Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.


6.3.5 C-terminal modifications
A cause of heterogeneity in some proteins arises from the partial cleavage
of terminal lysine or arginine by an intracellular carboxypeptidase. For
mAbs the C-terminal lysine of heavy chains may be removed, giving rise
to a mixture of Lys0 (absence) or Lys1 (presence) heavy chain variants.
Analysis by mass spectrometry showed that the Lys0 variant can amount
to 40–45% of the total (Lazar et al., 2004). Removal of C-terminal
arginine has been shown for human recombinant EPO and also for the
two-chain form of tPA following zymogen activation (Harris, 1995). It is
considered that the removal of either C-terminal lysine or arginine is
unlikely to cause loss of biological activity because they are found as
natural variants of these proteins under physiological conditions in vivo.
6.3.6 Hydroxylation
The most well-known example of protein hydroxylation arises from the
activity of the enzyme, prolyl-4-hydroxylase. This enzyme can convert
proline to hydroxyproline when the substrate is contained in an amino
acid motif (X-Pro-Gly). This is known to occur in collagens and requires
four co-substrates: ferrous ion, 2-oxoglutarate, oxygen, and ascorbate
(Kivirikko et al., 1989). An example of this type of conversion has also
been found in the N-terminus of the prion protein in which there is a
domain containing poly (L-proline) (Gill et al., 2000). An asparagine and
proline hydroxylase may serve to regulate the activity of a hypoxia-
inducible factor (HIF) in vivo (Lando et al., 2002). Under normoxic
conditions these enzymes are active and are capable of hydroxylating
specific asparagine and proline residues in HIF, which is then targeted for
destruction via the ubiquitin proteasome pathway. However, hypoxia
inhibits the activity of the enzymes, allowing the HIF to function as a
transcriptional regulator of genes, whose products play a role in adapting
for oxygen deficiency in vivo.
6.4 Conclusions
Many biopharmaceuticals are produced as secreted glycoproteins from
mammalian cell culture. The post-translational modification of these
proteins is essential to insure structural stability and biological and clinical
activity. The best characterized of these modifications is N-glycosylation.
This gives rise to a significant heterogeneity of structural forms and it is
important to monitor these to insure consistency of production. However,
the ability to control the glycosylation is limited by our understanding of
the parameters that affect the heterogeneity of added glycan structures. It
is clear that the glycosylation process is affected by a number of factors
including the three-dimensional structure of the protein, the enzyme
repertoire of the host cell, the transit time in the Golgi, and the availability
of intracellular sugar-nucleotide donors. From a process development
perspective there are many culture parameters that can be controlled to
enable a consistent glycosylation profile to emerge from each batch
culture. A further, but more difficult goal is to control the culture
142 Animal Cell Technology
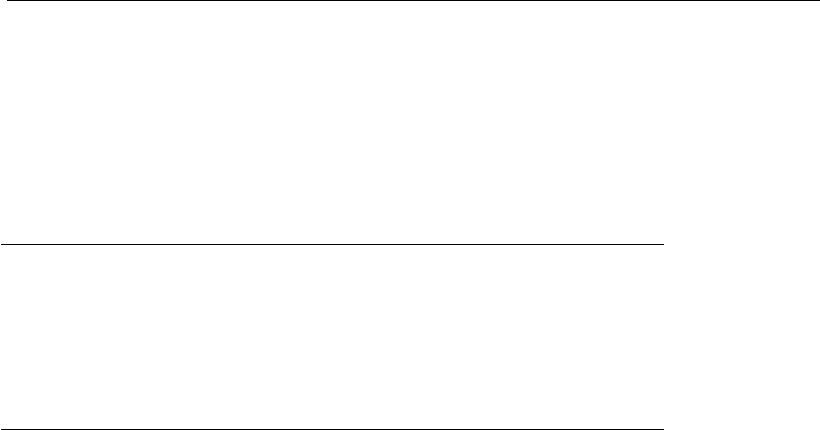
conditions to enable the enrichment of specific glycoforms identified with
desirable biological activities. There are also a number of other possible
post-translational modifications of proteins that can be characterized as
the addition or removal of small organic residues. These may be important
for the structural integrity of the protein and should be monitored in
culture bioprocesses designed for biopharmaceutical production.
