase
Mucopolysaccharidosis VI
Pulmozyme
1
DNAse I Cystic fibrosis
Replagal
1
/Agalsidase ÆÆ-Galactosidase Fabry disease
fi
TNKase/Tenecteplase
TM
/
Metalyse
tPA Acute myocardial infarction
Xigris
1
/Drotrecogin Æ Activated protein C Severe sepsis
394 Animal Cell Technology

r
Cell line Manufacturing
process data
First
approval
date
Company Countries where
approved
CHO Bioreactor 1996 Biogen Europe, USA
CHO Bioreactor with
microcarriers
1998 Serono Europe, USA
CHO Roller bottles 1989 Amgen Several
CHO – 1990 Chugai Japan, Europe
CHO – 1997 Roche Europe
CHO – 2001 Amgen Europe, USA
CHO – 1991 Chugai Japan, Europe
CHO – 1995 Serono Europe, USA
CHO Bioreactor 2000 Serono Europe, USA
CHO – 2000 Serono Europe, USA
CHO Bioreactor 1996 Organon Europe, USA
CHO Repeated batches 1998 Genzyme Europe, USA
CHO Suspension bioreactor 2002 Wyeth Europe
CHO – 2001 Howmedica International Europe
CHO 2500 L bioreactor 2003 Baxter Europe, USA
CHO Fed-batch bioreactor 1997 Genetics Institute Europe, USA
BHK Perfusion bioreactor 2000 Bayer USA, Australia,
Europe,
Switzerland
BHK Perfusion bioreactor 1993 Bayer Several
BHK Bioreactor, repeated
batches
1996 Novo Nordisk Europe, USA,
Switzerland
CHO – 1992 Baxter Several
CHO Perfusion bioreactor 1999 Genetics Institute/Biovitrum AB Europe, USA
CHO – 1986 Genentech Several
CHO – 1994 Genzyme Europe, USA
CHO Continuous bioreactor 2001 Genzyme Europe, USA
CHO – 2005 Halozyme Therap. USA
CHO – 2006 Genzyme Europe, USA
CHO – 2005 BioMarin Europe, USA
CHO – 1993 Genetech Sweden, USA,
Switzerland
Human
fibroblasts
– 2001 Shire Human Genetic Therapies Europe
CHO 12 000 L bioreactor 2000 Genentech/Boehringer Ingelheim Europe, USA
HEK-293 Bioreactor 2001 Eli Lilly Europe, USA
Continued
Recombinant therapeutic proteins 395

Table 16.1 Continued
Classification Product name Protein Indication
Fusion proteins Amevive/Alefacept Fusion protein (IgG1/LFA3) Psoriasis
or immuno- Enbrel
1
/Etanercept Fusion protein (IgG1/TNFR) Rheumatoid arthritis
conjugates Myoscint
1
/Imciromab
Pentetate
Immunoconjugate
(IgG2a/DTPA)
Cardiac imaging (in vivo
diagnostics)
ProstaScint
1
/Capromab
Pendetide
Immunoconjugate
(IgG1/DTPA)
Prostate in vivo diagnostics
Zevalin
1
/Ibritumomab
Tiuxetan
Immunoconjugate
(IgG1/MX)
NonHodgkin’s lymphoma
Antibodies Avastin
1
/Bevacizumab Immunoglobulin G1 Colorectal cancer
Bexxar
1
/Tositumomab Immunoglobulin G2a NonHodgkin’s lymphoma
Campath
1
/
MabCampath/
Alemtuzumab
Immunoglobulin G1 Chronic lymphocytic
leukemia
Erbitux
1
/Cetuximab Immunoglobulin G1 Head, neck, and colorectal
cancer
Herceptin
1
/Trastuzumab Immunoglobulin G1 Breast cancer
Humira
1
/Adalimumab Immunoglobulin G1 Rheumatoid arthritis
Panorex
1
Immunoglobulin G2a Colorectal cancer
Raptiva
1
/Efalizumab Immunoglobulin G1 Psoriasis
Remicade
1
/Infliximab Immunoglobulin G1 Rheumatoid arthritis,
Crohn’s disease, among
others
ReoPro
1
/Abciximab Immunoglobulin G
(fragment)
Ischemic cardiac
complications
Rituxan
1
/MabThera Immunoglobulin G1 NonHodgkin’s lymphoma,
rheumatoid arthritis
Simulect
1
/Basiliximab Immunoglobulin G1 Prophylaxis of organ
rejection in renal
transplantation
Synagis
1
/Palivizumab Immunoglobulin G1 Prevention of respiratory
tract diseases
Xolair
1
/Omalizumab Immunoglobulin G1 Asthma
Zenapax
1
/Daclizumab Immunoglobulin G1 Prophylaxis of organ
rejection in renal
transplantation
Others GenHevac B Pasteur
1
HBsAg Hepatitis
Data from: European Medicines Agency (2006); Food and Drug Administration (2006); Castilho & Medronho
(2002).
