Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

exceptions. For example, in Brazil living things (or parts thereof) are not
patentable, except transgenic microorganisms (Art. 18, III). The majority
of countries, however, follow the European example, where only varieties
of plants and animals are not eligible for patent protection.
In Brazil, for instance, the recombinant form of molecules such as IFN-
alpha and human erythropoietin can be patented, as well as the processes
for obtaining them using animal cells. However, the natural forms of these
molecules, as well as the animal cells used to obtain these molecules, are
not patentable.
15.6 Industrial property and technology transfer offices
The search by the business sector for new sources of technology and the
avidity of universities for new sources of funding have started to give a
new face to some academic environments. Academic–business relations
take many forms: investment of companies in complete projects or parts
of projects, licensing for exploitation of patents, among others.
In whatever form, a company will be much more willing to fund
research if there are guarantees that the technology will not wind up in the
public domain, and will instead receive adequate patent protection. In the
United States, where these movements began to gather steam in the 1970s,
universities had to prepare themselves to deal with these new questions.
On the other hand, the US Government found that the research financed
with public funds often wound up on the ‘‘shelves.’’ This prompted the
need for legislation, which culminated in 1980 with passage of the Bayh-
Dole Act. This act authorizes universities and small companies to retain
the patent rights that result from research funded by the federal govern-
ment.
As an indication of the effect of the Bayh-Dole Act, before 1980 fewer
than 250 patents a year were issued to American universities. In 2004, a
survey conducted by the Association of University Technology Managers
indicated that 4458 new licensing agreements were signed that year, and
the number of patent applications filed by universities was 13 021. Be-
tween 1991 and 2004, the survey reported an increase of over 100% in
patent filings, as well as a 142% jump in the number of licensing and
option agreements signed. These option agreements permit a company to
acquire the right to evaluate a technology for a given period, called the
option period, before deciding whether or not to actually license it.
The survey also found, however, that universities and research institu-
tions need to be patient and should not expect millionaire returns in short
time-frames, since the generation of revenues from licensing is not im-
mediate. Fewer than 1% of the 20 968 licenses active in 2000 generated a
million dollars or more. Of the institutions responding to the survey, only
10% reported profit greater than 20 million dollars from licensing, and
80% of these licenses had already been in force for at least 10 years.
In academic environments, the establishment of industrial property and
technology transfer offices has arisen from the increased activities to
protect inventions, the need to transfer technologies to enable their
production, and from legal requirements for obtaining patents, all driven
384 Animal Cell Technology

by the search for profits from research and development investments
through commercialization of the technologies patented.
In Brazil, researchers have started to wake up to the need to patent their
inventions, albeit tardily and slowly. The number of patent applications
filed with the INPI (Brazilian PTO), even though small compared with
the number of journal publications and conference proceedings, is increas-
ing every year. Of the 22 000 applications filed in 2001, 6200 were by
Brazilians. In 2000, Brazilians filed 5800 of the 20 000 applications with
the INPI.
Besides seeking assistance from a qualified professional, researchers
from some institutions in the country can call on specialized units at their
institutions to support and guide them. Among the institutions that have
established such units are Embrapa, Fiocruz, Unicamp (Campinas/SP),
University of Sa
˜
o Paulo, Minas Gerais Federal University, Rio de Janeiro
Federal University, and Rio Grande do Sul Federal University, among
others. The Sa
˜
o Paulo State Research Support Foundation (Fapesp) main-
tains a Patent and Licensing Unit (Nuplitec) to guide researchers whose
projects are financed by Fapesp.
In 2004, Law 10,973 was enacted, called the Law of Innovation, which
among other provisions, requires public-funded scientific and technology
institutions to have an innovation unit to encourage patenting and licen-
sing of technology. This is an important instrument to protect the
intellectual property generated at these institutions, as well as the negotia-
tion for licensing and transfer to the market. Before negotiating with
prospective partners, it is necessary to sign a confidentiality agreement
between the parties to preserve the secrecy of the information exchanged
during the negotiating phase. All this guidance and support is the respon-
sibility of the internal intellectual property and technology transfer office.
According to Chamas (2001), although the debate and interest in
intellectual property rights have arrived tardily in Brazil, the legal barriers
are gradually being removed and the climate is becoming more receptive
to the new activities. In this sense, the move by Brazilian universities is
evident to protect and exploit their intellectual property. A portfolio of
patents and similar assets should be the object of a competent business
policy, including frequent analysis of the advisability of commercializing
scientific ideas. The construction of an ample portfolio of intellectual
property rights is justified to protect and reward scientific effort and allow
a period of protected commercialization. Nevertheless, in the case of the
public sector, this may not be the only motivation, since concern must be
given to the country’s social welfare and economic development.