Acknowledgment
The Natural Science and Engineering Research Council (NSERC) of
Canada is gratefully acknowledged for financial support for the study of
post-translational modification of proteins through a series of Discovery
and Collaborative Research and Development (CRD) grants.
References
Akagawa M, Sasaki T, Suyama K (2002), Oxidative deamination of lysine residue in
plasma protein of diabetic rats. Novel mechanism via the Maillard reaction, Eur. J.
Biochem. 269:5451–5458.
Allen S, Naim HY, Bulleid NJ (1995), Intracellular folding of tissue-type plasminogen
activator. Effects of disulfide bond formation on N-linked glycosylation and
secretion, J. Biol. Chem. 270:4797–4804.
Backstrom M, Link T, Olson FJ, Karlsson H, Graham R, Picco G, Burchell J, Taylor-
Papadimitriou J, Noll T, Hansson GC (2003), Recombinant MUC1 mucin with a
breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster
ovary cells, Biochem. J. 376:677–686.
Camire RM, Larson PJ, Stafford DW, High KA (2000), Enhanced gamma-carboxyla-
tion of recombinant factor X using a chimeric construct containing the prothrom-
bin propeptide, Biochemistry 39:14322–14329.
Chee F, Wong D, Tin Kam Wong K, Tang Goh L, Kiat Heng C, Gek Sim Yap M
(2005), Impact of dynamic online fed-batch strategies on metabolism, productivity
and N-glycosylation quality in CHO cell cultures, Biotechnol. Bioeng. 89:
164–177.
Curling EM, Hayter PM, Baines AJ, Bull AT, Gull K, Strange PG, Jenkins N (1990),
Recombinant human interferon-gamma. Differences in glycosylation and proteo-
lytic processing lead to heterogeneity in batch culture, Biochem. J. 272:333–337.
Davidson DJ, Fraser MJ, Castellino FJ (1990) Oligosaccharide processing in the
expression of human plasminogen cDNA by lepidopteran insect (Spodoptera
frugiperda) cells. Biochemistry. 29(23):5584-90.
Donaldson M, Wood HA, Kulakosky PC (1999), Glycosylation of a recombinant
protein in the Tn5B1-4 insect cell line: influence of ammonia, time of harvest,
temperature, and dissolved oxygen, Biotechnol. Bioeng. 63:255–262.
Ellgaard L, Helenius A (2003), Quality control in the endoplasmic reticulum, Nat.
Rev. Mol. Cell Biol. 4:181-191.
Farrell PJ, Lu M, Prevost J, Brown C, Behie L, Iatrou K (1998), High-level expression
of secreted glycoproteins in transformed lepidopteran insect cells using a novel
expression vector, Biotechnol. Bioeng. 60:656–663.
Friedman AR, Ichhpurani AK, Brown DM, Hillman RM, Krabill LF, Martin RA,
Zurcher-Neely HA, Guido DM (1991), Degradation of growth hormone releasing
factor analogs in neutral aqueous solution is related to deamidation of asparagine
residues. Replacement of asparagine residues by serine stabilizes, Int. J. Pept.
Protein Res. 37:14–20.
Post-translational modification of recombinant proteins 143

Furie B, Bouchard BA, Furie BC (1999), Vitamin K-dependent biosynthesis of
gamma-carboxyglutamic acid, Blood 93:1798–1808.
Gavel Y, von Heijne G (1990), Sequence differences between glycosylated and non-
glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering,
Protein Eng. 3:433–442.
Gill AC, Ritchie MA, Hunt LG, Steane SE, Davies KG, Bocking SP, Rhie AG, Bennett
AD, Hope J (2000), Post-translational hydroxylation at the N-terminus of the
prion protein reveals presence of PPII structure in vivo, EMBO J. 19:5324–5331.
