Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

borohydride. Reactive lysine can also be determined
after conventional acid hydrolysis, by the furosine
method. This method is based on the property of
fructosyl-lysine to produce upon acid hydrolysis a
new amino acid, i.e., furosine. The measurement of
the furosine level allows quantification of the
Amadori compound of lysine and calculation of
the amount of reactive lysine.
The Advanced Maillard Reaction
0011 This stage of the reaction starts with the degradation of
the sugar moiety of the Amadori compound involving
dehydration, scission, oxidation, and Strecker degrad-
ation, leading to the generation of new molecules (pre-
melanoidins) contributing to aromas and flavors that
vary in their characteristics according to the conditions
employed. Some of these molecules are chemically
more reactive than the initial sugar, e.g., a-dicarbonyls,
reductones, and aldehydes. They react not only with
free amino groups still present in the food, but also
with a larger number of radical groups such as hy-
droxyl, amines, and other nitrogen-containing mol-
ecules, vitamins, and the side chains of amino acids.
0012 Polymerization of premelanoidins leads to the for-
mation of the high-molecular-weight melanoidins,
the reactions involved being responsible for the devel-
opments of pigments.
0013 During the advanced stage of the Maillard reac-
tion, loss of lysine and of B-vitamins in foods is accel-
erated. Other amino acids become less bioavailable
because they are either chemically modified or a de-
crease in the total food nitrogen digestibility occurs.
Lysine lost as new unavailable addition product(s)
can be calculated by difference as ‘lysine in advanced
Maillard compound(s)’ (Table 2). These addition
products do not regenerate lysine on acid hydrolysis
and their chemical structures are still unknown.
Indirect Effects
0014 Associated with the presence of Maillard reaction
products, a good example of an indirect negative
nutritional effect of nonenzymatic browning is the
reduction in protein digestibility. This is due to the
inability of proteases and peptidases to hydrolyze
the peptide bonds now containing modified amino
acids. Feeding such a protein results in an increase
in the amount of fecal nitrogen and a decrease in the
bioavailability of the chemically unmodified amino
acids. Another indirect effect is a modification of the
metabolism (intestinal absorption, utilization, and
urinary excretion) of some minerals and trace elem-
ents (calcium, zinc, iron, and copper). The increase
in the urinary excretion of zinc, iron, and copper
in patients fed intravenously a sterilized aqueous
solution of free amino acids and glucose, and the
hyperzincuria observed in rats fed heated casein–
glucose and casein–lactose mixtures, would be due
to the chelating effect of Maillard reaction products.
However, this increase in urinary zinc being a rela-
tively minor route of excretion compared to fecal
excretion, zinc retention, and zinc status is not
reduced in rats.
Physiological Effects
0015Many physiological changes in tissues of animals
have been attributed to feeding Maillard reaction
products. It has not always been easy, however, to
distinguish between the changes that are directly due
to the reduced nutritive value of the reacted protein
and those that are probably induced by the Maillard
components of the test protein in the diet. Further-
more, in many of these studies which corresponded to
model systems, the reaction products tested were in-
corporated in the diets at such high levels that inter-
pretation of observation has remained difficult.
0016Reduced growth rate and increased relative weight
of liver, kidney, and cecum have been noted in rats fed
a severely reacted (brown) compared to an unreacted
mixture of egg protein and glucose. Parallel increases
in values for blood glucose and urea nitrogen, and
serum transaminases and alkaline phosphatase were
also observed with the reacted mixture. The lower
growth rate, attributable to the reduced nutritive
value of the reacted egg protein, may partly explain
the above differences. Long-term rat feeding trials,
designed to eliminate the influence of the nutritional
factors through increased dietary protein level, im-
proved protein quality, and a lower, more realistic
level of incorporation of the reacted egg protein
under test showed less dramatic effects. Nevertheless,
an apparent effect of the reacted brown Maillard
compound was evident in the depressed growth rate,
enlarged liver, spleen, and cecum, lowered serum tri-
glycerides, increased total iron, and histopathologi-
cally observed fatty changes in the liver.
tbl0002 Table 2 Lysine blockage in processed milk
Heat process Percentage lysine
as Amadori compound
a
Freeze-drying 0
Pasteurization 0
UHT sterilization 0–2
Spray-drying 0–2
Spray-drying infant formula 5–10
HTST sterilization 5–10
Conventional sterilization 10–15
Roller drying 20–50
a
According to the furosine method.
UHT, ultra heat-treated; HTST, high-temperature, short-time.
BROWNING/Toxicology of Nonenzymatic Browning 675

0017 Activities of intestinal mucosal dissacharidases
(lactase, sucrase, and maltase) were also reduced in
rats fed the reacted, brown egg protein, compared to
those fed the unreacted material. Some improvement
occurred on fortification of the diet with amino acids
(apparently) destroyed during reaction, although a
persistent decrease in the activity of these enzymes
demonstrated a specific inhibitory effect of the Mail-
lard compounds.
0018 Diarrhea is often observed in animals fed high
levels of severely browned Maillard products. This
is likely to be a direct consequence of the increased
amount of indigestible protein available for fermen-
tation in the cecum, which is often found to be
enlarged.
