Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


aggregates to be active. After being activated, generally by initiator
caspases, the effector caspases are responsible for the death signal amplifi-
cation, for example, caspase 9 activates others caspases, like caspase 3,
which in turn cleaves and activates more caspase 9, thus amplifying the
apoptotic signal (Slee et al., 1999).
Effector caspases are activated by a transactivation mechanism, which is
characterized by the catalytic action of a mature caspase on a procaspase
(Thornberry et al., 1997; Earnshaw et al., 1999; Slee et al., 1999). Never-
theless, their activation can also occur by the action of other proteases.
Granzyme B, a serine-protease, also has proteolytic specificity for aspartic
acid residues. It is able to cleave and directly activate caspase 3 (Darmon et
al., 1995). Cathepsin B, a lysosomal protease, cleaves and activates procas-
pase 11 (Schotte et al., 1998).
Effector caspases also interact with other molecules besides caspases.
These caspases interact and cleave key regulatory and structural proteins
(Earnshaw et al., 1999) that can be directly inactivated, directly activated,
or can modulate the function of other proteins as a result of cleavage. The
main substrates directly inactivated are structural proteins, which lose
their function, like cytoskeleton proteins (actin, gelsolin, Æ-foldrin); com-
ponents of gap junctions (-cathenin, plakoglobin), and nuclear proteins
(lamin A and B); proteins involved in metabolism and DNA repair (DNA
topoisomerase II, PARP); signaling proteins, like transcriptional activators
(NFkB) and kinases (Akt, FAK); and antiapoptotic proteins (Bcl-2, Bcl-
X
L
). The cleavage of cytoskeleton and gap junction proteins results in cells
becoming spherical and detaching from the surface and from neighbor
cells. The cleavage of lamin A and B contributes to the break up of the
nucleus into vesicles. Examples of proteins activated after cleavage are the
caspases themselves, proapoptotic proteins, like Bid and Bax, and kinases
(PAK2, MEKK1). These two kinases, when activated, are capable of
activating the SAPK/JNK pathway, which increases the transcription of
proapoptotic genes under the control of the transcription factor c-Jun.
Caspases can also modulate the activity of specific proteins by inactivating
inhibitors of these proteins, such as DNAse CAD/DFF40, which is
constantly inactivated by its inhibitor ICAD. This inhibitor is a caspase 3
substrate, and CAD/DFF40 release results in chromosome cleavage at
internucleosomal spaces. These irreversible proteolytic events are respon-
sible for the morphological changes displayed by apoptotic cells.
Caspase activation occurs as a late and common step in all cells under-
going apoptosis. Nevertheless, there are many initial pathways that can
result in caspase activation. Probably, each distinct pathway is triggered
by different apoptotic stimuli. In mammalian cells, the apoptotic response
is usually mediated by the intrinsic and extrinsic pathways, depending on
the origin of the death signal. The intrinsic pathway can further be divided
into mitochondrial and ER stress pathways.
The Bcl-2 family and the intrinsic mitochondrial pathway
Besides its role as the energy-generating organelle, the mitochondrion has
recently emerged as the center of conversion of cellular life and death
signals. This organelle contains, in its intermembrane space, apoptogenic
162 Animal Cell Technology

factors, like cytochrome c, AIF (apoptosis-inducing factor), procaspases 2,
3, and 9, Smac/DIABLO (second mitochondrial activator of caspases/
direct inhibition of apoptosis protein IAP binding protein with low pI),
Omi/HtrA2, and endonuclease G (Gross et al., 1999). In the presence of
apoptotic signals, these factors are released into the cytoplasm and a few
of them participate in caspase activation. This apoptotic pathway centered
on the mitochondria is known as the intrinsic mitochondrial or mitochon-
dria-dependent caspase activation pathway.
Proteins of the Bcl-2 family are responsible for the maintenance or
release of these factors from the mitochondria into the cytoplasm. For this
reason, this family and the caspase family are considered as the main
regulators of the apoptosis process (Gross et al., 1999; Cory and Adams,
2002; Kuwana and Newmeyer, 2003). To date, at least 20 members of the
Bcl-2 family have been identified, which can be divided into two main
groups, depending on their function. The proapoptotic group contains
members (e.g. Bax, Bcl-X
S
, Bak, Bad, Bid, Bik) that induce the release of
apoptogenic factors from the mitochondria into the cytoplasm, resulting
in apoptosis. On the other hand, the antiapoptotic group contains proteins
(e.g. Bcl-2, Bcl-X
L
, Mcl-1, Bcl-w, Boo) that are responsible for the
maintenance of these factors inside the mitochondria, inhibiting the
apoptotic process (Adams and Cory, 1998).
