Castilho Leda R., Moraes Angela Maria (Ed.) Animal Cell Technology: From Biopharmaceuticals to Gene Therapy
Подождите немного. Документ загружается.

Golgi. However, in lower eukaryotes such as fungi and yeast the high-
mannose structures are released from the cell as end products of glycosyla-
tion (Meynial-Salles and Combes, 1996).
There is a great deal of variability of N-glycan structures attached to
any protein. This arises through macroheterogeneity, which is defined as
variable occupancy of potential sites of attachment and microheterogene-
ity, which is defined as variable structural forms at each site.
6.2.1.1 Macroheterogeneity
The occupation of a sequon occurs cotranslationally in the ER and
involves the transfer of a consensus oligosaccharide structure
(Glc
3
Man
9
GlcNAc
2
) from a dolichol-linked pyrophosphate donor to the
Asn side chain of an available sequon. Only around 65% of all appropriate
glycoprotein sequons are occupied and the oligosaccharyl transfer reaction
appears to depend upon various cellular factors including the protein
translation rate, the availability of the oligosaccharyl donor, and the
activity of the enzyme. Petrescu et al., (2004) surveyed the occupation of
sequons on several thousand proteins and found a significantly large
number of occupied glycans that have low accessibility in the folded
protein. Although this may seem surprising, it must be realized that
protein glycosylation precedes folding and so the inaccessibility of a
sequon in the fully folded protein does not mean that it is necessarily
inaccessible to the OST enzyme. The glycans may play a part in maintain-
ing the folded structure by reducing the conformational freedom of the
local peptide backbone (Petrescu et al., 2004). However, disulfide bridge
Asn
Asn
Asn
Complex
Hybrid
High mannose
⫽ Fuc
⫽ GlcNAc
⫽ Man
⫽ Gal
⫽ NeuAc
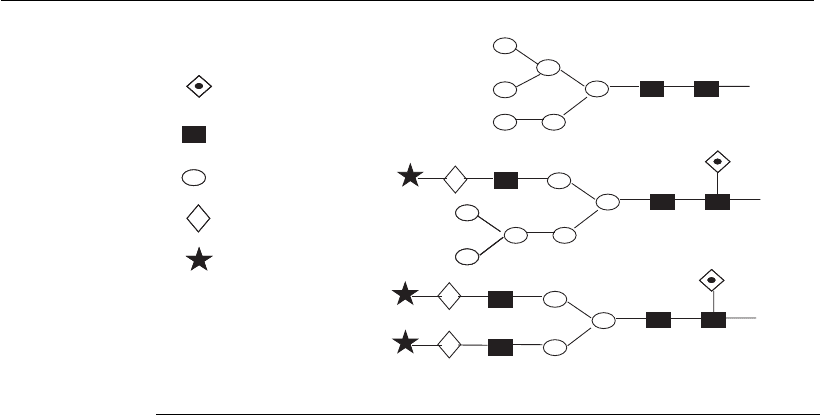
Figure 6.3
N-glycan structures of glycoproteins. There are three main types of glycan
structure, which are built on the core structure. The high mannose structures are
the main type of structures found in fungi. In animal cells these are generally
intermediates that are transferred to the Golgi for further processing. The complex
glycans consist of a variety of structures added in sequence to the trimannosyl
core. The hybrid structures consist of at least one antenna from the trimannosyl
core with a complex structure and one with a high mannose structure.
132 Animal Cell Technology

formation is also co-translational in the ER and may interfere with the
accessibility of some sequons, leaving them unoccupied. For some proteins
such as tissue-type plasminogen activator (tPA) this may lead to variable
occupancy of a specific site and macroheterogeneity of glycoforms (Allen
et al., 1995).