0019 During the progress of the nonenzymatic browning
reaction, additional glycosyl residues become fixed to
specific milk proteins leading to an increase in their
allergenicity. Using a skin reactivity test, a good
correlation has been shown between the degree of
browning and allergenic response of a modified
bovine b-lactoglobulin. Whilst this may explain one
of the mechanisms responsible for the enhancement of
the allergenicity of a protein, it does not explain the
‘how and why’ of allergy to cows’ milk protein.
0020 Maillard reaction products of low molecular
weight which are partially absorbed can affect the
activity of detoxifying enzymes. Rats fed browned
egg albumin exhibited increased hepatic benzo[a]-
pyrene hydroxylase activity and decreased colonic
aminopyrine N-demethylase activity compared to
animals fed nonbrowned egg albumin. This suggests
that Maillard reaction products may modify the
metabolism of endogenous substrates, exogenous
drugs, and other xenobiotics.
0021 Dietary melanoidins appear to decrease plasma
cholesterol in rats and to modify the composition of
fecal, neutral steroids, suggesting that melanoidins
influence the intestinal metabolism of cholesterol.
0022 Maillard reaction products also interact with
the microorganisms of the digestive tract. Thus they
strongly inhibit the activity of glycosyl transferase of
Streptococcus mutans which has an active role in the
development of dental caries. This inhibition would
hence reduce the adherence of this microorganism to
the tooth surface. Also, in the gut, the microflora is
able to regenerate the amino acids from their Ama-
dori compound and oxidize the advanced Maillard
reaction products.
Toxicity
0023 Studies of the toxicity of Maillard reaction products,
both in vitro and in vivo, are a recent develop-
ment. This interest comes from the observation that
Maillard reactions involving creatinine (a typical
molecule of animal tissues) or protein pyrolysis rep-
resent a carcinogenic potential due to the formation
of heterocyclic amines. In addition to these amines,
there exist mutagenic products of the premelanoidin
and melanoidin types resulting from heat-processed
carbohydrate-rich foods such as bakery products,
caramel, coffee, and other milk and meat products.
Mutagenic/Carcinogenic Heterocyclic Amines
0024Using the short-term Ames microbiological test, it
has been possible to detect mutagenic activity in
broiled fish and meat. A series of heterocyclic
amines has been isolated from cooked foods and
from protein and amino acid pyrolysates. Some of
them, 2-amino-3-methylimidazo [4,5-f ]quinoline
(IQ), 2-amino-3,4-dimethylimidazo [4,5-f]quinoline
(MeIQ), 2-amino-3-methylimidazo [4,5-f ]quinoxa-
line (IQx), 2-amino-3,8-dimethyl-imidazo [4,5-f ]qui-
noxaline (MeIQx), 2-amino-3,4,8-
trimethylimidazo[4,5-f ]quinoxaline (4,8-DiMeIQx),
and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyri-
dine (PhIP), possess an imidazole ring which is
formed from creatinine. The remaining parts of the
molecules (pyridines and pyrazines) are derived from
Maillard reaction products formed by amino acids
and sugars. Since creatinine is present only in animal
protein sources, this class of substances is found only
in these foods, albeit at low levels in the charred
portions of broiled fish and the surface of grilled
meat (PhIP 1 p.p.b; MeIQx 1 p.p.b.) and in beef ex-
tracts (IQ 70 p.p.b; MeIQx 8–90 p.p.b.).
0025The other heterocyclic amines detected in foods
are pyrolysis products formed from trypto-
phan: 3-amino-1,4-dimethyl-5H-pyrido[4,5-b]indole
(Trp-P-1), 3-amino-1,4-dimethyl-5H-pyrido[4,5-
b]indole (Trp-P-2), from glutamic acid: 2-amino-6-
methyldipyrido-[1,2-a:3
0
,2
0
-d]imidazole (Glu-P-1),
2-aminodipyrido-[1,2-a:3
0
,2
0
-d]imidazole (Glu-P-2),
from phenylalanine: 2-amino-5-phenylpyridine
(Phe-P-1), and from soya bean globulin: 2-amino-
9H-pyrido[2,3-b]indole (AaC), 2-amino-3-methyl-
9H-pyrido[2,3-b]indole (MeAaC). The heterocyclic
amines which are produced in very small amounts in
foods have been found to be carcinogenic in rats and
mice (Table 3). The major target organs are the small
and large intestines, liver, lung, and blood vessels.
Mutagenicity of Melanoidin-Type Products
0026Heated mixtures of free amino acid or food proteins
and sugars possess a weak mutagenic activity in the
Ames test. This activity varies with the composition
of the mixture and the conditions of the reaction.
Generally, the mutagenic activity observed in vitro
correlates quite well with the formation of brown
676 BROWNING/Toxicology of Nonenzymatic Browning
pigments and with the presence of molecules with
high antioxidant capacity.