Goochee CF, Gramer J, Andersen DC, Bahr JB, Rasmussen JR (1991), The oligosac-
charides of glycoproteins: bioprocess factors affecting oligosaccharide structure
and their effect on glycoprotein properties, Bio/Technology 9:1347–1355.
Guarna MM, Fann CH, Busby SJ, Walker KM, Kilburn DG, Piret JM (1995), Effect of
cDNA copy number on secretion rate of activated protein C, Biotechnol. Bioeng.
46:22–27.
Harris RJ (1995), Processing of C-terminal lysine and arginine residues of proteins
isolated from mammalian cell culture, J. Chromatogr. A 705:129–134.
Hayter PM, Curling EMA, Baines AJ., Jenkins N, Salmon I, Strange PG, Tong JM,
Bull AT (1992), Glucose-limited chemostat culture of chinese hamster ovary cells
producing recombinant human interferon-. Biotechnol. Bioeng. 39:327–335.
Hersecovics A, Orlean P (1993), Glycoprotein biosynthesis in yeast. FASEB J. 7:
540–550.
Huang L, Lu J, Wroblewski VJ, Beals JM, Riggin RM (2005), In vivo deamidation
characterization of monoclonal antibody by LC/MS/MS, Anal. Chem. 77:1432–
1439.
Jarvis DL, Finn E (1996), Modifying the insect cell N-glycosylation pathway with
immediate early baculovirus expression vectors, Nat. Biotechnol. 14:1288–1292.
Jarvis DL, Kawar ZS, Hollister JR (1998), Engineering N-glycosylation pathways in
the baculovirus-insect cell system, Curr. Opin. Biotech. 9:528–533.
Jenkins N, Curling EM (1994), Glycosylation of recombinant proteins: problems and
prospects. Enzyme Microb. Technol. 16:354–364
Jenkins N, Parekh RB, James DC (1996), Getting the glycosylation right: implications
for the biotechnology industry. Nat. Biotechnol. 14:975–981.
Kivirikko KI, Myllyla R, Pihlajaniemi T (1989), Protein hydroxylation: prolyl 4-
hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit.
FASEB J. 3:1609–1617.
Kornfeld R, Kornfeld S (1985), Assembly of asparagine-linked oligosaccharides, Annu.
Rev. Biochem. 54:631–664.
Kulakosky PC, Shuler ML, Wood HA (1998), N-Glycosylation of a baculovirus-
expressed recombinant glycoprotein in three insect cell lines, In Vitro Cell. Dev.
Biol. 34:101–108.
Kunkel JP, Jan DC, Jamieson JC, Butler M (1998), Dissolved oxygen concentration in
serum-free continuous culture affects N-linked glycosylation of a monoclonal
antibody, J. Biotechnol. 62:55–71
Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML (2002), Asparagine
hydroxylation of the HIF transactivation domain a hypoxic switch. Science
295:858–861.
Lazar AC, Kloczewiak MA, Mazsaroff I (2004), Matrix-assisted laser desorption/
ionization mass spectrometry for the evaluation of the C-terminal lysine distribu-
tion of a recombinant monoclonal antibody, Rapid Commun. Mass Spectrom.
18:239–244.
Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ (1990),
Assignment of intrachain disulfide bonds and characterization of potential glyco-
sylation sites of the type 1 recombinant human immunodeficiency virus envelope
glycoprotein (gp120) expressed in Chinese hamster ovary cells, J. Biol. Chem.
144 Animal Cell Technology

265:10373–10382.
Lopez M, Tetaert D, Juliant S, Gazon M, Cerutte M, Verbert A, Delannoy P (1999),
O-glycosylation potential of lepidopteran insect cell lines, Biochim. Biophys. Acta
1427:49–61.
Maras M, Saelens X, Laroy W, Piens K, Claeyssens M, Fiers W, Contreras R (1997), In
vitro conversion of the carbohydrate moiety of fungal glycoproteins to mamma-
lian-type oligosaccharides. Evidence for N-acetylglucosaminyltransferase-I-
accepting glycans from Trichoderma reesei. Eur. J. Biochem. 249:701–707.