A further consideration is the position of the sequon in relation to the
primary structure of the protein. Statistical analysis of a large number of
glycoproteins has indicated that the frequency of non-glycosylated se-
quons increases toward the C-terminus (Gavel and von Heijne, 1990). The
critical distance appears to be 60 amino acid residues from the C-terminus
when reduced glycan occupation occurs. This distance corresponds to the
distance between the ribosome P-site and the active site of the OST and it
has been hypothesized that the protein chain is not available for N-glycan
attachment once it is released from the ribosome. However, this phenom-
enon of poor glycosylation efficiency toward the C-terminus does not
appear to be universal for all proteins (Walmsley and Hooper, 2003).
6.2.1.2 Microheterogeneity
The transferase reactions in the Golgi do not always go to completion and
so give rise to heterogeneity of the final glycan structure (microhetero-
geneity). This heterogeneity is evident as variable antennarity, with the
number of branches from the central mannose of the core structure
ranging typically between two (biantennary) and four (tetra-antennary).
Terminal sialylation of the antenna, fucosylation of the innermost core
GlcNAc (proximal) or the outer arm GlcNAc (peripheral), and addition
of a ‘‘bisecting’’ GlcNAc to the central core Man residue are also examples
of glycan processing that is variable and may be incomplete in the Golgi.
A diverse range of glycan forms may be produced in some recombinant
proteins. For example, the HIV envelope glycoprotein (gp120) produced
from CHO cells contains 24 occupied sequons, of which 13 are complex
glycans and 11 are high mannose or hybrid structural forms (Leonard
et al., 1990).
6.2.2 O-linked glycans
Although most attention has been focused on N-linked glycosylation of
proteins, O-linked glycans are smaller structurally but equally ubiquitous
among eukaryotic glycoproteins. The most common form of O-glycan
attached to glycoproteins from mammalian cells is the mucin-type, which
involves the addition of N-acetylgalactosamine to a serine or threonine
residue in a protein. The O-glycan is added post-translationally to the
fully folded protein. No consensus sequence has been identified, although
glycosylation often occurs in a region of the protein that contains a high
proportion of serine, threonine, and proline (Van den Steen et al., 1998).
These residues probably enable the region of the protein to assume a
conformation that is accessible to the GalNAc transferase enzyme respon-
sible for the addition of the GalNAc. The first step for the assembly of the