Members of the Bcl-2 family share one or more Bcl-2 homology (BH)
domains, named BH1, BH2, BH3, and BH4 (Adams and Cory, 1998). It is
not yet clear which structural features determine if these proteins possess
pro- or anti-apoptotic activities. However, some studies revealed that the
BH3 domain is a critical domain for the proapoptotic members (Chitten-
den et al., 1995). Besides BH domains, some contain a hydrophobic
domain in the C-terminal region, which is essential for the attachment to
intracellular membranes, like the outer mitochondrial, nuclear, and endo-
plasmic reticulum membranes (Krajewski et al., 1993; Nguyen et al., 1993).
In the absence of a death signal, most of the pro- and anti-apoptotic
members are located in separate subcellular compartments. Anti-apoptotic
proteins are inserted in intracellular membranes, mainly the mitochondrial
membrane, while some proapoptotic members are located in the cyto-
plasm or cytoskeleton in an inactive form. They are activated and translo-
cated by apoptotic stimuli to their place of action to perform their
functions (Gross et al., 1999).
A remarkable feature of the Bcl-2 proteins is their ability to interact
with one another to form either homo- or hetero-dimers (Oltvai et al.,
1993). The formation of hetero-dimers between pro- and anti-apoptotic
members suggests a competitive neutralization of their activities. A
healthy cell maintains a balance between these groups of proteins and a
destabilization in their relative concentration determines, at least in part,
the decision for cell survival or cell death (Gross et al., 1999).
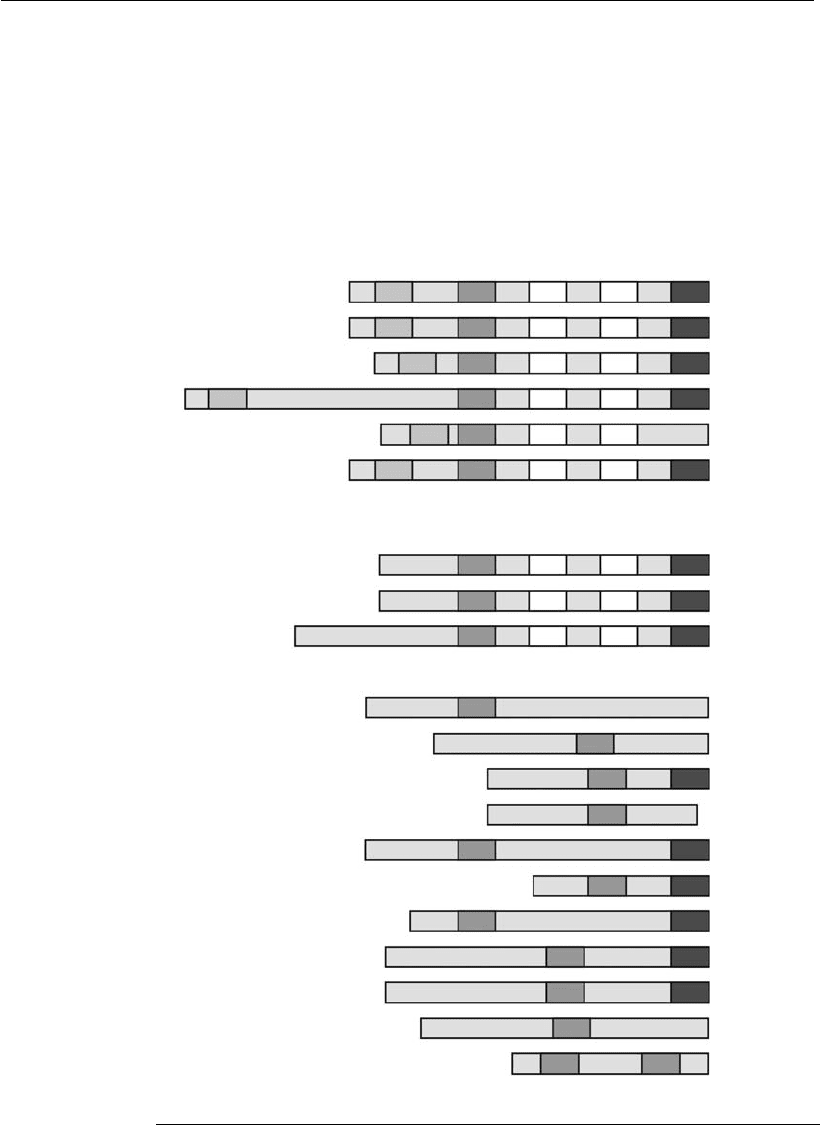
Proapoptotic proteins can be further classified according to their BH
domains (Figure 7.5). Some members, such as Bax and Bak, contain multi-
ple domains and others, like Bid, Bad, Bim, and Bmf, contain only the
BH3 domain (Gross et al., 1999; Kuwana and Newmeyer, 2003). These
structural differences also reflect differences in their function. Multi-
domain proteins directly induce outer mitochondrial membrane permeabi-
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
163
lization with consequent release of apoptogenic factors into the cytoplasm
(Desagher et al., 1999; Zong et al., 2001). On the other hand, the proteins
with only the BH3 domain are considered the essential initiators of the cell
death program (Huang and Strasser, 2000; Bouillet and Strasser, 2002).