Meynial-Salles I, Combes D (1996), In vitro glycosylation of proteins: an enzymatic
approach. J. Biotechnol. 46:1–14.
Nilsson MR, Driscoll M, Raleigh DP (2002), Low levels of asparagine deamidation can
have a dramatic effect on aggregation of amyloidogenic peptides: implications for
the study of amyloid formation. Protein Sci. 11:342–349.
Nyberg GB, Balcarcel RR, Follstad BD, Stephanopoulos G, Wang DI (1999), Meta-
bolic effects on recombinant interferon-gamma glycosylation in continuous cul-
ture of Chinese hamster ovary cells, Biotechnol. Bioeng. 62:336–347.
Palacpac NQ, Yoshida S, Sakai H, Kimura Y, Fujiyama K (1999), Stable expression of
human 1,4-galactosyltransferase in plant cells modifies N-linked glycosylation
patterns, Proc. Natl Acad. Sci. U S A 96:4692–4697.
Parekh RB, Dwek RA, Edge CJ, Rademacher TW (1989), N-glycosylation and the
production of recombinant glycoproteins, Tibtech 7:117–122.
Pels Rijcken WR, Overdijk B, Van den Eijnden DH, Ferwerda W (1995), The effect of
increasing nucleotide-sugar concentrations on the incorporation of sugars into
glycoconjugates in rat hepatocytes, Biochem. J. 305:865–870.
Petrescu AJ, Milac AL, Petrescu SM, Dwek RA, Wormald MR (2004), Statistical
analysis of the protein environment of N-glycosylation sites: implications for
occupancy, structure, and folding, Glycobiology 14:103–114.
Raju TS, Briggs JB, Borge SM, Jones AJ (2000), Species-specific variation in glycosyla-
tion of IgG: evidence for the species-specific sialylation and branch-specific
galactosylation and importance for engineering recombinant glycoprotein thera-
peutics, Glycobiology 10:477–486.
Requena JR, Chao CC, Levine RL, Stadtman ER (2001), Glutamic and aminoadipic
semialdehydes are the main carbonyl products of metal-catalyzed oxidation of
proteins. Proc. Natl Acad. Sci. U S A 98:69–74.
Rudd PM, Dwek RA (1997), Glycosylation: heterogeneity and the 3D structure of
proteins, Crit. Rev. Biochem. Mol. Biol. 32:1–100.
Smales CM, Pepper DS, James DC (2002), Protein modification during anti-viral heat-
treatment bioprocessing of factor VIII concentrates, factor IX concentrates, and
model proteins in the presence of sucrose, Biotechnol. Bioeng. 77:37–48.
Spellman MW (1990), Carbohydrate characterization of recombinant glycoproteins of
pharmaceutical interest, Anal. Chem. 62:1714–1722.
Stark NJ, Heath EC (1979), Glucose-dependent glycosylation of secretory glycopro-
tein in mouse myeloma cells, Arch. Biochem. Biophys. 192:599–609.
Storring PL (1992), Assaying glycoprotein hormones – the influence of glycosylation
on immunoreactivity, Tibtech 10:427–432.
Sugiura T, Maruyama HB (1992), Factors influencing expression and post-translational
modification of recombinant protein C, J. Biotechnol. 22:353–360.
Thornalley PJ, Langborg A, Minhas HS (1999), Formation of glyoxal, methylglyoxal
and 3-deoxyglucosone in the glycation of proteins by glucose, Biochem J.
344:109–116.
Van den Steen P, Rudd PM, Dwek RA, Opdenakker G (1998), Concepts and principles
of O-linked glycosylation, Crit. Rev. Biochem. Mol. Biol. 33:151–208.
Walmsley AR, Hooper NM (2003), Glycosylation efficiency of Asn-Xaa-Thr sequons
is independent of distance from the C-terminus in membrane dipeptidase, Glyco-
Post-translational modification of recombinant proteins 145

biology 13:641–646
Xie L, Wang DI (1997), Integrated approaches to the design of media and feeding
strategies for fed-batch cultures of animal cells, Trends Biotechnol. 15:109–113.