mucin type O-glycans is the addition of N-acetylgalactosamine (GalNAc)
residue to a Ser/Thr by a GalNAc transferase (GalNAcT) from UDP-
Post-translational modification of recombinant proteins 133
GalNAc (Van den Steen et al., 1998). Further elongation leads to a large
number of structures, synthesized by various glycosyltransferases, produ-
cing eight different core structures (Figure 6.4). These core structures can
be further modified by sialylation, fucosylation, sulfation, methylation, or
acetylation. The most common is the core 1 structure (Gal1-3GalNAc)
that may be monosialylated or disialylated. These are the most prominent
O-glycan structures found in glycoproteins produced from CHO cells
(Backstrom et al., 2003).
6.2.3 Patterns of glycosylation in non-mammalian cells
The prokaryotes (mainly E. coli) were the first cells to be used for gene
expression of recombinant proteins. These cells can be manipulated
genetically and show high productivity in large-scale systems. However,
the cells lack any metabolic pathways for glycosylation and so the proteins
produced are not glycosylated. Lower eukaryotes (yeast, insect, and plant
cells) are capable of glycosylation of proteins. However, the glycans
produced in these cells differ significantly from those present in mamma-
lian glycoproteins (Jenkins et al., 1996). Yeast, insect, plant, and mamma-
lian cells share the feature of N-linked oligosaccharide processing in the
ER, including attachment of Glc
3
Man
9
GlcNAc
2
-P-P-Dol and subsequent
truncation to Man
8
GlcNAc
2
structure. However, oligosaccharide proces-
sing by these different cell types diverges in the Golgi apparatus (Goochee
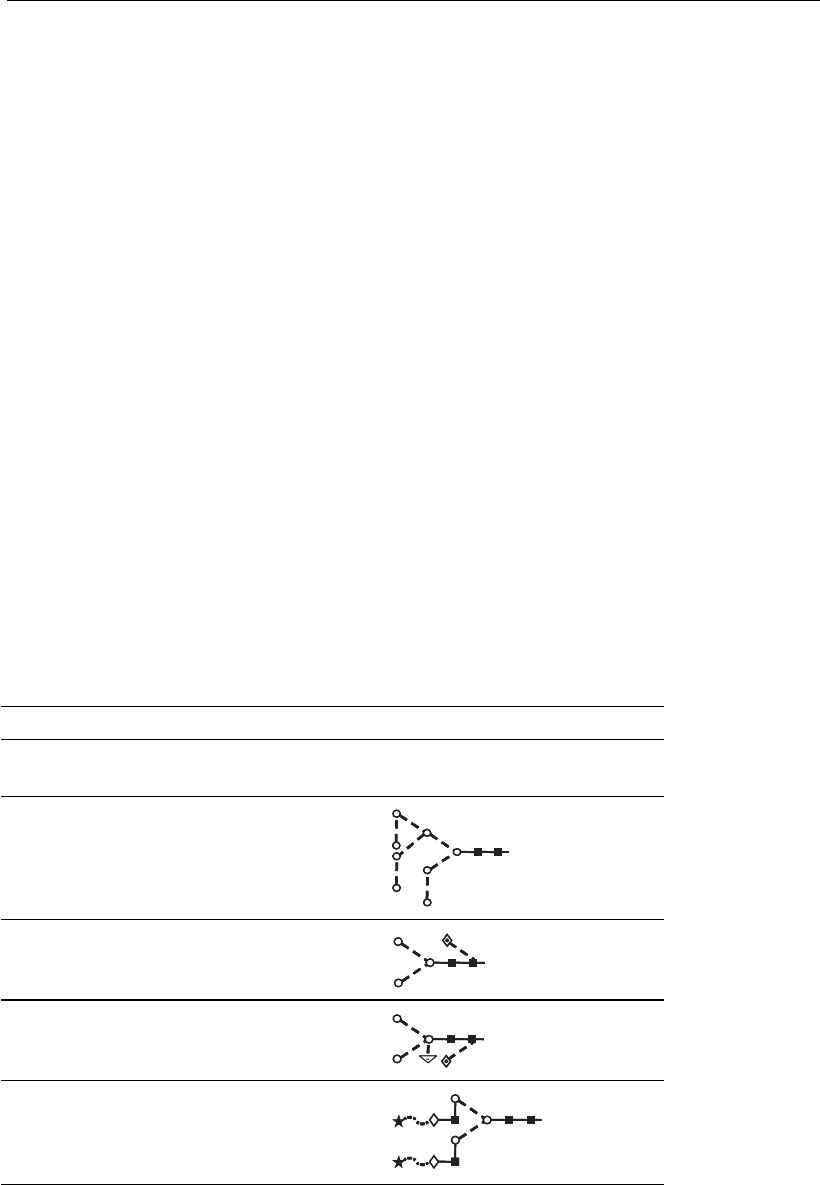
S/T
S/T
S/T
S/T
Core VIII
Core V
Core VI
Core VII
S/T
S/T
S/T
S/T
S/T
Core I
Core II
Core III
Core IV
Tn antigen
2
3
4
8
6
Linkage position
GlcNAc
Gal
GalNAc
Figure 6.4
Core structures of mucin-type O-linked glycans. These represent a series of
characteristic structures attached to a serine (S) or threonine (T) amino acid
residueofaprotein.Thereareeightcorestructures,eachofwhichmayhave
further monosaccharide additions. Tn is a precursor of the T (Thomsen–
Freidenreich) antigen which is commonly used as a marker for tumor cells.
134 Animal Cell Technology
et al., 1991). Although there is extensive heterogeneity of structures arising
from any cell type, examples of predominant N-glycans that might occur
from different systems are shown in Table 6.1.
Insect cells. Lepidopteran insect cell lines (such as Spodoptera frugiper-
da, Sf-9) have been used extensively for expression of recombinant
proteins using the baculovirus as an expression vector (Jarvis et al., 1998).