Mention must also be made of the crucial role of support agencies for
the structuring and consolidation of these internal offices.
An article by Kingston (2000) reports the history of the ‘‘antibiotics
revolution’’ and how the discovery of Pasteur in 1877 that certain bacteria
were able to inhibit the growth of anthrax organisms supplies important
lessons for economic innovation. According to the article, questions such
as the establishment of strategies for patenting and ownership of intellec-
tual property, cooperation between universities and industries, and the
role of support agencies must be considered when seeking successful
innovation of a product/process.
Intellectual property 385

The distinction between the profile of an inventor and innovator is quite
clear from the report involving the invention and innovation of penicillin
and streptomycin. According to Schumpeter (1988), an inventor produces
ideas, while an innovator makes things happen, and materializes ideas.
Personal commitment and willpower are characteristics of an innovator.
This is the difference between Fleming and Florey in relation to the
discovery and innovation of penicillin, respectively. The researcher/inven-
tor has to deal with resistance to new ideas without the ability to make
these new ideas accepted. In contrast, an innovator has the ability neces-
sary to promote ideas and transform them into reality.
Researchers need to be capable of perceiving that an experiment may
generate more information than it is possible to foresee before starting.
On this point, Kingston (2000) shows the misleading effect that a partial
success can have, which he exemplifies by the fact that Fleming was only
able to perceive a limited use of his discovery of penicillin, allowing this
partial success to mask the possibility of its use as an antibiotic.
This is where the innovator comes in, capable of noting this previously
unidentified potential, and especially knowing how to use his or her
personal characteristics to find the means to overcome difficulties and
convince others to invest in developing and materializing the idea.
15.7 Patent and technology transfer specialists
An internal intellectual property and technology transfer office will have
two types of professionals: the industrial property specialist and the
technology transfer specialist. These activities require specific qualifica-
tions and extensive training, involving expertise normally not mastered by
scientists. For this reason, the interactions between universities and re-
search institutions on the one hand and companies on the other regarding
patenting and marketing of inventions warrant specific handling.
The intellectual property specialist, usually with a professional back-
ground in engineering, chemistry, physics, or biomedical sciences, needs
to have an understanding of international law, treaties, and conventions as
well as national laws, decrees, edicts, etc., and the entire bureaucratic
apparatus in the field. He or she must also interact with scientists to obtain
the information necessary to draft the patent application, study the state of
the art to prepare the diagrams, determine the scope of claims, see to the
deposit of biological material with an international depositary authority,
develop patenting strategies, deal with industrial property agents, follow
the progress of the application, see to maintenance of patents already
granted, monitor the patents and applications of competitors, contest
negative opinions from patent offices, and respond to the various technical
and legal demands involving the whole process, including any violation of
intellectual property rights (Chamas and Mu
¨
ller, 1998).
Regarding monitoring patent applications of third parties, Brazilian
legislation allows any interested party to submit documents and informa-
tion during an application process period, to assist the technical examina-
tion. This is provided in Article 31 of the Industrial Property Law, and can
be done up to the final examination. The tools for this are the Industrial
386 Animal Cell Technology

Property Review and the INPI site. Once a patent has been granted, no
appeal is possible, but administrative cancellation can be sought within 6
months. Court action to nullify a patent can be taken at any time during
its lifetime.
Technology transfer specialists, in contrast, are more focused on busi-
ness aspects. They need to be able to work closely with and understand
the work of the industrial property people, to help them check on the
patent applications filed by the institution. But they also, and more
importantly, must follow market trends involving the portfolio of patents
and applications, orient the preparation of technical cooperation, detect
and contact potential partners for future technology transfers, negotiate
and draft contractual instruments applicable to each case, monitor the
partnerships formed, deal with external law offices specializing in intellec-
tual property, and act in cases of breach of contractual clauses. In relation
to this last item, when a contractual infraction or other compliance
problem is noted, a choice must be made as to what action to take. Will it
be by judicial action or some form of alternative dispute resolution?
There is a range of alternative dispute resolution (ADR) methods.
Among the most common are arbitration and mediation, although con-
ciliation and direct negotiation are also possible.
Dispute resolution is an important process for business, since it permits
a quick, fair, and economic way to solve conflicts, without the need for
prolonged litigation. Hence, it is possible to obtain a more satisfactory
outcome and often to preserve a suitable commercial relationship.