Yan A, Lennarz WJ (2005), Unraveling the mechanism of protein N-glycosylation,
J. Biol. Chem. 280:3121–3124.
Yang M, Butler M (2000), Effects of ammonia on CHO cell growth, erythropoietin
production, and glycosylation, Biotechnol. Bioeng. 68:370–380.
146 Animal Cell Technology

7
Mechanisms of cell
pr oliferation and cell death
in animal cell culture
in
vitro
Maı
´
ra Peixoto Pellegrini, Rodrigo Coelho Ventura Pinto, and
Leda dos Reis Castilho
7.1 Introduction
The advances attained in the last decades in the knowledge of the bio-
logical fundamentals underlying animal cell culture have enabled signifi-
cant improvements in cell culture processes in vitro. In this chapter, the
mechanisms that determine cell proliferation and cell death are discussed.
Aspects concerning the kinetics and the mathematical description of cell
growth and cell death are dealt with in Chapter 8.
7.2 Cell proliferation mechanisms
Cell proliferation occurs through a sequence of coordinated events aimed
at doubling the cellular material, so that cells can divide. This is known as
the cell cycle and is the essential mechanism governing reproduction of all
eukaryotic cells. The basic function of the cell cycle is the error-free
duplication of the genetic material (DNA – deoxyribonucleic acid) con-
tained in the chromosomes, followed by a precise segregation of the copies
into two genetically identical daughter cells. During the cell cycle, total
proteins and RNA are duplicated and two cells of similar size with respect
to volume and mass are formed (Mitchison, 2003).
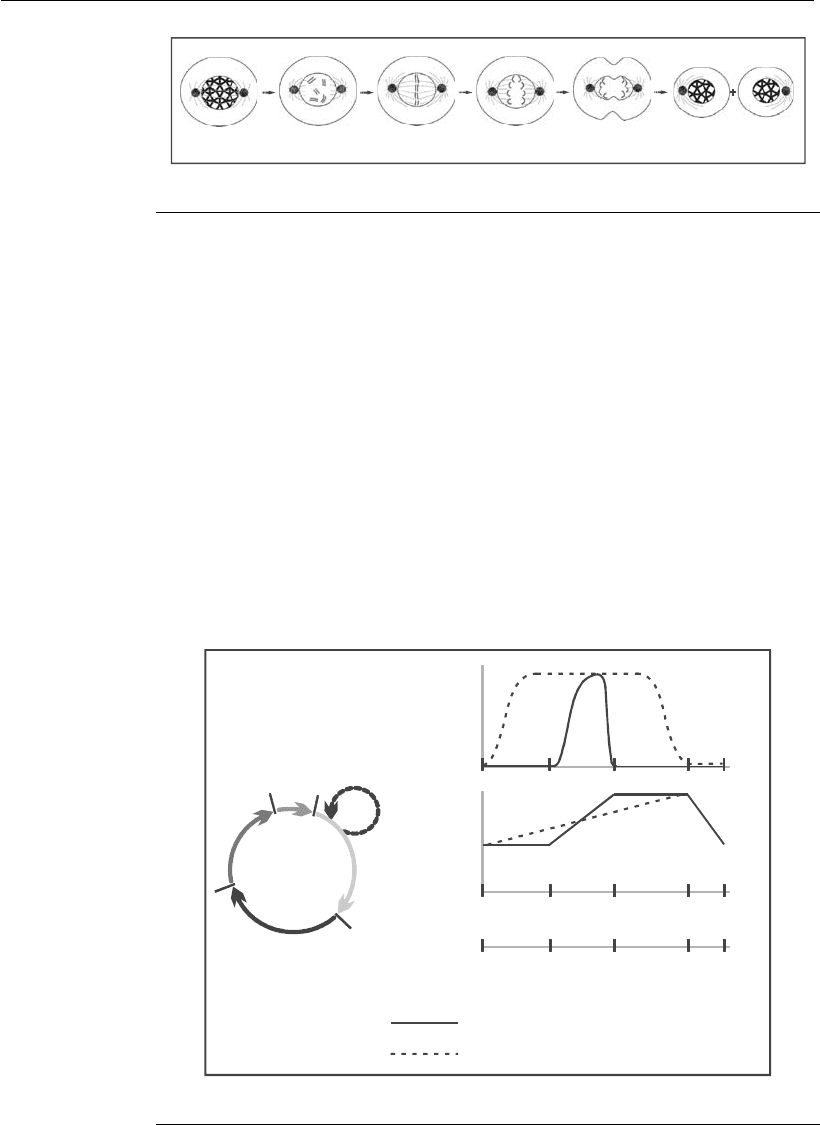
The duplication process defines two important phases of the cell cycle
(Griffiths, 1984; Alberts et al., 2002). In the S phase (
synthesis), which in
mammalian cells lasts 10–12 hours (representing half of the cell cycle),
DNA duplication occurs. In the M phase (
mitosis), which lasts less than 1
hour in mammalian cells, segregation of chromosomes and cell division
take place. This phase is divided into six stages: prophase, prometaphase,
metaphase, anaphase, telophase, and cytokinesis (Figure 7.1). In the initial
stages, the duplicated DNA strands are condensed into more compact
chromosomes, which are necessary for segregation. Subsequently, the
chromosome copies bind to the mitotic spindle, which consists of a bundle
of microtubules. Its function is to segregate chromosomes during cell
division. The chromosomes are aligned and move to opposite poles of the
cell, that is, to the opposite ends of the mitotic spindle. At this stage they
are less compact and they form two new nuclei. The final stage of this
process, known as cytokinesis, consists of the complete division into two