Alternative methods of protein expression are also available using efficient
insect-associated promoter systems (Farrell et al., 1998). The advantage of
the use of these cells is the high expression level and growth rate of the
cells in culture. However, the glycosylation of proteins expressed by insect
cells is limited. These cells can add Glc
3
Man
9
GlcNAc
2
precursors to
appropriate N-glycan sites in a nascent polypeptide and convert them to
Man
9
GlcNAc
2
. They also have the enzymes necessary to trim this
oligosaccharide all the way down to Man
3
GlcNAc
2
. The formation of this
product requires the action of the enzymes ManI, GnTI, and ManII.
However, there is little structural processing beyond the Man
3
GlcNAc
2
core oligosaccharide apart from the possibility of fucosylation (Jarvis and
Finn, 1996; Donaldson et al., 1999).
Glycosyltransferase enzymes are either absent or at a low level of
activity (Jarvis et al., 1998). Therefore, generally the insect expression
system is incapable of synthesizing sialylated lactosamine complex-type
N-glycan or sialylated O-glycans. However, some insect cells have been
found to produce recombinant glycoproteins with elongated trimannosyl
core structures containing terminal GlcNAc or Gal, and one recombinant
glycoprotein acquired complex biantennary N-linked glycan containing
sialic acid (Davidson et al., 1990; Kulakosky et al., 1998).
Table 6.1 Typical predominant N-glycan structures from different c ell types.
Cell-type N-glycan Structure
Bacteria, E.coli None
-
-------------
-
-------------
Yeast High mannose
Insect Fucosylated core structure
Plant Xylosylated and fucosylated
core structure
Mammalian Complex biantennary
Post-translational modification of recombinant proteins 135

Jarvis et al. (1998) proposed a model to explain the N-linked oligosac-
charide structures found in insect cell-produced glycoproteins. In this
model the enzyme GlcNAcTII competes with N-acetylglucosaminidase
(GlcNAdase) at a branch point. Depending upon the relative activities of
these competing processing enzymes, trimannosyl core or complex
N-linked glycans may be produced. It would appear that insect cells have
a high level of N-acetylglucosaminidase activity, which can remove
GlcNAc from a terminal position. The production of complex sialylated
N-linked structures could only occur when the recombinant protein is a
very poor substrate for GlcNAdase or excellent substrate for the low level
activities of the glycosyltransferases. The enzymic activity may vary con-
siderably and this explains the differences in the N-glycan pattern of the
same glycoprotein secreted by different insect cell lines (Kulakosky et al.,
1998).
The potential of insect cells for O-glycosylation was reported in a study
in which three lepidopteran cell lines were shown to produce predomi-
nantly short O-glycan structures (Lopez et al., 1999). All three cell lines
expressed GalNAcÆ1-O-Ser/Thr (Tn antigen), whereas the ability to
synthesize Gal1-3GalNAcÆ1-O-Ser/Thr (T antigen) and GalÆ1-4Gal1-
3GalNAcÆ1-O-Ser/Thr (PT antigen) was more limited. This indicated
low activity of 1-3-galactosyltransferase and Æ1-4-galactosyltransferase.
There was no indication of sialylation of these structures, suggesting the
absence of sialyltransferase activity. The culture medium used to grow
these cell lines had a major effect on the O-glycans expressed. The use of a
semi-defined rich medium enhanced glycosyltransferase activity signifi-
cantly compared with a minimal nutrient medium. The limitations in the
ability of insect cells for glycosylation have restricted their use for
production of human therapeutics. Genetic engineering of these systems is
under study in an attempt to improve the glycosylation machinery to
produce more ‘‘humanized’’ glycoproteins (Jarvis et al., 1998). In particu-
lar the transformation of insect cells with the gene for mammalian 1,4-N-
acetylgalactosyltransferase with a baculovirus expression vector leads to
expression of the enzyme and results in more extensively processed N-
glycans (Jarvis and Finn, 1996).