Some activities need to be carried out jointly by the industrial property
and technology transfer specialists. An example is the decision on whether
or not an invention is patentable, or should be patented, based on technical
and economic considerations, and industrial property policies.
Some aspects of intellectual property that need to be evaluated by these
specialists when analyzing a research project are listed below (Mu
¨
ller,
2003).
(i) Searching for prior art and verifying the satisfaction of patentability
requirements.
(ii) Confirming with inventors the absence of disclosure of the result of
the research, even in articles submitted for publication.
(iii) If the patentability requisites are met, preparing the first draft of the
application from the technical information sent by the inventors.
(iv) Whenever possible, including functional equivalents of the invention
to enlarge the scope of the protection.
(v) In parallel, confirming who the inventors are and to what companies/
institutions they belong.
(vi) In the case of external inventors, verifying if there is a formal partner-
ship agreement.
(vii) If so, verifying whether the agreement contains ownership clauses.
(viii) Identifying the percentage of intellectual contribution of each inven-
tor in materializing the invention, information that will influence the
percentage that will accrue to each company/institution.
(ix) Verifying whether there were or will be other external sources of
Intellectual property 387
funding for the project besides the outlay of the company/institution
itself.
(x) Whenever possible, negotiating the ownership of the company/insti-
tution and proposing an agreement between the parties stipulating
the percentage that belongs to the other party or parties as a result of
commercial exploitation of the invention.
15.8 Conclusions
Based on the above observations, the importance of patent protection of
biotechnology inventions is clear, as well as the need for professionals
trained to take care of the protection and licensing of the intellectual
property generated by universities, research institutions, and companies.
Public–private partnerships should be encouraged as essential for the
development of products and processes, and especially to leverage invest-
ment seeking to reduce the dependence on imported biopharmaceuticals.
For this, it is vital that institutions adequately project their research results,
to enable them to better attract investors to fund technological develop-
ment. Hence, it will be possible to increase the chances that an invention
will turn into an innovation.
References
Association of University Technology Managers (1999), AUTM Licensing Survey FY
1996. Norwalk: AUTM.
Beier FK, Straus J (1986), Genetic engineering and industrial property, In: The
Sixteenth Bitburg Colloquium on Genetic Engineering and Law, Bitburg,
Abstracts.
Bull AT, Holt G, Lilly MD (1982), Biotechnology: International Trends and Perspec-
tives, OECD, Paris.
Chamas CI (2001), Protec¸a
˜
o e Explorac¸a
˜
o Econoˆmica da Propriedade Intelectual em
Universidades e Instituic¸o
˜
es de Pesquisa, COPPE/UFRJ, PhD Thesis.
Chamas CI, Mu
¨
ller ACA (1998), Gereˆncia da Propriedade Industrial e da Transfer-
eˆncia de Tecnologia, In: XX Simpo´ sio de Gesta
˜
o da Inovac¸a
˜
o Tecnolo´ gica, Sa
˜
o
Paulo, Abstracts.
Gama Cerqueira JDA (1982), Tratado da Propriedade Industrial, 2nd Ed., Editora
Revista dos Tribunais, Rio de Janeiro.
Kingston W (2000), Antibiotics, invention and innovation, Research Policy 29:
679–710.
Mu
¨
ller ACA (2003), Patenteamento em Biotecnologia: Abrangeˆncia e Interpretac¸a
˜
ode
Reivindicac¸o
˜
es, EQ/UFRJ, PhD Thesis.
Schumpeter JA (1988), A teoria do desenvolvimento econoˆmico, Nova Cultural, Sa
˜
o
Paulo.
388 Animal Cell Technology

16
Recombinant therapeutic
pr oteins
Maria Candida Mai a Mellado and Leda dos Reis Castilho
16.1 Introduction
Biopharmaceuticals are proteins and nucleic acid-derived molecules that
are used for therapeutic purposes or in vivo diagnostics, and are produced
by means other than direct extraction from a native (non-engineered)
biological source (Walsh, 2002). When considered together with vaccines
and biomolecules extracted from natural sources, they are designated
‘‘biologicals’’ by the pharmaceutical industry.