cells, each containing part of the original cytoplasm.
Cell division also requires duplication of the protein mass and of
organelles, which takes longer to be accomplished than DNA replication.
Thus, for the cell to be able to grow and duplicate its contents, there are
two additional phases in the cell cycle: the G
1
phase (between the M and
the S phase), and the G
2
phase (between the S-phase and mitosis). The cell
cycle is divided into four sequential phases: G
1
,S,G
2
, and M (Figure 7.2).
The part of the cycle that consists of phases G
1
, S, and G
2
is designated the
interphase. In a typical proliferating mammalian cell, the interphase can
Prometaphase
TelophaseAnaphase
Cytokinesis
Prophase Metaphase
Figure 7.1
The nuclear division process during the M phase lasts less than an hour. The M
phase can be divided into six distinct stages, according to the progression of
nuclear division (prophase, prometaphase, metaphase, anaphase, telophase, and
cytokinesis) (adapted from Alberts et al., 2002).
S
G2
M
G0
1
2
Relative
contents
Synthesis
rate
G1 S
M
HeLa
cell line
8.0 0.5 h
DNA
RNA protein⫹
G1
G2
9.5 4.8
Figure 7.2
Cell cycle phases versus synthesis rate and concentration of macromolecules in the
cell, as well as average duratio n of cell cycle phases for the tumor HeLa cell line
(adapted from Griffiths, 1984).
148 Animal Cell Technology

last 23 hours and mitosis 1 hour, resulting in an average doubling time (t
d
)
of 24 hours (Griffiths, 1984; Alberts et al., 2002). Depending on the
environmental conditions and the characteristics of the cell (e.g. tumor
cells), the doubling time can vary.
The two G phases represent mainly time intervals when the cell can
grow, but important regulatory phenomena also occur during G
1
and G
2
.
In these phases, the cell monitors the intra- and extracellular environment
to ensure that there are adequate conditions and that the cell itself is
prepared to move forward to the next phase. G
1
has a rather variable
duration, which depends on extracellular conditions and external signals.
If the conditions are not suitable, the cell delays its progression through
G
1
and may even enter a quiescent phase designated G
0
, where it can
remain for an indefinite period of time until restarting cell proliferation. If
the extracellular environment is favorable and there are growth factors
available, a cell in G
1
and G
0
proceeds up to a point where it cannot be
detained any more, known as point of restriction. After transposing this
point, the cell is fated to replicate its DNA, even if the environment
becomes adverse for its development (Griffiths, 1984; Alberts et al., 2002).