396 Animal Cell Technology

Cell line Manufacturing
process data
First
approval
date
Company Countries where
approved
CHO – 2003 Biogen USA
CHO – 1998 Immunex/Wyeth Europe, USA
Hybrid-
oma
– 1989 Centocor Europe, USA
Hybrid-
oma
Hollow-fiber bioreactor 1996 Cytogen USA
CHO – 2002 Idec Europe, USA
CHO – 2004 Genentech/Roche Europe, USA
Hybrid-
oma
– 2003 Corixa/GlaxoSmithkline USA
CHO Suspension bioreactor 2001 ILEX/Genzyme Europe, USA
SP2/0 10 000L batch
bioreactor
2004 ImClone/Merck Europe, USA
CHO 12 000 L batch
bioreactor
1998 Genentech/Roche Europe, USA
CHO Batch bioreactor 2002 Abbott Laboratories Europe, USA
– – 1995 Glaxo Germany
CHO – 2003 Genentech/Serono Europe, USA
SP2/0 Perfusion bioreactor 1998 Centocor Europe, USA
SP2/0 Perfusion bioreactor 1997 Centocor USA
CHO Suspension bioreactor 1997 Idec/Genentech/Roche Europe, USA
Murine
myeloma
– 1998 Novartis Europe, USA
NS0 Fed-batch bioreactor 1998 MedImmune/Abbott Europe, USA
CHO Suspension bioreactor 2003 Genentech/Novartis Europe, USA
NS0 Suspension bioreactor 1997 Hoffmann-La Roche Europe, USA
CHO – 1989 Pasteur-Me
´
rieux France
Recombinant therapeutic proteins 397

(Alteplase and Tenecteplase
TM
) are used in the treatment of acute myocar-
dial infarction.
Urokinase is also a thrombolytic agent, used for treating pulmonary
embolism. Two variants of this protease have already been isolated: one of
54 kDa and another of 33 kDa, both displaying proteolytic activity over
plasminogen. Until recently, the only exogenous source for this enzyme
was urine. However, in 2002 the product called Abbokinase
1
, which is
produced in neonatal kidney tissue culture, was approved in the USA.
Gaucher’s disease is a disorder of genetic origin, characterized by the
absence of a vital enzyme called glucocerebrosidase. In this case the
glucocerebroside, the lipid component of cell membranes, accumulates in
the organism, leading to an exaggerated enlargement of the liver and other
organs. Cerezyme
1
, produced by the company Genzyme, is the recombi-
nant version of glucocerebrosidase, expressed in CHO cells (Table 16.1).
Another genetic metabolic disorder is Fabry disease, which is related to
a deficiency in the enzyme Æ-galactosidase A. This enzyme is involved in
the metabolism of lipids, and its deficiency leads to the accumulation of
lipids in the eyes, kidneys, and nervous and cardiovascular systems. In
2001, the biopharmaceutical called Fabrazyme
1
was approved, which
consists of recombinant Æ-galactosidase A and is produced by genetically
modified CHO cells.