A survey carried out by the Pharmaceutical Research and Manufacturers
of America (PhRMA) verified that in 2004 over 100 biopharmaceuticals
had already gained approval from the US Food and Drug Administration
(FDA) (PhRMA, 2004). This survey found that 324 new biotechnology
medicines, targeting nearly 150 diseases, were undergoing human clinical
trials or under review by the FDA. Among the products under develop-
ment, almost half (154) were intended for cancer treatment, and another
significant portion for infectious diseases, AIDS/HIV and related condi-
tions, and autoimmune and neurological disorders. Despite the long
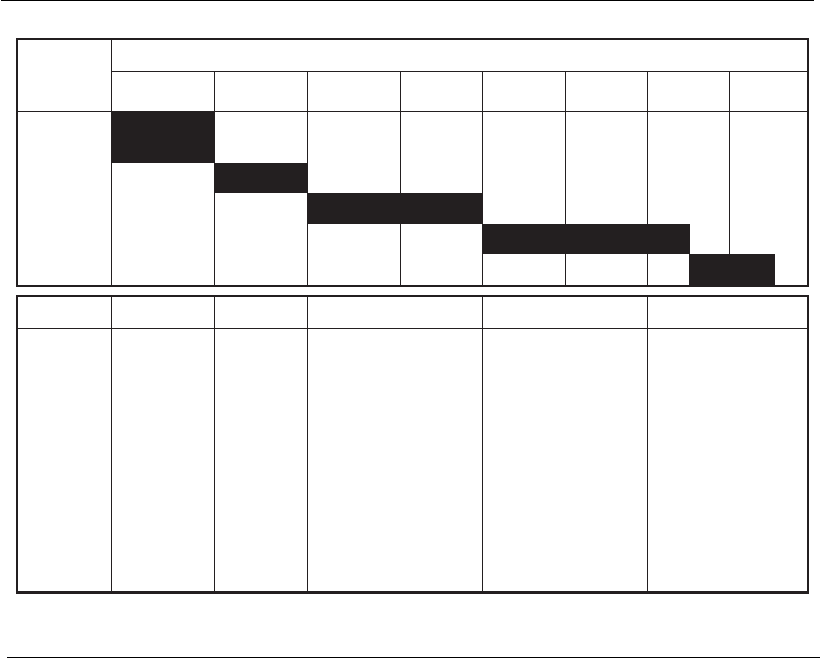
development time for biopharmaceuticals (Figure 16.1) and the fact that
most molecules under clinical trials do not gain final approval from the
regulatory agencies, a considerable increase in the number of commercia-
lized biopharmaceuticals is expected in the next few years.
It is estimated that to date more than 250 million people worldwide
have benefited from the biologicals already approved for the treatment or
prophylaxis of cardiac diseases, multiple sclerosis, several types of cancer,
hepatitis, arthritis, and diabetes, among others. Since biopharmaceuticals
focus their action on the molecular basis of diseases, they are providing
physicians and patients with new powerful tools for the treatment of
diseases, changing fundamentally the way diseases are treated (PhRMA,
2004).
16.2 Main therapeutic proteins
Therapeutic proteins can be divided into seven different groups: cytokines,
hematopoietic growth factors, growth factors, hormones, blood products,
enzymes, and antibodies (Walsh, 2003). Most of these proteins have
complex structures and undergo different post-translational modifications,
such as glycosylation, which usually is essential for biological activity, as
discussed in Chapter 6. For this reason, most of the biopharmaceuticals
already approved or under development are produced by mammalian cells,
since microorganisms and insect cells are not able to correctly perform the
required post-translational modifications. Among mammalian cells, the
cell line most usually employed in the production of recombinant ther-
apeutic proteins is the CHO cell line (Chinese hamster ovary cells), which
provides proteins with a glycosylation pattern very similar to that of
human native proteins. Besides, CHO cells are considered safe because the
major viruses that cause disease in humans are not able to replicate in these
cells. The manufacturing processes for these proteins are all based on
principles discussed in the previous chapters of this book.
16.2.1 Cytokines
Cytokines play a central role in regulating the immune and inflammatory
response. Interferons (IFNs) were the first family of cytokines to be
CommercialPhase IIIPhase IIPhase I
IND
Duration (years)
Develop-
ment
phase
123
4
5
6
7
8
Preclinical
Phase I
Phase II
Phase III
BLA
Phase
Premises
Expression
system
defined
Process
scalability
Primary
definition
of process
No
validation
Refinement of
operational control
parameters
Development of
scale-down process
models for validation
Process out-of-limit
definition
Finalization of
process control
parameters
Fixed and defined
process and
products
Pivotal process
validation and
characterization
studies
Validated
production
process
Well-characterized
product
Robust process
Figure 16.1
Phases in the development of a biopharmaceutical and the concept of time-to-market. IND,
Investigational New Drug Application; BL A, B iologics License Application
(adapted from Werner, 2004).