The cell division process is governed by a complex regulatory mechan-
ism, which comprises signals from different sources that indicate when
division is necessary and appropriate. These signals originate from both the
extracellular and intracellular environment. The signals consist of growth
factors and hormones, which help cells to determine when it is necessary for
them to divide, if the necessary nutrients are available, and if there are any
signals contrary to the start of the reproductive cycle. The signals relating to
the intracellular verification points, at the end of phases G
1
and G
2
, are
necessary to coordinate in time and space the different cell cycle events,
ensuring that a complete sequence of events occurs before a new sequence
begins. If DNA damage or poor assembly of the mitotic spindle occurs, the
cycle progression is interrupted until repair measures are successfully
accomplished (Fussenegger and Bailey, 1998; Li and Zou, 2005).
The intracellular control of the cell cycle is carried out by a family of
proteins known as cyclin-dependent kinases (CDKs). The activity of these
kinases varies according to the progression of the cell in the cycle.
Alterations in the activity of these proteins result in cyclic changes in
phosphorylation of intracellular proteins, which indicate or regulate the
main cell cycle events. These cyclic changes that may occur in the activity
of CDKs are in turn controlled by other proteins, known as cyclins. As
the name suggests, CDKs depend on cyclins for appropriate activity, since
they are only active when bound to a specific cyclin. Unlike the level of
CDKs, which is approximately constant, cyclins present cycles of synth-
esis and degradation, which result in a cyclic formation and activation of
cyclin–CDK complexes. This activation, in turn, activates the cell cycle
events of animal cells (Alberts et al., 2002). Other important proteins and
intracellular factors also participate in the regulation of cell proliferation
and cell death. The protein known as p53 is activated upon DNA damage
during replication, interrupting cell cycle progression and activating cell
death or senescence mechanisms (Harris and Levine, 2005). The family of
polo-like kinases (PLKs) mediates the control of the checkpoints that
monitor events such as replication and segregation (Xie et al., 2005).
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
149

When cells from normal mammalian tissues are cultivated in vitro under
adequate conditions, they usually stop dividing after a given number of
cycles, and this is called replicative cellular senescence. Hayflick and
Moorhead (1961) first recognized that cells have a limited lifespan in vitro.
They worked with human fibroblasts (WI-38 cell line) and observed that
these cells died after about 50 population doublings (Hayflick and Moor-
head, 1961; Hayflick, 1965). Senescence is related to changes in the
structure of telomeres, which are repetitive DNA sequences associated
with proteins at the tips of the chromosomes. When cell division occurs,
the telomere sequences are not replicated in the same way as the rest of the
DNA. These sequences are synthesized by the telomerase enzyme, which
is also responsible for the addition of proteins that protect the chromo-
some ends. In the germ line, during early development, and in highly
proliferative organs, telomere shortening and elongation is balanced.
However, when cells become fully differentiated or mature, the shortening
process becomes dominant due to telomerase repression (Bekaert et al.,
2004). Thus, the telomeres become shorter and their protective proteins
deteriorate with time. With their ends exposed, the chromosomes become
prone to damage, and this may cause an arrest of the cell cycle or induce
cell death mechanisms (Alberts et al., 2002). Normal rodent cells possess
active telomerase throughout their proliferative lifespan, and this explains
why they are so readily immortalized and transformed in culture com-
pared with their human counterparts. Telomerase is present in 90% of
human cancer cells, and therefore it represents an attractive target for
developing new anticancer drugs (Newbold, 2002).
Although the use of immortalized cell lines has allowed the establish-
ment of different cell culture technologies for the large-scale production of
biopharmaceuticals, an enhanced capacity for proliferation may become
disadvantageous. In batch cultures, for instance, the aim is to reach high
cell concentrations, but this results in exhaustion of essential nutrients and
accumulation of toxic metabolites, leading to sub-optimal nutritional
conditions that lead to cell death and to the end of the production process
(Fussenegger and Bailey, 1999). However, in many cases, the synthesis of a
product is not associated with cell growth and maximum productivity is
reached under conditions in which cell proliferation is decreased, but high
viabilities are maintained. Therefore, some cultivation processes are based
on operational strategies that allow cells to remain viable, but in a non-
proliferative state, so as to prolong the productive phase and to increase
the productivity of the process (Suzuki and Ollis, 1989, 1990; Fussenegger
and Bailey, 1999).