When activated by apoptotic stimuli, the BH3-only proteins have two
fates: (1) some are targeted to the outer mitochondrial membrane and
heterodimerize with Bcl-2 and Bcl-X
L
, neutralizing the action of these
BH3BH4 BH1 BH2
BH3BH4 BH1 BH2
BH3BH4 BH1 BH2
BH3BH4 BH1 BH2
BH3BH4 BH1 BH2
BH3BH4 BH1 BH2
BH3 BH1 BH2
BH3 BH1 BH2
BH3 BH1 BH2
BH3
BH3
BH3
BH3
BH3
BH3
BH3
BH3
BH3
BH3
BH3 BH3
Bcl-2
Bcl-x
L
Bcl-w
Mcl-1
A1
Boo/Diva
Bax
Bak
Bok/Mtd
Bid
Bad
Bim
Bmf
Bik
Hrk/DP5
Blk
Nip3
BNip3/Ni
x
Puma
Noxa
Antiapoptotic
TM
Proapoptotic
Multidomain
BH3-only
Figure 7.5
Classification scheme of the Bcl-2 family members. TM refers to the hydrophobic
C-terminal region, which is probably a transmembrane domain (adapted from
Kuwana and Newmeyer, 2003).
164 Animal Cell Technology

antiapoptotic proteins and allowing, indirectly, multidomain proapoptotic
Bax and Bak proteins to release apoptogenic factors from the mitochon-
dria; (2) others, besides the previously described function, are also respon-
sible for the direct activation of the multidomain proteins, as is the case for
Bid (Desagher et al., 1999; Eskes et al., 2000) and probably Bim (Letai
et al., 2002). At least two events seem to be critical for Bax and Bak to
release apoptogenic factors into the cytoplasm homodimerization and
insertion into the outer mitochondrial membrane (Bouillet and Strasser,
2002).
It is not clear whether each member of this subgroup is activated by a
particular stimulus and through a specific mechanism, or whether their
roles are redundant. Nevertheless, it is possible that different BH3-only
domain proteins, or their combinations, are critical for apoptosis in differ-
ent cell types.
Tridimensional structure analysis of some Bcl-2 family members, such
as Bcl-X
L
, Bcl-w, Bax, and Bid, surprisingly revealed that pro- and anti-
apoptotic proteins share common structures (Kuwana and Newmeyer,
2003). The BH3 domain is buried inside the molecule, and it has been
suggested that it is essential for activity of the proapoptotic members and
has to be exposed to render the protein active. Therefore, in a healthy cell,
proapoptotic members are inactive, with the BH3 domain hidden inside
the molecule. However, by receiving apoptotic signals, they undergo a
conformational change, exposing this domain and, thus, acquiring pro-
apoptotic activity.