390 Animal Cell Technology

discovered, and are used as biopharmaceuticals to enhance the immune
response against infectious agents (viruses, bacteria, and protozoa) and to
treat some autoimmune conditions and some types of cancer (Table 16.1).
There are distinct classes of IFNs, and humans produce at least three;
IFNÆ, IFN, and IFNª (Walsh, 2003).
The IFNÆ class consists of approximately 20 subtypes of mole-
cules, most of them having 165–172 amino acids and a molar mass of
approximately 20 kDa. Depending on the subtype, they can be either
O-glycosylated or non-glycosylated, and present over 70% amino acid
homology with each other. Beyond being abundant in leucine and gluta-
mate, they are characterized by conserved cysteines, usually at positions 1,
29, 99, and 139, which generally form two disulfide bonds. The secondary
structure is characterized by several Æ-helices and no -sheets. The major
application of IFNÆ as a biopharmaceutical is the treatment of hepatitis,
but some commercial preparations have already been approved for leuke-
mia and other types of cancer.
IFN is produced in vivo normally by fibroblasts. In humans, only one
IFN is found, which has 166 amino acids and a molar mass larger than 20
kDa. The molecule has a disulfide bond as well as an N-linked carbo-
hydrate chain bound to asparagine 80. Structurally, it is characterized by
the presence of five Æ-helices. Recombinant IFN is marketed under the
names of Betaferon
1
, Betaseron
1
, Avonex
1
, and Rebif
1
, being indicated
for the treatment of multiple sclerosis, since it blocks the secretion of other
cytokines involved in the pathogenesis of this disease.
IFNª, also known as immune interferon, is produced in vivo predomi-
nantly by lymphocytes. Human IFNª has 143 amino acids, with a molar
mass varying between 17 and 25 kDa, depending on the level of N-
glycosylation. The secondary structure consists of six Æ-helices. The most
important therapeutic application of IFN ª is the treatment of chronic
granulomatous disease (CGD), a rare genetic condition characterized by
the deficiency of phagocytic cells. CGD is characterized by repeated
infections in sufferers because of the absence of phagocytes to ingest or
destroy infectious agents (Walsh, 2003).
Until the 1970s, the source of exogenous IFN for therapeutic use was
human blood itself. However, recombinant DNA technology made possi-
ble the cloning of IFN genes into several expression systems, such as
Escherichia coli, yeast, and mammalian cells, facilitating the large-scale
production of these proteins and increasing safety. The level of glycosyla-
tion of these proteins determines the expression system in which they
should be expressed. IFNÆ, for example, is not glycosylated in its native
form and therefore can be expressed in E. coli. Mammalian cells, especially
CHO cells, may be used to produce the other IFN classes, which are
glycosylated.
Another cytokine class used as a biopharmaceutical is interleukin (IL),
which consists of at least 25 different subtypes (IL-1 to IL-25). Except for
IL-1, most interleukins are glycosylated and have a molar mass in the
range of 15–30 kDa. IL-2 is the most well studied interleukin, and its
recombinant form is approved for the treatment of renal cell carcinoma.
Since the absence of glycosylation does not affect its biological activity,
rIL-2 is produced in genetically engineered E. coli.
Recombinant therapeutic proteins 391

16.2.2 Hematopoietic growth factors
Hematopoietic growth factors are also cytokines and play an essential role
in the formation and differentiation of blood cells (hematopoiesis). The
main molecules of this family are erythropoietin (EPO), granulocyte
colony-stimulating factor (G-CSF), macrophage colony-stimulating factor
(M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-
CSF).
GM-CSF, also known as pluripoietin-Æ, is a glycosylated polypeptide
with 127 amino acids and a molar mass of approximately 22 kDa. The
secondary structure exhibits four Æ-helices and two anti-parallel -sheets.
G-CSF (or pluripoietin) is a glycoprotein with two splicing variants, the
predominant one presenting 174 amino acids and 21 kDa, with only one
O-glycosylation site. Its structure is characterized by the presence of two
disulfide bonds and four Æ-helices. M-CSF (or CSF-1) has three N-
glycosylation sites and several disulfide linkages. There are three forms of
this protein, with molar masses varying from 45 to 90 kDa and the largest
one having 522 amino acids (Walsh, 2003).
The three types of recombinant CSFs (GM-CSF, G-CSF, and M-CSF)
are used as hematopoietic stimulators, in the treatment of infectious
diseases, some types of cancer, bone marrow transplants, and neutropenia,
a disease characterized by a reduced level of neutrophils, the precursors of
white blood cells (Table 16.1).