16.2.6 Blood coagulation factors
There are 13 factors involved in the blood coagulation process. Some of
them, such as factors VII, VIII, and IX, are proteins. The absence of these
proteins constitutes a genetic disease known as hemophilia. A hemophilic
person is constantly at risk of undergoing a hemorrhage because of the
incapacity to correctly carry out coagulation. Until the development of
recombinant blood coagulation factors in the 1990s, hemophiliacs were
treated by blood transfusion and with proteins obtained from the plasma
of human donors. However, both solutions presented high risk of trans-
mission of pathogenic agents, especially viruses. This is why a significant
number of persons affected with hemophilia were contaminated with HIV
and hepatitis viruses in the 1980s. Since then, although an additional virus
inactivation step has been added to the manufacturing of plasma-derived
molecules to increase product safety, there is still the risk of transmitting
unknown viruses and pathogens of other types. Apart from that, limit-
ations related to the availability of raw material (plasma from donors) also
pose problems for plasma-derived coagulation factors. For these reasons,
an increasing demand for recombinant coagulation factors has occurred in
recent years and, in some countries such as Canada and Ireland, the
exclusive use of recombinant factors is obligatory.
Blood factor VIII (FVIII) is a glycoprotein with 2351 amino acids and
330 kDa. Its deficiency causes hemophilia A. The first products based on
recombinant factor VIII to reach the market were Recombinate and
Kogenate
1
, expressed in CHO and BHK cells, respectively. Over the last
decade, other rFVIII products were approved, with modifications to the
molecule (e.g. deletion of the B-domain), in the formulation or in the
production processes.
398 Animal Cell Technology

In the production process of Kogenate
1
, BHK cells are cultivated in
perfusion bioreactors up to 500 L in size. The first step of purification
involves anion exchange chromatography. After that, an immunoaffinity
chromatographic step and gel filtration are carried out, followed by
another immunoaffinity and another anion exchange chromatography step
(Boedeker, 1992; Bhattacharyya et al., 2003).
Recombinate is produced in CHO cells cultivated in 2500 L bioreactors,
operated in fed-batch mode. The purification process starts by removing
cells by a filtration step, followed by three chromatographic steps: im-
munoaffinity, anion exchange, and cation exchange (Bhattacharyya et al.,
2003).
When hemophilia is due to a deficiency in factor IX (FIX), it is
designated hemophilia B. In the same way as hemophilia A, the alternative
to treatment based on blood transfusion or plasma-derived products is the
use of recombinant FIX, which was approved for commercialization in
1997 with the name of BeneFix
1
.
It is produced in recombinant CHO cells cultured in a medium free of
animal-derived components. The BeneFix
1
production process involves
an ultrafiltration/diafiltration step, followed by four chromatographic
steps: ion exchange (Q resin), pseudo-affinity (Cellufine sulfate resin),
hydroxyapatite, and affinity (immobilized Cu
2þ
ions). After these chro-
matographic steps, there are membrane processes (nanofiltration for viral
clearance and diafiltration for solvent exchange), after which the purified
protein is formulated (Edwards and Kirby, 1999).
Some hemophilia A and B patients develop antibodies against FVIII and
FIX, respectively. This complicates the direct administration of these
proteins. An alternative in these cases is treatment with active factor VII
(FVIIa), which complexes with factor III in the presence of phospholipids
and Ca
2þ
, activating factor X, which, in normal patients, is activated by
active factors VIII and IX. The commercial name of recombinant FVIIa
expressed in BHK cells and produced by Novo-Nordisk is NovoSeven
1
(Table 16.1).
16.2.7 Antibodies
Therapeutic antibodies constitute the most studied and commercialized
class of biopharmaceuticals nowadays. Their applications range from in
vitro and in vivo diagnostics, to the treatment of cancer, cardiovascular
diseases, asthma, and infectious and autoimmune diseases.
In the case of tumor treatment, in most cases, the mechanism of action
of a monoclonal antibody (mAb) is based on the specific recognition and
binding to specific surface receptors that are found on the tumor cells.
However, in 2004, the antibody Avastin
1
(Bevacizumab), which acts as an
inhibitor of the vascular endothelial growth factor (VEGF), was approved
for the treatment of metastatic colorectal cancer. In this way, Avastin
1
inhibits angiogenesis around tumors, avoiding the abnormal formation of
blood vessels that support tumor expansion. Additionally, mAbs can be
conjugated to drugs, to direct these molecules to the target tumor cells,
decreasing any adverse effects on normal cells.
Recombinant therapeutic proteins 399

In the case of cardiovascular diseases, clot formation in the vascular
system not only interrupts blood flow but also leads to embolism with
fatal consequences. Antibodies against fibrin are able to detect these clots
in their initial stage of formation.