In some molecules, such as Bid, Bax, Bak, Bmf, and Bim, the N-terminal
region acts as an inhibitory domain, hiding the BH3 domain (Gross et al.,
1999). Bid must be cleaved by caspase 8, and its truncated form (without
the N-terminal) translocates to the mitochondria to interact with Bax and/
or Bak, activating them (Li et al., 1998; Desagher et al., 1999). Prior to the
apoptotic signal, Bmf and Bim are found associated with cytoskeleton
complexes by the N-terminal region. In the presence of these signals, they
dissociate from these complexes and translocate to the mitochondria to
bind Bcl-2 and Bcl-X
L
, antagonizing their antiapoptotic activity (Puthala-
kath et al., 1999, 2001). Bax and Bak require the interaction with some
BH3-only proteins to derepress their N-terminal domain, exposing not
only their BH3-only domain, but also a C-terminal hydrophobic domain,
which allows them to become integral proteins in the outer mitochondrial
membrane and induce the release of apoptogenic factors (Goping et al.,
1998; Desagher et al., 1999). Bad is phosphorylated at two serine residues
(Ser-112 and Ser-136), which allows it to be sequestered by the cytosolic
protein 14-3-3, keeping it inactivated (Zha et al., 1996). In the presence of
apoptotic signals, Bad is dephosphorylated, resulting in its dissociation
from the 14-3-3 protein and its translocation to the outer mitochondrial
membrane to bind to Bcl-2 and Bcl-X
L
(Kelekar et al., 1997; Ottilie et al.,
1997). It is suggested that Bad phosphorylation regulates the BH3 domain
exposure (Zha et al., 1997). Antiapoptotic proteins can also be converted
to proapoptotic if they expose their BH3 domains (Cheng et al., 1997).
However, not all proapoptotic members are regulated post-translation-
ally. Some, such as Noxa, Puma, and HRK, are regulated transcriptionally.
Noxa and Puma are regulated by the p53 protein and, therefore, are critical
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
165

for an apoptosis process induced by DNA damage (Oda et al., 2000;
Nakano and Vousden, 2001), while HRK is regulated by the JNK-
dependent mechanism (Harris and Johnson, 2001). Some post-translation-
ally regulated proteins can also be regulated transcriptionally, such as Bax,
which can also be regulated by p53 (Miyashita and Reed, 1995). Antiapop-
totic members, like Bcl-X
L
, Mcl-1, A-1 and, less frequently, Bcl-2, can
also be regulated transcriptionally (Gross et al., 1999).
The exact manner of how proapoptotic proteins induce the release of
apoptogenic factors from the mitochondrial intermembrane space into the
cytoplasm and how antiapoptotic proteins prevent it remains obscure.
The protection conferred by antiapoptotic members may occur by
their direct binding to proapoptotic members, sequestering the BH3-
only proteins and therefore preventing the activation of Bax and Bak or
directly neutralizing the activity of the multidomain proteins (Oltvai
and Korsmeyer, 1994). The heterodimerization between members of
Bcl-2 family occurs by the insertion of the BH3 domain of the
proapoptotic protein into a hydrophobic pocket formed by the BH1,
BH2, and BH3 domains on the surface of the antiapoptotic protein
(Sattler et al., 1997). Bcl-2 and Bcl-X
L
do not prevent Bid-induced
conformational change of Bax and Bak (Desagher et al., 1999). How-
ever, they block the release of apoptogenic factors from the mitochon-
dria. Some mutants of Bcl-X
L
that have lost the ability to form
heterodimers with Bax and Bak can still suppress cell death by apopto-
sis, suggesting the existence of a protection mechanism independent of
heterodimer formation (Cheng et al., 1996).
In the literature, two main mechanisms have been proposed that could
explain how Bax and Bak induce the release of apoptogenic factors,
especially cytochrome c. The first model of outer mitochondrial mem-
brane permeabilization predicts the occurrence of homo-oligomerization
of Bax (probably four molecules) and Bak, resulting in the formation of
channels just wide enough for the passage of cytochrome c. The passage
of other apoptogenic factors is still contested (Antonsson et al., 1997;
Saito et al., 2000; Kuwana et al., 2002; Kuwana and Newmeyer, 2003).
The second model is based on the activity regulation of pre-existent
channels, such as the permeability transition pore (PTP) (Marzo et al.,
1998; Narita et al., 1998; Kuwana and Newmeyer, 2003). PTP is a
multiprotein channel, formed by components of both the outer and inner
mitochondrial membranes and matrix proteins, including VDAC (vol-
tage-dependent anion channel, also known as mitochondrial porin), ANT
(adenine nucleotide translocator), and cyclophilin D, respectively
(Crompton et al., 1999). Bax, Bak, and Bcl-X
L
have been found to
interact with VDAC (Narita et al., 1998; Shimizu et al., 1999), and Bax
to interact with ANT (Marzo et al., 1998). The PTP opening would be
followed by the swelling of the mitochondrial matrix and the rupture of
the outer membrane. In this context, the release of apoptogenic factors
would not be specific and would occur indirectly, as a result of the
rupture of the outer membrane. When PTP is induced, the inner
mitochondrial membrane potential (˜łm) becomes dissipated, leading to
the loss of mitochondrial functions, such as energy production and
protein import.