EPO is a globular glycoprotein, characterized by four Æ-helices and two
anti-parallel -sheets, which contains 166 amino acids. It has a molar mass
between 34 and 40 kDa, 40% of which is due to carbohydrate chains. The
molecule has three N-glycosylation sites (asparagine residues 24, 38, and
83) and an O-glycosylation site (serine 126). The carbohydrate chains
contribute to the solubility, in vivo metabolism, and cellular processing of
the molecule. However, its complex glycosylated tetra-antennary structure
and the high degree of sialylation enhance the importance of a careful
control of operational conditions during the manufacturing process. This
aims at minimizing the micro- and macroheterogeneity and, consequently,
maximizing the yield in terms of isoforms with high biological activity
(see Chapter 6).
The first approval of a therapeutic use for recombinant EPO was in
1989 for the treatment of anemia related to chronic renal failure. The
treatment with EPO stimulates production of erythrocytes and improves
the patient’s quality of life, as well as reducing or eliminating the need for
blood transfusion. There are other non-renal applications, such as the
minimization of blood transfusion after surgery, the prevention of anemia
after bone marrow transplantation, and the treatment of anemia caused by
the use of antiretroviral drugs, by chemotherapy, and by prematurity.
16.2.3 Growth factors
Apart from the hematopoietic growth factors, discussed in Section 16.2.2
above, there are other types of growth factors, such as transforming
growth factor (TGF), platelet-derived growth factor (PDGF), insulin-like
growth factor (IGF), and epidermal growth factor (EGF).
392 Animal Cell Technology

TGF consists of a family of growth factors employed in the treatment
of bone fractures and skin ulcers. TGF molecules of type exist in the
form of homodimers of 112 amino acids. Two members of this family have
their recombinant form approved for treating tibia fractures (Osigraft
1
and InductOs
TM
), being produced in mammalian cells (Table 16.1).
PDGF, which plays an important role in wound healing (cicatrization),
is a dimer formed by two polypeptides. There are two isoforms of this
protein that are most commonly encountered in the human body: one
with 110 amino acids and another with 125. Both isoforms have one
glycosylation site and three disulfide bonds.
IGF is a polypeptide of 70 amino acids, with three disulfide bonds and a
molar mass of 7.6 kDa. Its therapeutic use is still under study and future
medical applications for this protein are likely to be the treatment of
dwarfism, diabetes type 2, and renal diseases, among others.
EGF has a sequence of 1208 amino acids divided into seven domains,
the largest being a glycosylated peptide of 53 amino acids. The secondary
structure is characterized by the presence of two anti-parallel -sheets and
three disulfide bonds, which confer stability to the molecule. This protein
is indicated for the treatment of wounds and skin ulcers.
16.2.4 Hormones
Hormones are proteins responsible for regulating molecular synthesis in
vivo. The most well known therapeutic hormones are insulin, glucagon,
growth hormones, and gonadotropins.
Recombinant human insulin and recombinant human growth hormone,
both produced by microbial cells, were the first biopharmaceuticals to
obtain approval from the regulatory agencies. Insulin is widely used for
diabetes treatment, whereas growth hormone is used in the treatment of
short stature, obesity, and for stimulating ovulation.
The gonatropins are a family of hormones that include follicle stimulat-
ing hormone (FSH), luteinizing hormone (LH), and chorionic gonadotro-
pin (CG), among others. These three proteins are heterodimers that
contain an identical polypeptide subunit (Æ) and another specific subunit
(), which confer the respective biological activity. FSH has a molar mass
of 34 kDa, 14% of it being due to carbohydrate side chains. LH has a
molar mass of 28.5 kDa.
The recombinant forms of these proteins (Gonal-f
1
, Luveris
1
,
Ovitrelle
1
, and Puregon
1
), produced in CHO cells, are indicated for
treating female infertility (Table 16.1).
16.2.5 Therapeutic enzymes
There is a great range of recombinant enzymes used for therapeutic
purposes, but not all of them are expressed in mammalian cells.
Tissue plasminogen activator (tPA) is a protease with 527 amino acids
and 4 glycosylation sites that acts in vivo as a thrombolytic agent. Its
function is to proteolytically convert the zymogen, plasminogen, into
active plasmin, which in turn degrades fibrin strands, thus dissolving
the clots (Walsh, 2003). For this reason, recombinant tPA molecules
Recombinant therapeutic proteins 393