0027 The demonstration that carbonyl compounds can
produce free radicals lends confirmation to the ob-
servation that the mutagenicity of the antioxidant
fraction of the Maillard products is deactivated by
catalases. This apparent contradiction suggests that,
when applied at high concentration to in vitro tests,
Maillard products can induce mutations due to an
‘overpowering’ of their prooxidant activity, a phe-
nomenon already observed with other antioxidants,
such as the antioxidant vitamins and phenolic
compounds.
0028 When considering model systems reflecting normal
cooking conditions a relatively weak mutagenic ac-
tivity was observed compared to that of heterocyclic
amines. The typical mutagenic effects of this class of
compounds reflect base pair, in contrast to frameshift
mutations, as caused by heterocyclic amines. Further-
more, their mutagenic effect has been found to be
effectively inactivated by the liver microsomal
enzymes (S9 liver fraction), in contrast to heterocyc-
lic amines which require S9 activation to mutagenic/
carcinogenic species. (See Mutagens.)
0029Examples of heated foods with such a weak
mutagenic activity that is metabolically deactivated
in vitro are some commercial caramel preparations
(ammonia and sulfite/ammonia processes), hydro-
lyzed plant proteins and roasted coffee. However, it
has been confirmed in animal feeding studies that
melanoidin-containing foods such as roasted coffee
and roasted cocoa are neither mutagenic nor carcino-
genic. Moreover, tested in vivo under well-controlled
physiological conditions, Maillard reaction products
with antioxidant activity are found to act as anti-
mutagens and/or anticarcinogens.
Antioxidant and Antimutagenic Activity of
Melanoidin-Type Products
0030Many Maillard reaction products exhibit antioxidant
properties which are utilized with success in different
types of food. Such reaction products include preme-
lanoidins which are rich in carbonyl compounds as
well as melanoidins. The observed antioxidant prop-
erty depends on the conditions of the reaction and on
the amino acids. Thus, reaction mixtures containing
histidine have been found to have the highest antioxi-
dant activity. The mechanism itself of the antioxidant
tbl0003 Table 3 Mutagenic and carcinogenic effects of heterocyclic amines
Compound Source Mutagenicity Carcinogenicity
Revertants
(mg) TA 98
Species Concentration (%) Experimental
period(weeks)
Tar g e t o r ga n s
Products formed
from creatinine
IQ Broiled sardines 433 000 Rats 0.03 72 Liver, intestine, skin
Mice 0.03 96 Liver, lung
MeIQ Broiled sardines 661 000 Rats 0.03 40 Large intestine,
mammary gland
Mice 0.04 91 Liver, forestomach
MeIQx Fried beef 145 000 Rats 0.04 61 Liver, skin
Mice 0.06 84 Liver, lung
Pyrolysis products
formed from
tryptophan
Trp-P-1 Tryptophan pyrolysate 39 000 Rats 0.02, 0.015 52 Liver
Mice 0.02 89 Liver
Trp-P-2 Tryptophan pyrolysate 104 200 Mice 0.02 89 Liver
Pyrolysis products
formed from
glutamic acid
Glu-P-1 Glutamic acid pyrolysate 49 000 Rats 0.05 67 Liver, small
and large intestines
Mice 0.05 68 Liver, blood
vessels
Glu-P-2 Glutamic acid pyrolysate 1 900 Rats 0.05 104 Liver, small
and large,
intestines
Mice 0.05 84 Liver, blood vessels
IQ, 2-amino-3-methylimidazo [4,5-f ]quinoline; MeIQ, 2-amino-3,4-dimethylimidazo-[4,5-f ]quindine; MeIQx, 2-amino-3,8-dimethyl-imidazo [4,5-
f ]quinoxaline; Trp-p-1, 3-amino-1,4, dimethyl-5H-pyrido [4,5-b] indole; Trp-P-2, 3-amino-1-methyl-5H-pyrido[4,5-b]indole; Glu-P-1, 2-amino-6-
methyldipyrido-[1,2-a:3
0
,2
0
-d]imidazole; Glu-P-2, 2-aminodipyrido-[1,2-a:3
0
2
0
-d]imidazole.
BROWNING/Toxicology of Nonenzymatic Browning 677
activity in food systems involves both the reducing
capacity of the carbonyl compounds in the premela-
noidins and melanoidins, and the property of the
melanoidin brown pigments to chelate prooxidant
metals, copper, and iron.
0031 That Maillard reaction products possess antimuta-
genic and/or anticarcinogenic properties has been
evidenced. Thus, such reaction products inhibit
mutagenic activity of several foodborne heterocyclic
amines and of aflatoxin B
1
. Antimutagenic and pos-
sible anticarcinogenic activities have been attributed
to melanoidins and a-dicarbonyl compounds gener-
ated either during the aminocarbonyl or carameliza-
tion reactions. The scavenging of active oxygen by
melanoidins, demonstrated by means of electron spin
resonance, can explain their antimutagenic effects.
The involvement of antioxidant reactions in the anti-
mutagenicity of heat-reaction products suggests
that these compounds might have anticarcinogenic
properties which include inhibition of nitrosamine
formation, scavenging of active oxygen involved in
cancer initiation and promotion, and also change in
the chemical structure of carcinogens during the
browning process.