Fungi. The early steps in the addition of carbohydrate to proteins
appear to have been remarkably conserved during evolution. The synthesis
of the Glc
3
Man
9
GlcNAc
2
-P-P-Dol precursor, the transfer to the polypep-
tide, and early processing in the ER are common events shared by
eukaryotic cells. However, although relatively few N-linked sites have
been characterized, it was noted that there is a trend in favor of the use of
Asn-X-Thr sites over Asn-X-Ser in yeast glycoproteins. In contrast to
mammalian cells, where several Man residues may be removed during
processing, in Saccharomyces cerevisiae, a single specific Man residue is
cleaved to form Man
8
GlcNAc
2
. Most yeast and filamentous fungi synthe-
size carbohydrate chains of the high mannose type. Complex glycan
structures are not observed among fungal glycoproteins. Addition of Man
residues to core oligosaccharides occurs very rapidly in the Golgi, forming
the characteristic high mannose structures (mannan), which can consist of
more than 50 mannose residues and resulting in high molecular weight
glycoproteins (Hersecovics and Orlean, 1993).
136 Animal Cell Technology

Proteins synthesized in yeast may also contain O-glycans consisting of
linear poly-mannose structures attached to Ser or Thr. Similar to mamma-
lian cells, O-glycosylation in yeasts has no obvious consensus sequence.
However, unlike mammalian cells O-glycosylation in yeast is initiated
with covalent attachment of mannose via a dolichol phosphate mannose
precursor. Maras et al., (1997) showed that if the high mannose structures
are trimmed in vitro by mannosidase, they can become acceptors for the
recombinant processing enzymes, N-acetylglucosaminyltransferase I,
1,4-galactosyltransferase, and Æ2,6-sialyltransferase. Mutant strains of
yeast also may synthesize truncated mannose structures.
Plants. Plant cells also conserve the early stages of N-glycosylation.
However, the processing of the oligosaccharide trimming and further
modification of glycans in the Golgi differ from mammalian cells. Plant-
derived oligosaccharides do not possess sialic acid and frequently contain
xylose (Xyl), not normally present in mammalian N-linked oligosacchar-
ides. Typically processed N-glycans in plants have a Man
3
GlcNAc
2
structure with 1,2 xylose and/or Æ1,3 fucose residues attached to the
reducing terminal GlcNAc (Palacpac et al., 1999). Xylose is not present in
mammalian glycan structures and fucose is attached to proximal (core)
GlcNAc by an alternative linkage (Æ1,6) in mammalian cells. The presence
of these two residues (Xyl and Æ1,3 fucose) in plant recombinant glyco-
proteins and their absence in mammalian proteins makes them highly
immunogenic if present in therapeutic glycoproteins (Parekh et al., 1989;
Storring, 1992; Palacpac et al., 1999).
6.2.4 Glycosylation in animal cells: the effect of the host cell line
The pattern of protein glycosylation is dependent on the expression of
various glycosyltransferase enzymes that are present in the Golgi of the
cell. Differences in the relative activity of these enzymes among species
can account for significant variations in structure. In one systematic study
of glycan structures of IgG produced from cells of 13 different animal
species significant variation was found in the proportion of terminal
galactose, core fucose, and bisecting GlcNAc (Raju et al., 2000).
The fact that glycoproteins normally exist as mixtures of glycoforms
suggests that the protein structure is not the primary determining factor in
glycosylation. The glycoforms that emerge from the Golgi are end
products of a series of incomplete enzymic reactions. Thus, the choice of
the host cell line is a particularly important factor in the glycoform profile
of a recombinant protein (Rudd and Dwek, 1997). Sialylation patterns of
the secreted protein are particularly affected by the host cell.