166 Animal Cell Technology

Some authors suggest that the mitochondria, especially the inner mem-
brane, remain intact during apoptosis. Thus, it is suggested that PTP
activation is not involved or that cells starting to die by apoptosis could
switch to necrosis, therefore activating the PTP (Antonsson, 2001). Never-
theless, it is important to remember that these contradictory results can
occur since apoptosis can be activated by distinct mechanisms in different
cell types by different apoptotic signals. It is also possible that both
mechanisms are correct. Bax and Bak oligomers can form channels for the
initial release of cytochrome c, followed by a larger flux through PTP. In
both cases, antiapoptotic proteins Bcl-2 and Bcl-X
L
seem to inhibit the
formation of both kinds of channels.
Following the outer mitochondrial membrane permeabilization, the
apoptogenic factors are released into the cytoplasm. Among them, cyto-
chrome c has an important role in caspase activation, because it is the
cofactor for assembling a large caspase 9 activating complex in the
cytoplasm, called apoptosome. Along with cytochrome c, the Apaf-1
protein and dATP or ATP are required to form this complex in the
cytoplasm (Hill et al., 2003).
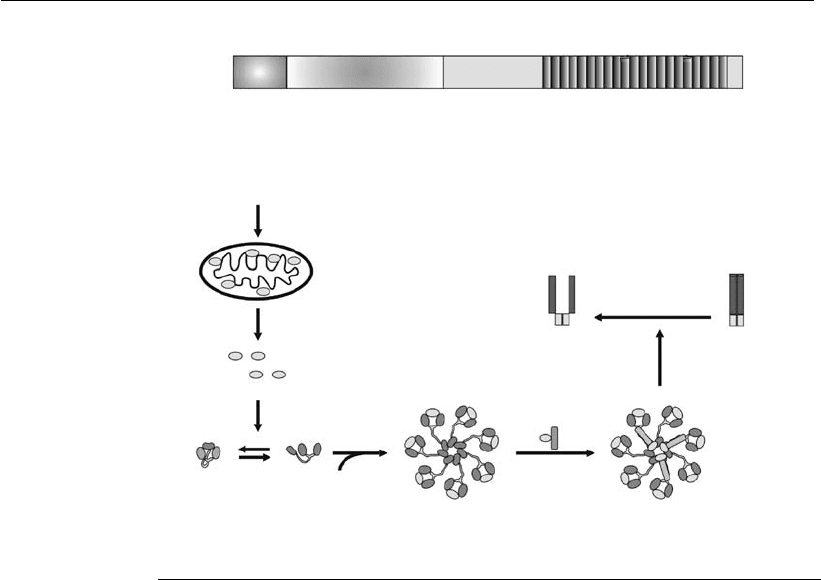
Apaf-1 consists of three functional domains: an N-terminal CARD, a
central NBD (nucleotide-binding domain), and WD-40 repeats at the C-
terminal region (Figure 7.6). In the absence of cytochrome c, Apaf-1 exists
as a monomer in a compact, auto-inhibited form (Hu et al., 1998; Acehan
et al., 2002). When cytochrome c and dATP (or ATP) are present, Apaf-1
is forced into a more open conformation, facilitating the oligomerization
with adjacent Apaf-1 molecules (Jiang and Wang, 2000) and the cyto-
chrome c association with the WD-40 repeats (Acehan et al., 2002). The
apoptosome assembly is illustrated in Figure 7.6.