Nitrosamines of Amadori Compounds
0032 Amadori compounds have been shown to be nitro-
sated to mutagenic N-nitroso derivatives. This has led
to the postulation that such compounds, if present in
human food or if formed in the stomach, will consti-
tute a health hazard. However, heated foods rich in
Maillard reaction products with antioxidant proper-
ties can both inhibit nitrosamine formation and
reduce nitrosamine-induced carcinogenicity. (See
Nitrosamines.)
See also: Amines; Browning: Nonenzymatic;
Nitrosamines
Further Reading
Eriksson C (ed.) (1981) The Maillard Reaction in Food.
Progress in Food and Nutritional Science 5. Oxford:
Pergamon Press.
Finot PA, Aeschbacher HU, Hurrell RF and Liardon R (eds)
(1990) The Maillard Reaction in Food Processing,
Human Nutrition and Physiology. Advances in Life
Sciences. Basel: Birkhauser.
Friedman M (ed.) (1991) Nutritional and Toxicological
Consequences of Food Processing. Advances in Experi-
mental Medicine and Biology 289. New York: Plenum
Press.
Lee TC, Pintauro SJ and Chichester CO (1982) Nutritional
and toxicological effects of non-enzymic Maillard
browning. Diabetes 31 (suppl. 3): 37–46.
Enzymatic – Biochemical
Aspects
J Nicolas and C Billaud, Conservatoire National des
Arts et Me
´
tiers, Paris, France
J Philippon (Retired), Centre National du Machinisme
Agricole du Ge
´
nie Rural des Eaux et des Fore
ˆ
ts,
Antony, France
M A Rouet-Mayer, Centre National de la Recherche
Scientifique, Gif sur Yvette, France
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The discoloration which occurs in plant material after
cell disruption and results in the formation of brown
or sometimes yellow, black, or pink pigments is
mainly due to the process of enzymatic browning.
Besides plant materials which are primarily con-
cerned, enzymatic browning also affects some animal
materials, e.g., melanosis (black spot) of shrimp.
0002Cell disruption can arise either from mechanical
injury or temperature changes which lead to physio-
logical disorders or even cell death (e.g., for deep-
frozen products). This loss of cell integrity results
in the decompartmentation of phenolic substrates
and enzymes and then, in the presence of molecular
oxygen, the oxidative production of colored pig-
ments.
0003Brown pigmentation following this enzymatic
reaction, and subsequent nonenzymatic reactions, is
generally considered to be detrimental to food quality
from both the organoleptic and nutritional points of
view. The prevention of enzymatic browning has
always been a challenge to food scientists owing to
the loss of quality that it causes in many food prod-
ucts, e.g., fruits and vegetables during either storage
or processing. It is only in a few exceptional cases that
the enzymatic browning is desirable (prunes, dates,
tea, tobacco, etc.).
0004With a better knowledge of the mechanism of
browning reactions, it will be possible to propose
processes which avoid or at least minimize this
discoloration and which can be adapted to each par-
ticular product. (See Colorants (Colourants): Proper-
ties and Determination of Natural Pigments).
Nomenclature
0005Two kinds of enzymes are able to act upon diphenols
in the presence of molecular oxygen according to
the reaction scheme shown in Figure 1. Both have
the trivial name polyphenol oxidases, but they are
somewhat different in nature. The first class of
enzymes, catechol oxidases (1,2-benzenediol: oxygen
678 BROWNING/Enzymatic – Biochemical Aspects
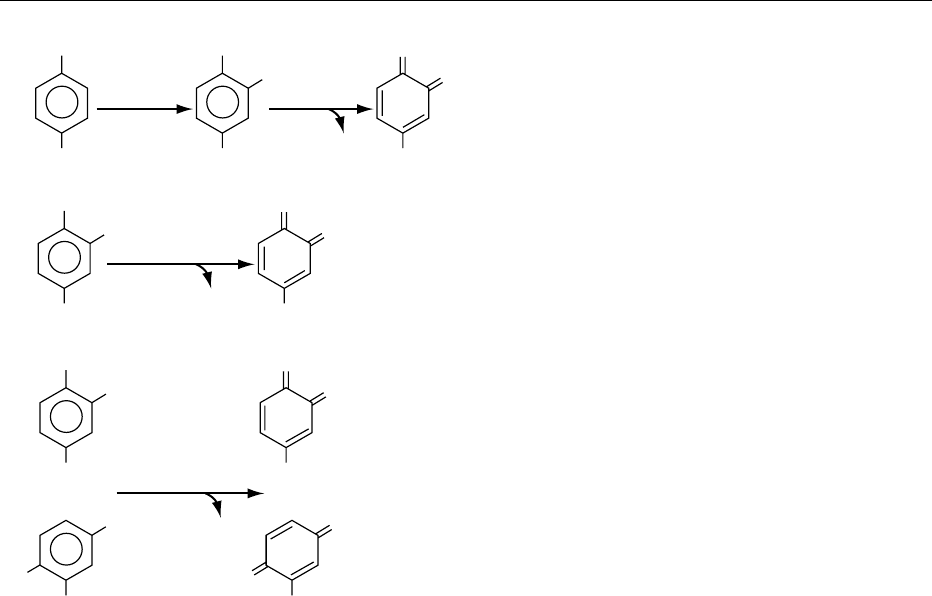
oxidoreductase, EC1.10.3.1), catalyze two distinct
reactions (reactions (1) and (2) of Figure 1), namely
hydroxylation of monophenols into o-diphenols (cre-
solase activity) and oxidation of o-diphenols into o-
quinones (catecholase activity). Both reactions con-
sume oxygen and the overall stoichiometry is 1 mol
of oxygen for 1 mol of monophenol, giving 1 mol of
o-quinone and 1 mol of water. The second class, lac-
cases (benzenediol: oxygen oxidoreductase,
EC1.10.3.2), oxidize o-diphenols as well as p-diphe-
nols, forming their corresponding quinones (reaction
(3) of Figure 1). The stoichiometry is one atom of
oxygen for 1 mol of diphenol giving 1 mol of quinone
and 1 mol of water. The unique ability to oxidize p-
diphenols can be used to distinguish the laccase activ-
ity from that of the first class of polyphenol oxidases.