In viral infectious diseases, antibodies are used to bind to viral surface
antigens, inhibiting disease propagation. Among the viruses most widely
studied as potential antibody targets are the hepatitis B and HIV viruses.
One of the worst consequences of a bacterial infection is sepsis. Because
of the release of bacterial endotoxins (lipopolysaccharides), the immune
system acts to produce cytokines, which are only efficient for localized
infections. However, in the case of septicemia, the excessive release of
cytokines leads to dilatation of blood vessels, causing a drop in blood
pressure and insufficient tissue irrigation. Antibodies against endotoxins
can be used to minimize this condition.
Another application of antibodies is to treat autoimmune diseases,
which occur due to a deficiency of the immune system that recognizes an
endogenous molecule as a foreign element. The most common immu-
notherapeutic approach in these cases is that the antibody is directed
against the antigen on the surface of the lymphocytes responsible for the
immune response.
Finally, some antibodies are used in prophylaxis to prevent the rejection
of transplanted organs, such as kidney, liver, and heart. An example is
Zenapax
1
, which was approved for use in 1997.
Some examples of therapeutic mAbs are presented in Table 16.1. How-
ever, because of the clinical and commercial importance of this class of
therapeutic proteins, an entire chapter is dedicated to monoclonal anti-
bodies (Chapter 17).
16.3 Economic aspects
The biopharmaceutical global market grew from 2000 to 2004 at an annual
rate of 19%, and was evaluated at US$ 48 billion in 2005. This represents a
much higher growth rate than that experienced by the pharmaceutical
industry as a whole. The forecast is that the biopharmaceutical market will
reach US$ 100 billion by 2010 (Research and Markets, 2005a).
Erythropoietin (Epoetin, EPO) had a global market of US$ 11.1 billion
in 2004 (Research and Markets, 2005b) and was the top-selling biopharma-
ceutical worldwide. The patent on this biopharmaceutical, owned by the
company Amgen, expired in 2004, paving the way for new companies to
enter the market.
The colony stimulating factors (G-CSF, GM-CSF, and M-CSF) are also
commercially relevant. The annual sales growth rate of these proteins in
the period 2000–2004 was 16% and in 2004 the sales totalled US$ 3.6
billion (Research and Markets, 2005c).
However, the most promising class of biopharmaceuticals consists of
the therapeutic mAbs. In 2004, total sales of therapeutic mAbs reached
approximately US$ 11.2 billion, with an impressive annual growth rate of
42% in the period 2000–2004 (Research and Markets, 2005d). The forecast
is that in 2010 this market will reach US$ 34 billion.
400 Animal Cell Technology
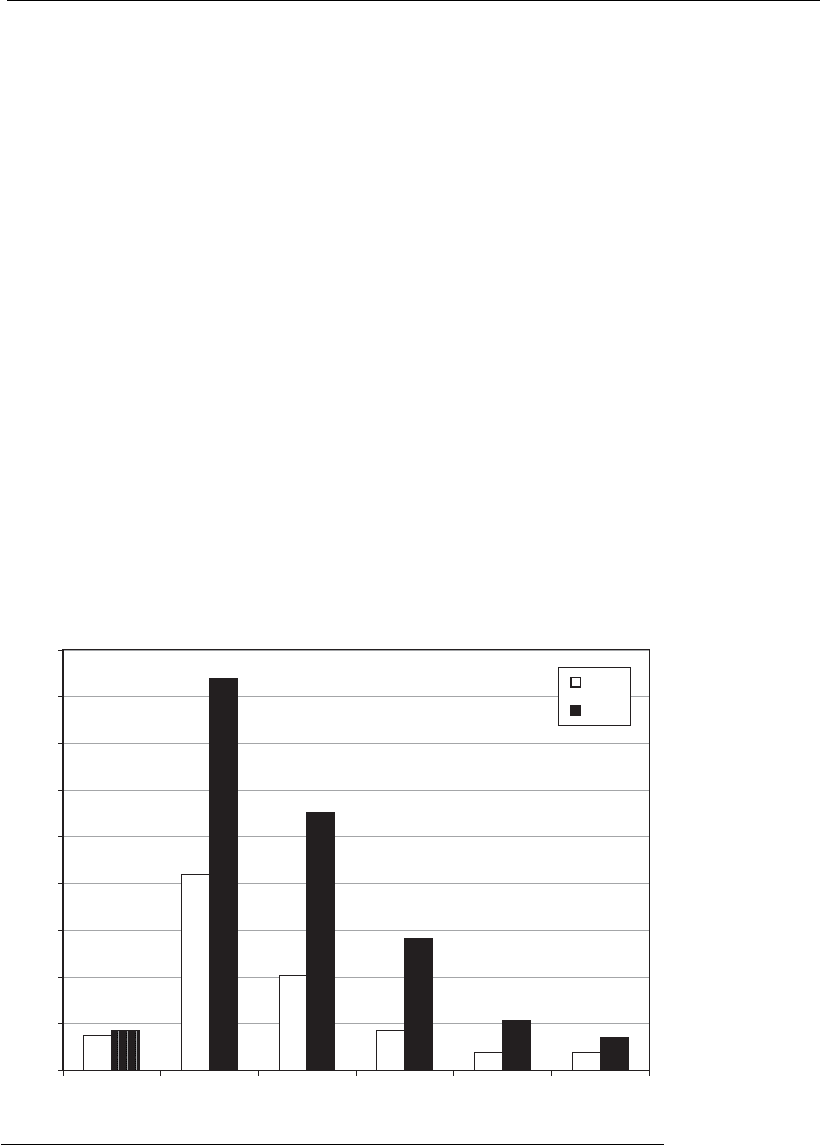
According to Reichert and Pavlou (2004), 17 therapeutic mAbs, grouped
into 4 different types, had been approved by the FDA at that time: 3
murine, 5 chimeric, 8 humanized, and 1 human. Among them, Remicade