It has been suggested that the apoptosome is formed by the oligomer-
ization of seven Apaf-1 molecules, resulting in a wheel-like structure. This
structure comprises a central hub connected to seven radial spokes, as
shown in Figure 7.6. The model suggests that the ring is composed of
seven Apaf-1 CARD domains held together in close proximity. Apaf-1
central and C-terminal regions form spokes projecting outward from the
hub (Qin et al., 1999; Acehan et al., 2002). The procaspase 9 is recruited
into the apoptosome through interaction with Apaf-1 with the procaspase
9 CARD domains, at a 1:1 proportion (Budihardjo et al., 1999; Jiang and
Wang, 2004). The procaspase 9 aggregation leads to autoproteolysis (Saleh
et al., 1999). Caspase 9 and the apoptosome form an active holoenzyme,
responsible for the activation of downstream effector caspases, such as
caspases 3 and 7 (Bratton et al., 2001).
After caspase 9 activation, the death signal is propagated by downstream
caspase activation. Caspase 9 directly activates caspases 3 and 7. Caspase 3,
in turn, processes and activates caspases 2 and 6 and also caspase 9,
therefore amplifying the death signal. Caspase 6 cleaves and activates
caspases 8 and 10 (Slee et al., 1999).
Besides cytochrome c and procaspase 9, other important apoptogenic
factors are also released from the mitochondria, such as SMAC/DIABLO,
Omi/HrtA2, AIF, and endonuclease G. The function of SMAC/DIABLO
and Omi/HrtA2 is to activate caspase by suppressing the caspase inhibi-
tory activity of IAP (Du et al., 2000; Verhagen et al., 2000). The protein
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
167
family IAP, which contains members like survivin, xIAP, cIAP1, cIAP2,
inhibits caspase activity by directly binding to the active enzymes. These
proteins contain one or more BIR (baculovirus IAP repeat) domains,
which are responsible for the caspase inhibitory activity. In healthy cells,
it is likely that IAP proteins serve to inhibit residual or unwanted caspase
activity. SMAC/DIABLO directly binds to BIR domains of IAPs, inhibit-
ing their functions. Since Omi/HtrA2 is a serine-protease, it proteolyti-
cally cleaves and inactivates IAP proteins.
When AIF and endonuclease G are released into the cytoplasm, they
directly translocate to the nucleus and induce DNA fragmentation and
subsequent chromosomal condensation, a remarkable morphological fea-
ture of the apoptotic process. AIF induces chromatin digestion into large
fragments of approximately 50 kb, probably by activating a nuclear
DNAse. Therefore, these proteins are important for the caspase-indepen-
dent apoptosis pathway.
CARD
NBD
WD-40 repeat region
A
Apoptotic stimulus
Mithocondrion
Cytochrome c
Apaf-1
dATP/ATP
Apoptosome
Procaspase-9
Active caspase-3/7 Procaspase-3/7
B
Figure 7.6
Scheme of the mechanism of apoptosome formation. (A) The Apaf-1 molecule
possesses three domains: CARD, NBC, and WD-40 repeats. (B) In the absence of
cytochrome c, Apaf-1 may exist in a compact, autoinhibited form, with the CARD
region buried between the lobes of WD-40 repeats. The binding of cytochrome c
displaces the CARD region from the WD-40 repeats, forcing the molecule into a
more open conformation. The interaction with dATP/ATP prevents the
reassociation of CARD to the lobes of WD-40 repeats, facilitating the interaction
with other Apaf-1 molecules for the apoptosome assembly. Procaspases 9 are
recruited by a CARD:CARD interaction, leading to their autoprocessing (adapted
from Hill et al., 200 3, and Jiang and Wang, 2004).
168 Animal Cell Technology

The intrinsic ER stress-induced pathway
The ER is an organelle in which most proteins acquire their tertiary and
quaternary structures. It is also the Ca
2þ
storage site (Rao et al., 2004).
Under certain conditions, such as disruption of Ca
2þ
homeostasis, hypoxia
or ischemia, or when there is an overload of proteins, an accumulation of
unfolded proteins occurs in the ER. This accumulation activates a com-
pensatory mechanism called ER stress response or unfolded protein
response (UPR). This response consists of four distinct steps: a cell cycle
arrest in G
1
/S phase; attenuation of protein synthesis to prevent further
protein aggregation and accumulation in this organelle; induction of ER-
localized chaperone proteins to assist protein folding; and activation of a
protein degradation mechanism to eliminate unwanted protein aggregates.
However, if the ER stress cannot be bypassed, it culminates in apoptosis
(Szegezdi et al., 2003).
Since this ER pathway was discovered recently, not much is known
about its signaling mechanism. However, it is clear that this pathway can
unleash the apoptotic process through three distinct mechanisms. The first
mechanism is dependent on a transcription factor, the GADD153/CHOP.