In all cases, the quinones formed are very reactive
and, depending on the nature and the concentration
of the reactive species in the medium, they can enter
in numerous secondary enzymatic and nonenzymatic
reactions which will be described later.
0006 The nomenclature of these enzymes is some-
what confusing since, besides the two designated
as EC1.10.3.1 and EC1.10.3.2, a third one,
EC1.14.18.1 (monophenol, l-DOPA: oxygen oxido-
reductase), exists. It is referred to as monophenol
monooxygenase (tyrosinase) and corresponds to the
same enzymes as EC1.10.3.1 but which always cata-
lyze the hydroxylation of monophenols. For the sake
of simplicity, we will use the general terms of poly-
phenol oxidase (PPO) for the first class (EC1.10.3.1)
and laccase for the second class (EC1.10.3.2). (See
Enzymes: Functions and Characteristics.)
Enzymatic Browning Factors
0007The different factors of enzymatic browning are
examined, including some minor ones which may
occasionally play an important role, e.g., peroxidases.
Enzymes
0008Polyphenol oxidases (EC1.10.3.1) PPOs are copper
oxidoreductases exhibiting both cresolase and cate-
cholase activities. However, many enzymatic prepar-
ations have a very low cresolase activity or none at
all. It is difficult to insure that the latter activity was
originally absent in the plant or has not either been
extracted or has been destroyed during extraction
owing to its lability. Cresolase activity is often lost
during purification. Thus, in a preparation, the ratio
of catecholase to cresolase activity (when the latter is
present) can vary from 1 to 40.
0009PPO is present in a wide variety of plants. In a
particular plant, PPO activity varies from one organ
to another and varies inside an organ, depending on
the tissue considered. PPOs have been found in differ-
ent cell fractions, in organelles (chloroplasts and,
more precisely, in thylakoids, mitochondria, peroxi-
somes) where the enzymes are tightly bound to mem-
branes and in the soluble fraction of the cell. The
degree of binding to membranes varies with the tissue
and its ontogenic state. Thus, the overall PPO activity
is higher and mostly present in bound forms in
young green fruits, whereas it generally decreases
and the proportion of soluble forms increases in ripe
fruits.
0010Extraction of the PPO activity from plant sources is
complicated by the presence of endogenous phenolic
substrates which are oxidized and then interact with
proteins. Besides destroying activity, this may induce
‘new’ enzymatic forms corresponding to artifacts.
As a consequence, this oxidation has to be prevented
by adding a reducing agent (e.g., ascorbic acid or
a thiol) and/or a phenol-complexing compound
(e.g., polyamide, polyvinylpolypyrrolidone (PVPP),
or polyethylene glycol) to the extracting solution.
Solubilization after preparation of an acetone powder
is a frequently used procedure. Detergents (Triton
OH
PPO
(cresolase)
1
/
2
O
2
PPO
(catecholase)
1
/
2
O
2
Laccase
1
/
2
O
2
PPO
(catecholase)
1
/
2
O
2
(1)
(2)
R
OH
OH
R
(3)
OH
OH
R
OH
HO
O
O
R' R'
or
O
O
R
O
O
R
OH
R
O
R
OH
H
2
O
H
2
O
H
2
O
O
fig0001 Figure 1 Reactions catalyzed by polyphenol oxidases. Repro-
duced from Browning: Enzymatic-Biochemical Aspects. Encyclo-
paedia of Food Science, Food Technology and Nutrition, Macrae R,
Robinson RK and Sadler MJ (eds), 1993, Academic Press.
BROWNING/Enzymatic – Biochemical Aspects 679

X100 or X114) are also used, but probably induce
modifications of the enzyme structure and properties.
Most of the purification procedures are based on
fractional precipitation by ammonium sulfate
followed by one or several chromatographic steps.