By these strategies cell proliferation may be controlled by adding
chemical additives that arrest the cell cycle, usually in the G
1
phase,
increasing specific productivity. However, concomitantly undesirable ef-
fects such as cytotoxicity may be observed, which result in a decrease in
cell viability and in the impossibility of maintaining the culture in a
nonproliferative state for long periods of time (Suzuki and Ollis, 1990;
Al-Rubeai et al., 1992). Deprivation of specific nutrients and growth
factors can also stop cell proliferation, but in this case cell viability
decreases and programmed cell death – apoptosis – is activated (Mercille
and Massie, 1994a, 1994b; Singh et al., 1994; Fussenegger and Bailey,
150 Animal Cell Technology
1999). Currently, much research on the biochemical control of cell
cultures based on preventing the cell death mechanisms, to avoid cell death
instead of inhibiting cell growth, is being carried out with the aim of
prolonging the productive period of a cell culture process (Al-Rubeai and
Singh, 1998; Fussenegger et al., 1999; Fussenegger and Bailey, 1999). This
biochemical control is based on the knowledge and manipulation of the
molecular basis of cell death, as discussed in detail in Section 7.6 of this
chapter.
7.3 Cell death mechanisms: apoptosis and necrosis
Cell death occurs by two different mechanisms: necrosis or apoptosis. The
term ‘‘apoptosis’’ was first used in 1972 by Kerr, Wyllie, and Currie to
describe a form of cell death that differed from cell death by necrosis in
that no damage to the cell membrane and no inflammatory responses were
observed (Kerr et al., 1972). Apoptosis was later recognized as an impor-
tant physiological process during the life of all multicellular organisms,
where cells are regularly lost. These cells are removed when they are no
longer needed or when they represent a danger. This allows the tissue to
function normally. The perfect balance between proliferation and cell
death maintains homeostasis. Examples of apoptosis during the life of an
organism are: tissue renovation, positive and negative selection of lympho-
cytes, elimination of cells during embryogenesis (such as the formation of
limbs), and development of the nervous system of vertebrates. An appro-
priate control of apoptosis is crucial for higher eukaryotes, and any failure
in its regulation can lead to diseases. The lack of a mechanism for
apoptosis in tumor cells permits them to survive and, thus, contributes to
cancer development. It can also be responsible for autoimmune diseases.
Excessive or inappropriate activation of apoptosis may cause degenerative
diseases, such as osteoporosis and Alzheimer’s or Parkinson’s diseases.
Necrosis is considered an accidental death, and thus a non-physiological
process, which is characterized by changes in morphology and in mito-
chondrial function, as well as by the inability of the plasma membrane to
regulate osmotic pressure inside the cell. Initially, there is an increase in
the cytoplasmic volume due to the influx of liquid into the cell (Figure
7.3). This is followed by membrane and organelle rupture, leading to the
release of lysosomes and cytoplasmic material, and culminating in random
nuclear fragmentation (Al-Rubeai, 1998). The damage to the plasma
membrane includes alterations in the ion transport system, followed by an
increase in the permeability of the membrane and cell swelling (Buja et al.,
1993).
Apoptosis in turn is characterized by a series of morphological altera-
tions, in an autodestruction process that results in the formation of vesicles
containing cell material, known as apoptotic bodies, which, in vivo, are
subsequently phagocytized by specialized cells. This series of events is
initiated by a given stimulus that elicits a cascade of intracellular signals,
activating enzymes (caspases) that will mediate the morphological and
physiological changes experienced by the cell. In general, apoptosis differs
from necrosis by the absence of cytoplasmic material leakage, by the
Mechanisms of cell proliferation and cell death in animal cell culture
in vitro
151