6.2.5 Culture parameters that may affect glycosylation
It is important to control culture parameters to insure consistency of
glycosylation of a recombinant protein in a culture bioprocess. However,
this may not be so easy given that the extent of glycosylation may decrease
over time in a batch culture (Curling et al., 1990). This is likely to be due
to the continuous depletion of nutrients (particularly glucose or gluta-
mine) and accumulation of metabolic byproducts, which have been shown
Post-translational modification of recombinant proteins 137

to limit the glycosylation process (Hayter et al., 1992; Jenkins and Curling,
1994; Nyberg et al., 1999). Culture conditions such as nutrient content,
pH, temperature, oxygen, or ammonia, may have a significant impact on
the distribution of glycan structures found on the resulting recombinant
protein (microheterogeneity). This of course is of major concern in trying
to produce consistent biopharmaceuticals. It can lead to enhanced glyco-
form heterogeneity and significant batch to batch variation in the produc-
tion process. To maintain product quality it is important to understand the
parameters that cause the variation in glycosylation.
Glucose starvation may result in an intracellular depleted state with a
shortage of glucose-derived precursors of glycans that could result in
reduced site occupancy. This has been shown at low glucose concentration
(, 0.5 mM) for the synthesis of antibody (Stark and Heath, 1979) and
recombinant gamma-interferon (Hayter et al., 1992). Fed-batch strategies
may be designed to insure that the concentrations of these key nutrients
do not decrease below a critical level that could compromise protein
glycosylation (Xie and Wang, 1997). The lower levels of nutrients for the
production of recombinant protein from CHO cells were found to be 0.1
mM glutamine and 0.7 mM glucose (Chee et al., 2005). Nutrient levels
below these critical concentrations led to decreased sialylation and an
increase in hybrid and high mannose-type glycans. A plausible mechanism
for reduced site occupancy of a recombinant protein produced by glucose-
depleted or glutamine-depleted CHO cell cultures was offered by Nyberg
et al. (1999). They showed that in both cases the low level of glycosylation
was related to a decreased intracellular concentration of UDP-GlcNAc.
This intracellular concentration of UDP-GlcNAc has also been shown
to be important in another respect. An elevated UDP-GlcNAc appears to
cause a decrease in sialylation, which may be explained metabolically by
the inhibition of CMP-sialic acid transport (Pels Rijcken et al., 1995). This
may be the mechanism for the observed decrease in sialylation that has
been shown to accompany high levels of ammonia in culture (Yang and
Butler, 2000).
The dissolved oxygen (DO) level of cultures has also been shown to be
a critical parameter to affect glycosylation. Terminal galactosylation of an
immunoglobulin (IgG) was changed significantly with a gradual decrease
in the digalactosylated glycans (G2) from 30% at the higher oxygen level
to around 12% under low oxygen conditions (Kunkel et al., 1998).
6.3 Other forms of post-translational modification
6.3.1 Deamidation
Non-enzymatic deamidation of glutamine or asparagine residues of pro-
teins can occur spontaneously, leading to potential structural changes and
loss of biological activity. Deamidation of Asn occurs more rapidly and
leads to Æ-linked (Asp) or -linked aspartyl (Isoasp) via a succinamide
derivative. This introduces an extra negative charge in the protein that can
result in alterations of properties. For example, deamidation of an Asn
residue of human growth hormone-releasing factor can lead to a 25–500-
138 Animal Cell Technology

fold loss in potency (Friedman et al., 1991). Low levels of deamidation can
lead to protein aggregation and are related to amyloid formation (Nilsson
et al., 2002). The presence of Isoasp may also enhance the immunogenicity
of a protein. Deamidation may occur during bioprocessing or storage of
biopharmaceuticals and is dependent upon a number of factors including
pH, temperature, solvent dielectric constant, and ionic strength, as well as
the structure of the protein. Deamidation half-times for Asn in synthetic
peptides at neutral pH and 37
o
C are in the range of 1–500 days. Huang
et al. (2005) described ‘‘hot spots’’ for spontaneous deamidation in a
humanized monoclonal antibody (mAb) that can lead to loss of binding
capacity.