Until the mid-1980s, the molecular mass of PPOs
was believed to be around 40–45 kDa. However,
PPOs from a number of plant species have now been
characterized with molecular masses ranging from 20
to 180 kDa. This disparate estimation of molecular
mass, traducing the heterogeneity and multiplicity
of forms of the enzyme, can partly be attributed to
artifacts of protein isolation and electrophoresis, as
exposed above. Moreover, they can correspond to
small multigene families for PPO. In fact, PPO genes
characterized from different leaf and fruit plant
species (including faba bean, apple, tomato, potato,
spinach, Virginian pokeweed, and grape berry) are
present in the plant genome as gene families. To
date, cDNAs for PPOs cloned from these various
plants have been found to encode mature peptides
of 56–67 kDa with 8–12 kDa putative transit pep-
tides. The nuclear encoded-plastid protein is thus
translocated as a precursor with this transit peptide
that can direct its transport into the thylakoid mem-
brane or lumen. The copper content of various plant
PPOs has not yet been well established, but their
deduced amino acid sequences from the cloned
PPOs genes have revealed two highly conserved
copper-binding domains, showing significant hom-
ology with that of closely related bacterial, fungal,
or mammalian tyrosinases.
0011 Most of the PPOs studied show an optimum activ-
ity between pH 4 and 7. However, there are often
discrepancies in the published values for PPOs from
the same source. In addition, several reports indicate
differences in pH optimum depending on cultivars
and maturity. These differences have been attributed
to variations in the proportions of isoenzymes which
have distinct pH optima. Moreover, pH optima were
found to vary with the phenolic substrate used for
several enzymatic preparations. The effect of tem-
perature on PPO activity has been less investigated
than that of pH. The optimum temperature ranges
from 15 to 40
C, depending on the same factors as
pH.
0012 Laccases (EC1.10.3.2) The laccases catalyze the
oxidation of o- and p-diphenols into their correspond-
ing quinones according to reaction (3) in Figure 1.
They occur much less frequently in the plant kingdom
than PPOs. Their presence has been mainly reported
in many fungi and in species of the genus Rhus (e.g.,
the Japanese lacquer tree). They are almost absent
from fruits and vegetables, with the exception of
certain peach cultivars and apricots. Nevertheless,
the distinction between PPO and laccase activities is
not always clear-cut since the presence of endogenous
phenols can induce the coupled oxidation of
p-diphenols and therefore leads to a false conclusion
on the presence of a laccase type of activity. Selective
inhibitors can be used to ascertain the type of activity.
Thus, cinnamic acid, salicylhydroxamic acid, 4-hex-
ylresorcinol, phenylhydrazine, and carbon monoxide
inhibit PPO activity more specifically, whereas cetyl
trimethyl ammonium bromide, a cationic detergent,
is more specific for laccases.
0013Most fungi laccases are glycoproteins with a basic
subunit consisting of a single polypeptide chain of 50–
70 kDa containing a considerable amount of carbo-
hydrate (10–45%) and four atoms of copper. The
effect of pH on laccase activity is similar to that
observed for PPO activity, i.e., an optimum pH
which ranges between 4 and 7.5 and depends on the
substrate being used.
0014Peroxidases (EC1.11.1.7) The peroxidases are en-
zymes whose primary function is to oxidize hydrogen
donors at the expense of peroxides. They are highly
specific for hydrogen peroxide, but they accept a wide
range of hydrogen donors, including polyphenols.
The overall reactions catalyzed are shown in eqns
(1) and (2).
AH
2
þ H
2
O
2
! A þ 2H
2
O ð1Þ
2AH þ H
2
O
2
! AA þ 2H
2
O ð2Þ
0015Peroxidases are glycoproteins with a hematin com-
pound as cofactor. Their molecular weights range
between 30 and 55 kDa. Depending on the enzyme
source, the isoenzyme considered, and the hydrogen
donor substrate, the optimum activity is between pH
4 and 7. In many, but not all cases, the peroxidase
isoenzymes are activated by calcium ions. The higher
thermostability of some isoenzymes is well known,
and the residual peroxidase activity after blanching
is often used as an index of thermal treatment.
0016Although peroxidases are widely distributed,
especially in plants, they generally appear to be little
involved in enzymatic browning of fruits and vege-
tables following a mechanical stress. The explanation
could be that the peroxidase activity is limited by
the internal level of hydrogen peroxide. However,
their involvement in slow processes such as internal
browning during cold storage of fruits is possible.
0017Nevertheless, the direct involvement of peroxidase
in browning still needs confirmation, just as does that
of laccase which may not be present in some fruits
and vegetables. Therefore, the following sections will
be mainly devoted to PPO activity.