1
/
Infliximab was the top-selling one (US$ 1.6 billion in 2002), representing
30.5% of mAb sales in that year.
It is forecast that up to the end of the current decade, the research focus
regarding therapeutic mAbs will be on two categories: oncology and
arthritis, and inflammatory and immune disorders. A significant increase
of sales is expected up to 2008 for chimeric mAbs, in absolute terms, and
for human and humanized mAbs, in relative terms (Figure 16.2). Up to
now, murine mAbs are those with the lowest approval rate (4.5%),
whereas the chimeric are the most successful (26%). The approval rates for
humanized mAbs (18%) and human antibodies (14%) were intermediate.
However, as most of the last two types are still under development, the
approval situation may change significantly in the future (Reichert and
Pavlou, 2004).
This rapidly growing market is very competitive. Companies in this area
must be highly innovative to be successful. There are key determinant
factors that should be optimized for competitive innovation: (a) product
development should be shortened by accelerating clinical trials; (b) well-
protected intellectual property should be built and maintained; (c) the
efficacy of products should be increased, for example, through new
formulations, conjugation, or pegylation; (d) drug delivery should be
Fragment
Conjugated
HumanHumanized
Chimeric
0
1
2
3
4
5
6
7
8
9
Murine
Sales (US$ billions)
2002
2008
Figure 16.2
Comparison of sales of therapeutic monoclonal antibodies (mAbs) in 2002 (in
white) and forecast for 2008 (in black) (adapted from Reichert and Pavlou, 2 004).
Recombinant therapeutic proteins 401
improved; and (e) cost-efficient manufacturing capabilities should be
developed (Pavlou and Belsey, 2005).
Cost-effective technologies have been gaining importance in the last few
years due to the expiration of patents of the first-generation biopharma-
ceuticals (Table 16.2). In the past, the concept of ‘‘time-to-market’’
dominated the industrial arena. Nowadays, companies are willing to focus
on process optimization and cost reduction. Important tools for this
purpose are the use of advanced genetic manipulation techniques to
increase the cell specific productivity, as well as the development of
perfusion processes, to increase volumetric productivity, and more
efficient purification processes, to improve yield (see Chapters 3, 9, 11
and 12).
16.4 Challenges and future perspectives
16.4.1 Formulation and delivery of biopharmaceuticals
Despite the significant development in the biopharmaceutical area in
recent years, many challenges have yet to be overcome. Among the main
obstacles faced by companies are the development of improved formula-
tions and new routes of administration, as well as the delivery of the active
molecules to the site of therapeutic action. Many biopharmaceuticals
present limitations of low stability in vitro (shelf-life) or in vivo (half-life
after injection in the patient), and low solubility and bioavailability
(Muller and Keck, 2004).