6.3.2 Deamination
Proteins subjected to metal-catalyzed oxidation can form carbonyl groups
that are essentially deamination products (glutamic and aminoadipic
semialdehydes) that result from proline, arginine and lysine residues. This
may lead to loss of function or structural alterations of the proteins that
are associated with the loss of a positive charge. Glutamic semialdehyde
(5 mmole/mol) accounted for the majority of carbonyl groups in proteins
from HeLa cells in culture. Treatment of the cells with an H
2
O
2
-generat-
ing system led to a 2.5-fold increase of these carbonyl groups (Requena
et al., 2001).
Oxidative deamination of lysine has also been observed by incubating a
model protein in the presence of high concentrations of glucose (50 mM)
or lower concentrations of the Æ-oxoaldehydes; methyl glyoxal, or
3-deoxyglucosone (1 mM; Akagawa et al., 2002). These Æ-oxoaldehydes
may be formed by the nonenzymatic degradation of glucose or from the
early stage of glycation (Thornalley et al., 1999). The presence of copper
(Cu
2þ
) increases the rate of this process.
6.3.3 Glycation
This is a non-enzymatic process of adding glycans that may contribute to
the post-translational modification of proteins. It is a complex process that
results in lysine- and arginine-derived glycation adducts in intracellular or
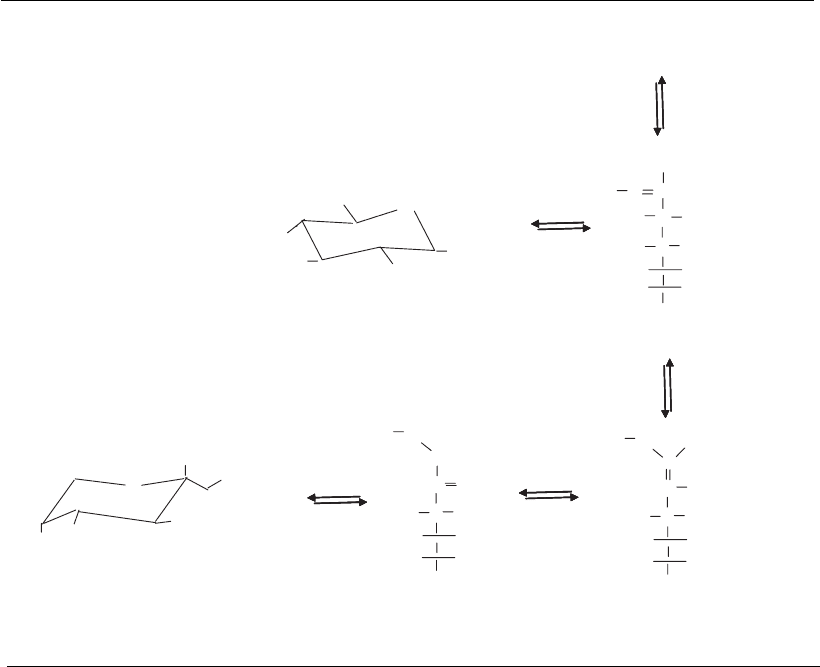
extracellular proteins. The initial Maillard reaction involves the formation
of a Schiff’s base between glucose and the -amino group of lysine ( Figure
6.5). This undergoes a rearrangement to form a more stable fructosamine–
protein adduct. However, a number of subsequent reactions are possible
that lead to advanced glycation end products (AGEs) that have been
associated with pathological complications of diabetes. The process has
been well studied in connection with pathological hyperglycemia. How-
ever, there are also some specific considerations for bioprocesses.
Biopharmaceuticals may be subjected to heat treatment as a means of
viral inactivation. Protein damage is prevented by the addition of high
concentrations of a thermostabilizing excipient such as sugars or polyhyd-
ric alcohols. The non-reducing polyhydric additives increase thermostabil-
ity of the protein by the formation of a hydrogen-bonded solvent shell.
The typical treatment for factor VIII consists of heat treatment at 608C for
Post-translational modification of recombinant proteins 139
10 h in the presence of high concentrations of sucrose and glycine. Such a
treatment will destroy most viruses but may cause glycation products that
could render the protein immunogenic (Smales et al., 2002). The observed
modifications take place by reaction adducts of glucose or fructose that are
derived from the hydrolysis of sucrose. The heat treatment can result in
deamidation, protein glycation, or AGE adduct formation.