680 BROWNING/Enzymatic – Biochemical Aspects
Substrates
0018 Susceptibility to browning varies widely from one
plant to another. This variation is linked to both
quantitative and qualitative aspects of their phenolic
content. Thus, browning after bruising of fruit culti-
vars with similar amounts of total phenolics can be
either more or less intense. Among the wide variety of
phenolic compounds found in fruits and vegetables,
only a small number serves as direct substrates for
PPO. Caffeic acid derivatives and monomeric flavan-
3-ols (mainly (þ)-(gallo)catechin and ()-(gallo)epi-
catechin) often appear to be the best substrates. Other
quantitatively important classes of phenols, namely
anthocyanins, flavonols, and condensed forms of
flavan-3-ols (tannins), are weakly if not directly
oxidized by PPO. The same holds for other less
important classes of phenols (flavones, flavanones,
flavononols, chalcones, and dihydrochalcones). This
restricted activity is probably related to the presence
of a sugar moiety in many of these molecules which
could cause steric hindrance since the aglycone forms
are often good substrates of PPO. Nevertheless,
phenolic compounds which are not direct substrates
can actively participate in browning through coupled
oxidation reactions. Thus, in model systems, it has
been shown that the degradation of anthocyanins,
procyanidins, and flavonols by PPO is greatly accel-
erated in the presence of caffeic acid derivatives or
catechins. The o-quinones enzymatically formed
from either of the latter compounds are able to pro-
mote cooxidation reactions leading to both the deg-
radation of the former compounds and the
regeneration of good substrates for the enzymatic
reaction. This degradation and the phenolic copoly-
merization resulting from nonenzymatic coupled oxi-
dations (reactions (2) and (3) of Figure 2) lead to
products which may be intensely brown.
0019Many studies have been devoted to the specificity
of PPO towards phenolic substrates. Usually, the ap-
parent Michaelis constant (K
m
) is higher than 1 mmol
l
1
, indicating a relatively low affinity. However, for a
particular substrate, the K
m
values can vary widely
depending on the PPO source, and the same holds
for the relative rate of oxidation among different
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
R
R
R
R
R
R
R
R
R
R
R
(1)
(2)
(3)
O
O
O
O
O
O
O
O
O
O
HO
HO
HO
HO
O
2
PPO
H
2
O
o-Quinone
Brown
polymers
or
R'
R'
R' R'
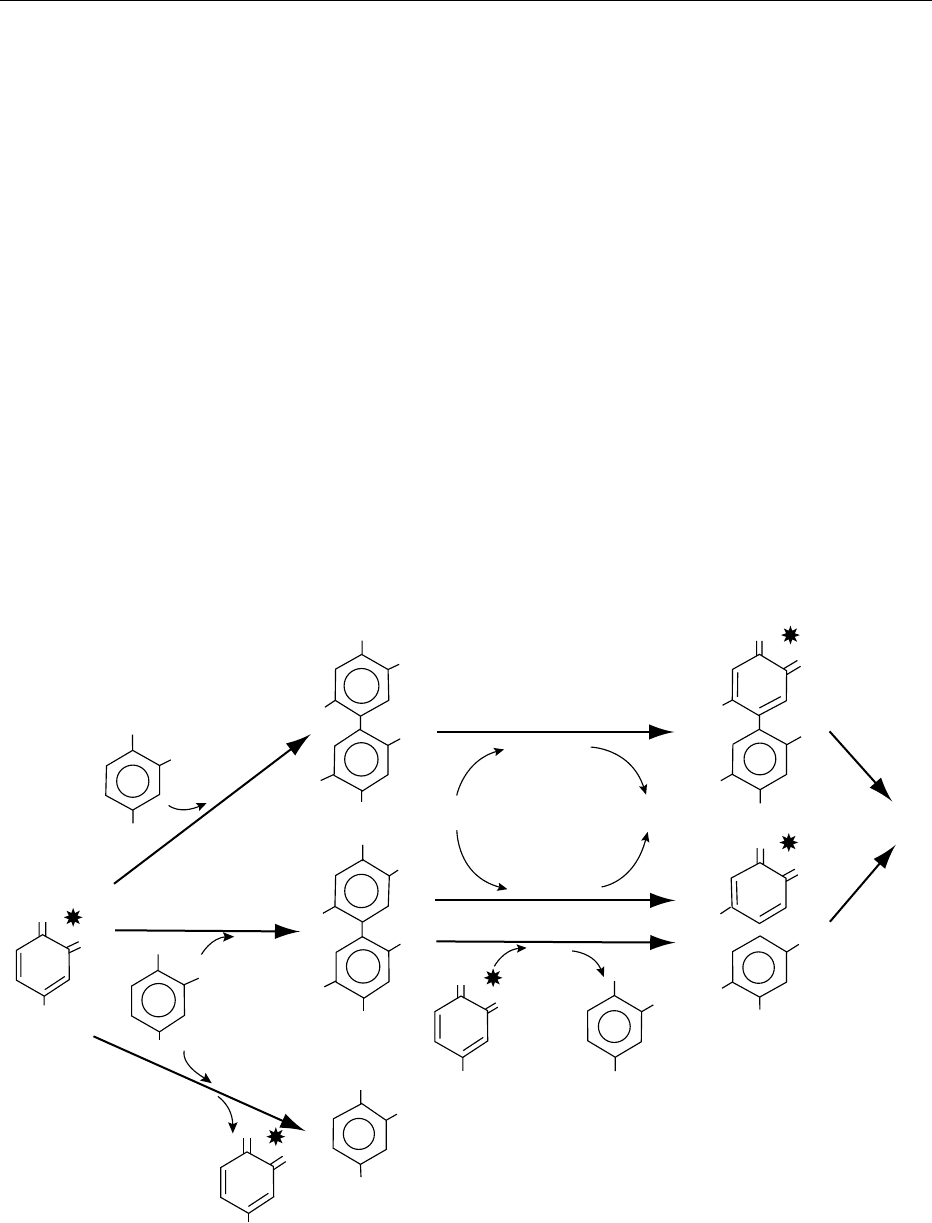
fig0002 Figure 2 Reactions of o-quinones with phenolic compounds. (All reactions are nonenzymatic except those with polyphenol oxidases
(PPOs). Reactions (2) and (3) are able to regenerate the original phenol.) Products with differing color intensities are indicated by
asterisks. Reproduced from Browning: Enzymatic-Biochemical Aspects. Encyclopaedia of Food Science, Food Technology and Nutrition,
Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic Press.