Inhalation/nebulization is an alternative method of administration of
some biopharmaceuticals, for example insulin and GM-CSF (Anderson
Table 16.2 Examples of biopharmaceuticals that have lost patent protection
Company Commercial name Protein Year of patent
expiration
Produced in animal cells
Biogen Avonex
1
Interferon -1a 2003
Amgen/J&J/Sankyo Epogen
1
/Procrit Epoetin Æ (erythropoietin) 2004
Genentech Activase
1
Alteplase (tPA) 2005
Genetics Institute Recombinate Factor VIII 2005
Genetics Institute/Wyeth Benefix
1
Factor IX 2005
Eli Lilly Xigris
1
Drotrecogin Æ (activated protein C) 2005
Produced in microorganisms
Schering-Plough Intron A
1
Interferon Æ-2a 2002
Novo Nordisk Novolin
1
Insulin 2002
Eli Lilly Humulin
1
Insulin 2002
Eli Lilly Humatrope
1
Human growth factor 2003
Genentech Nutropin
1
Human growth factor 2003
Genentech Protropin
1
Human growth factor 2004
Amgen Neupogen
1
Filgrastim (G-CSF) 2006
Adapted from Dudzinski (2004).
402 Animal Cell Technology

et al., 1999). The first biopharmaceutical administered by inhalation to be
commercialized is Exubera
1
, a powder insulin, which was approved by
FDA and EMEA in 2006 (European Medicines Agency, 2006; Food and
Drug Administration, 2006). This is the first alternative route of adminis-
tration since the discovery of insulin in the 1920s.
Another possible form of administration that is under study is through
the use of nanoparticles with diameters in the range of 200–400 nm,
obtained through the formation of nanocrystals or by creating nanoscale
structures that capture the biomolecules. Depending on the materials
employed and the preparation method, distinct particles can be used:
nanoparticles, liposomes, polymeric micelles, ceramic nanoparticles, and
dendrimers.
Nanocrystals can increase in vitro stability by transforming soluble
molecules into non-soluble forms. In this way, only the nanocrystal
surface is accessible to degrading factors, such as water and oxygen. This
means that an external monolayer of degraded molecules is formed to
protect the inner part of the nanocrystal. Soluble molecules, such as
peptides, nucleotides, and proteins can be transformed into particles by
dispersing them into oil. After oral administration, the oil is degraded by
lipases in vivo , releasing the drug. Alternatively, soluble molecules can be
transformed into non-soluble molecules by forming lipid conjugates
(LDC
1
). Conjugated particles can be prepared by either salt formation
(e.g. amino group containing molecule with fatty acid) or covalent bond
(e.g. ether, ester) (Muller and Keck, 2004).
Another challenge is the delivery of a biopharmaceutical to its site of
action, as the injection of molecules in solution leads to a partitioning of
the molecules according to their physicochemical properties. One ap-
proach to deliver particles injected intravenously is based on the concept
of ‘‘differential protein adsorption.’’ After injection the particles adsorb
blood proteins according to physicochemical surface properties of the
particles. The adsorbed proteins determine the cells to which the particles
will be directed (Muller and Keck, 2004).
A popular approach to confer enhanced stability and improve the
pharmacokinetics of therapeutic proteins is to conjugate them to differ-
ent polymers. Nowadays polyethylene glycol (PEG) is the most widely
employed polymer due to its low toxicity and cost. This technique,
known as pegylation, is able to increase protein stability, improve
pharmacokinetics, and potentially decrease immunogenicity (Chirino
and Mire-Sluis, 2004). Since the entry of the first pegylated protein in
the market in 1990, several products have been approved, such as
Neulasta
1
(pegylated G-CSF for the treatment of neutropenia induced
by chemotherapy) and PEG-Intron
TM
/Pegasys (IFNÆ for the treatment
of chronic hepatitis C). These products have enhanced stability, thus
reducing the number of injections patients need to receive. However,
the companies owning the patents commercialize pegylated biopharma-
ceuticals at prices much higher than the corresponding nonpegylated
ones, preventing a broader use of the pegylated version, mainly in
developing countries. The prices charged certainly do not represent the
additional cost of polymer conjugation, but rather a market value that
reflects a monopoly.
Recombinant therapeutic proteins 403