Although no target genes have been identified to date, it has been
speculated that GADD153/CHOP can decrease Bcl-2 expression
(McCullough et al., 2001).
The second mechanism is dependent on caspase 12 activation. Never-
theless, it is still not clear how this activation occurs. Preliminary
studies showed a caspase activating complex containing VCP and ALG-
2 proteins, which also possess apoptotic activities. This has suggested
the existence of a caspase 12 activating complex, similar to the apopto-
some, which was designated eraptosome (Hoppe et al., 2002; Kilic et
al., 2002). The adaptor protein TRAF2 may be involved in procaspase
12 aggregation, resulting in its cleavage and activation (Yoneda et al.,
2001). The procaspase 12 may also be processed by caspase 7, through a
cleavage in the middle of its prodomain, which leads to autoprocessing
between the prodomain and the large subunit, and between the large
and small subunits of caspase 12 (Szegezdi et al., 2003). Furthermore, it
was also suggested that caspase 12 can be processed by calpain. The
disruption of Ca
2þ
homeostasis leads to calpain activation, which in
turn translocates from the cytoplasm to the ER and cleaves off the
caspase 12 CARD prodomain. After this processing, this caspase is
autoprocessed into large and small subunits (Szegezdi et al., 2003).
Once activated, caspase 12 cleaves and activates the procaspase 9,
triggering the remaining caspase proteolytic cascade. Calpain can also
cleave Bid.
The third mechanism is Ca
2þ
-dependent and also involves the intrinsic
mitochondrial pathway. Some apoptotic stimuli induce Ca
2þ
release from
the ER, and this process may be regulated by the Bcl-2 family members
that reside in this organelle (Szegezdi et al., 2003). Bax and Bak seem to
induce Ca
2þ
release, while antiapoptotic members, such as Bcl-2, seem to
reduce this process. Ca
2þ
ions released from the ER eventually accumulate
in mitochondria, which induces permeabilization of the outer mitochon-
drial membrane through PTP formation. Release of apoptogenic factors
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
169

into the cytoplasm causes activation of caspase 9 and mitochondria-
mediated apoptosis. Apoptogenic Bap31 protein, which resides in the ER,
can be cleaved by caspase 8 and also seems to induce Ca
2þ
release from
this organelle (Breckenridge et al., 2003).
The extrinsic or death receptor-induced pathway
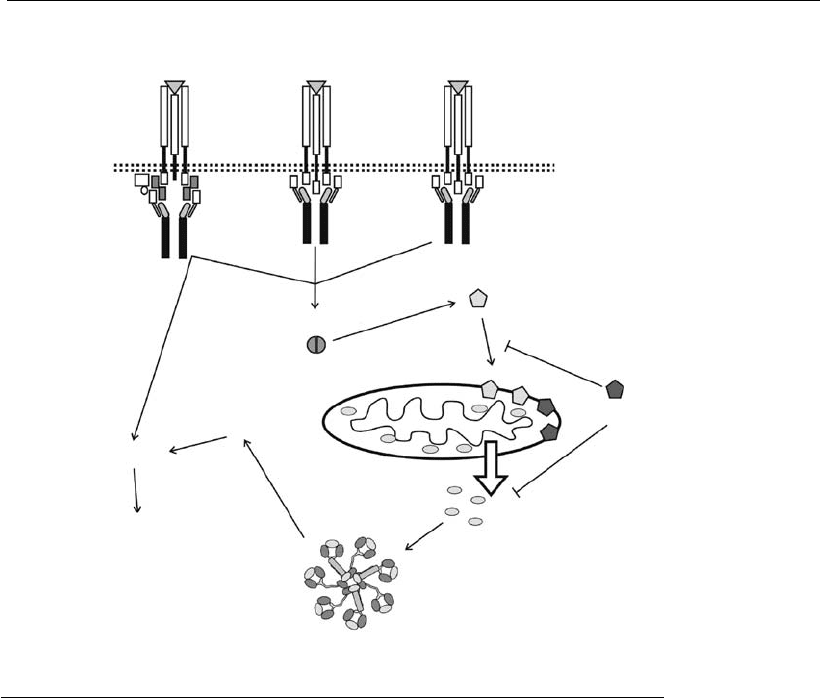
The extrinsic pathway consists of a series of events initially induced by
death receptors located on the cell surface. It is initiated by interaction of
extracellular death ligands with their respective receptors, located on the
surface of the plasma membrane. The death ligands are members of the
tumor necrosis factor (TNF)/nerve growth factor (NGF) superfamily.