6.3.4 Gamma-carboxylation
Gamma-carboxyglutamic acid (Gla) is an amino acid with a dicarboxylic
acid side chain that has metal-binding properties and is important for the
biological activities of a range of blood coagulation proteins that include
prothrombin, factor X, factor IX, and factor VII, as well as the regulatory
proteins, protein C and protein S. The extra negative charge provided by
the carboxylation aids in the calcium-induced interaction of these proteins
with membrane surfaces. Gamma-carboxyglutamic acid is synthesized by
the post-translational modification of glutamic acid residues (Glu) in a
reaction catalysed by a carboxylase enzyme with requirements for vitamin
R.NH glucose
2
⫹
H
HO
N
H
C
CC
CC
OH
R
H
OH
CH OH
2
H
H
OH
o
NH-protein
HO
OH
HOCH
2
Schiff’s base
H
NH
R
HO
H
C
CC
OH
OH
CH OH
2
H
H
OH
H
OH
C
OH
OH
OH
O
HO
NH-protein
NH
R
HO
H
C
C
O
OH
CH OH
2
H
H
OH
CH
2
C
H
OH
HO
Fructosamine
Amadori
re-arrangement
Figure 6.5
Glycation reactions. Glucose or other sugars may react non-enzymatically with proteins to form
glycation products. Early glycation products such as fructosamine–protein adducts may later be
transformed to the more complex advanced glycation end products (AGE products).
140 Animal Cell Technology
K, O
2
, and CO
2
(Figure 6.6).
:
The carboxylation of glutamic acid residues
of these proteins appears to be directed by a specific recognition sequence,
which is often found in the propeptide to insure binding to the vitamin
K-dependent carboxylase. Furie et al., (1999) identified five requirements
for gamma carboxylation:
(i) there is a recognition site that interacts with the carboxylase enzyme;
(ii) the protein is trafficked through the rough ER during synthesis;
(iii) the cell has a carboxylase enzyme associated with the rough ER;
(iv) there are Glu residues within 40 residues of the enzyme recognition
sequence;
(v) intracellular vitamin K is present.
The production of recombinant protein C has been studied from BHK
cells and it was shown that the protein secretion rate increased with cDNA
copy number. However, a higher secretion rate decreased the efficiency of
gamma-carboxylation (Guarna et al., 1995). Similarly, culture conditions
were shown to affect carboxylation of protein C, with a good supply of
oxygen favoring high efficiency of gamma-carboxylation (Sugiura and
Maruyama, 1992).
Factor X undergoes extensive post-translational modification including
gamma-carboxylation of 11 glutamic acid residues. However, the effi-
ciency of this process is variable and inevitably leads to a heterogeneity of
isoforms. HEK-293 cells have been shown to be more efficient for
gamma-carboxylation than CHO cells. This may depend on the availabil-
ity and activity of the carboxylase enzyme that binds to the propeptide.
Camire et al., (2000) improved the efficiency of carboxylation of factor X
by using a chimeric cDNA that contained a propeptide with reduced
affinity to the enzyme. Surprisingly, this increased the extent of carboxyla-
tion from a normal value of 32% to 85% by enhancing substrate turnover.
Nevertheless, in all situations there were two pools of protein produced: a
fully carboxylated and uncarboxylated recombinant factor X.
O
N
N
O
H
H
OHO
N
N
O
HO
OH
O
Glutamic acid Gamma-carboxyglutamic acid
Carboxylase
Vitamin K,
CO , O
22
Figure 6.6
Gamma carboxylation of glutamic acid residue in a protein. This is an enzymic
reaction catalyzed by a carboxylase to modify a glutamic acid residue of a protein
to form gamma-carboxyglutamic acid. The carboxylase reaction requires vitamin
K, O
2
,andCO
2
.
Post-translational modification of recombinant proteins 141