TNF-R1, Fas (Apo-1/CD95), TRAIL-R1, TRAIL-R2, and NGF-R are
examples of death receptors. They are transmembrane proteins consisting
of an external domain, where the ligand associates, and a cytoplasmic
domain, which contains the DD (death domain).
The death ligands, such as FasL, are typically homotrimeric molecules.
When they bind to their receptors, which are monomers, they induce
aggregation and trimerization of the receptors, resulting in their cytoplas-
mic domains becoming physically closer together. The close proximity of
the DDs results in the recruitment of adaptor proteins located in the
cytoplasm, such as FADD and TRADD. These adaptor proteins also
possess a DD and bind to the DD of the death receptors. FADD binds
mainly to Fas, while TRADD binds preferentially to TNF-R1. After
TRADD binds to TNF-R1, FADD can bind to TRADD. FADD also
possesses another domain, besides DD, which is called DED. Procaspases
8 and 10 (initiator caspases) also possess the DED and are able to bind to
the adaptor protein through their DED, therefore assembling the DISC
(death-inducing signaling complex) (Figure 7.7).
The DISC-induced procaspase assembly results in the autoactivation of
caspases 8 and 10. The DISC process of caspase activation seems to be
analogous to the apoptosome process of caspase 9 activation. Caspase 8, in
turn, cleaves and activates caspase 3, which is responsible for the apoptotic
signal amplification with subsequent cell collapse.
Some cell types maintain a low level of caspase 8 in the cytoplasm.
Therefore, in the presence of apoptotic stimuli, this small amount of
caspase 8 is activated in the DISC complex, and the subsequent activation
of effector caspases is not possible. These cell types are called type II and
also require the mitochondrial pathway activation. These two pathways
can communicate through the cleavage of a Bcl-2 member, Bid, by caspase
8 (Li et al., 1998). Cell lines that have a higher level of caspase 8 in the
cytoplasm are called type I. In these cells, Bcl-2 family members do not
regulate the death receptor-mediated pathway.
The proteolytic cleavage of Bid removes its N-terminal portion, exposing
the BH3 domain (Li et al., 1998). The truncated Bid protein, called tBid or
p15, translocates to the mitochondria, where it interacts with Bax or Bak,
inducing a conformational change in these proteins (Desagher et al., 1999).
This conformational change is necessary for the permeabilization of the
outer mitochondrial membrane and the subsequent release of the apopto-
genic factors into the cytoplasm, resulting in caspase 9 activation.
170 Animal Cell Technology
7.6.2 Molecular strategies for apoptosis control
Since apoptosis occurs under the control of several genes, the molecular
manipulation of the signaling cascade could block apoptosis progression
and prolong cell viability. Maintaining a high cell viability in bioreactors is
of great interest for biotechnological processes. Therefore, many research-
ers in this area are devoted to the development of genetically modified cell
lines with increased resistance to apoptosis.
The most common strategies consist of developing recombinant cell
lines expressing antiapoptotic genes that regulate the two main families of
proteins involved in the apoptotic cascade: the Bcl-2 and caspase families.
The Bcl-2 gene has been the most widely studied and its overexpression
in cells has been described. It has the ability to protect several industrially
important cell lines, such as hybridomas, myelomas and CHO, BHK, and
COS cells, against apoptotic stimuli that are typical of a cell culture
Death receptors
PM
TNFa
APO3L
TNFR1
APO3L/DR3
FAS
FasL FasL
DR4,5
TRADD
FADD
FADD
FADD ?
Caspase 8
Caspase 9
Caspase 9
Caspase 8
Caspase 10
Death receptors
pathway
Apoptosis
Bid cleavage
Mitochondrion Bax
Bax/BAK
Bak
Bcl-2/Bcl-x
L
Cytochrome c
Mitochondrial
pathway
Apoptosome
Figure 7.7
Caspase activation through the death receptor-induced pathway. The activation of
initiator caspases 8 and 10 by the death receptors results in t he activation of
effector caspases 3, 6, and 7. In type II cell lines, the activation of these initiator
caspases also results in Bid cleavage and, therefore, in the activation of the
mitochondrial pat hway.
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
171