BROWNING/Enzymatic – Biochemical Aspects 681

phenolics (Table 1). It is often suggested that the
preferred substrate is the most abundant phenolic,
although this is not always true.
0020 By contrast, few studies have concerned oxygen,
the other substrate. Steady-state kinetics carried out
on PPO indicate that it probably follows an ordered
Bi-Bi mechanism in which the oxygen binds first. The
reported values for equilibrium constant (K
mapp
O
2
)
are in the range 0.1–0.5 mmol l
1
, corresponding to a
rather low affinity for the oxygen (compared to, for
example, cytochrome oxidase: 0.5–1 mmol l
1
). (See
Oxidation of Food Components; Phenolic Com-
pounds; Tannins and Polyphenols.)
Reaction Products
0021 The primary products of enzymatic oxidation are
o-quinones. These molecules have different spectral
properties and their color depends on the pH and the
phenol from which they originate. Some of the molar
extinction coefficients at maximum wavelengths are
given in Table 2, and show a wide range of variation.
0022 The colors are different from their precursors, since
after oxidation catechin is bright yellow, chlorogenic
acid is dull orange-yellow, whereas DOPA is pink.
Moreover, the o-quinones are reactive compounds,
as illustrated in Figures 2 and 3. Consider Figure 2
for the reactions with phenolic compounds. o-Quin-
ones can react with another phenol molecule,
resulting in a dimer of the original phenol (reaction
(1) of Figure 2). This dimer, having an o-diphenolic
structure, can be subject to reoxidation either enzy-
matically or by another o-quinone and gives larger
oligomers with different color intensities. The o-quin-
ones can also react with a different phenol molecule,
either leading to a copolymer (reaction (2) of Figure 2)
or regenerating the original phenol and giving a dif-
ferent o-quinone (reaction (3): coupled oxidation of
Figure 2). In Figure 3, corresponding to reactions
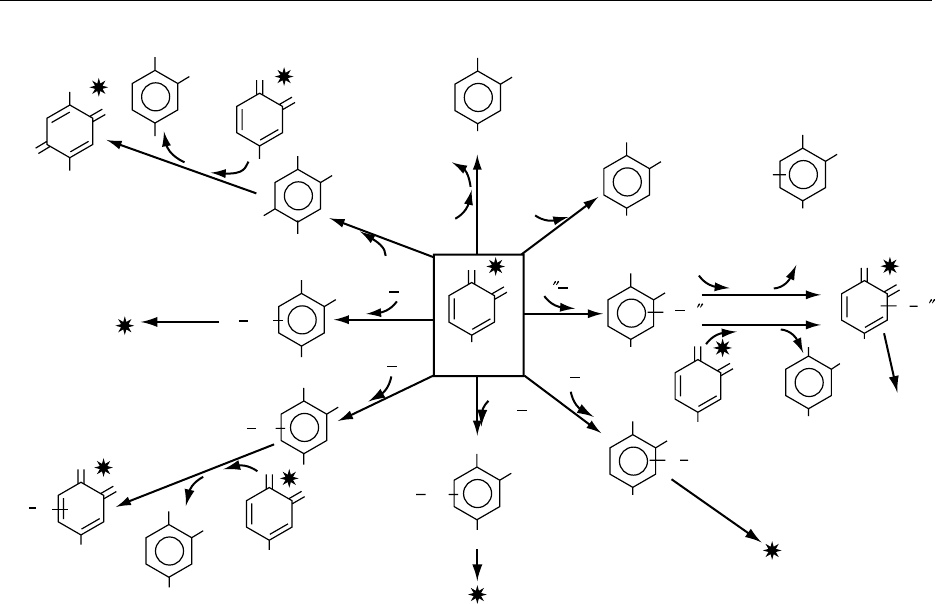
with nonphenolic compounds, another coupled oxi-
dation reaction is observed with ascorbic acid (reac-
tion (1) of Figure 3), since the phenol is regenerated
with the simultaneous formation of dehydroascorbic
acid.
0023With sulfites colorless addition compounds are
formed, together with regenerated diphenol (reaction
(2) of Figure 3). The o-quinones can form additional
compounds with thiol groups by nucleophilic substi-
tution (reactions (3) and (4) of Figure 3). Cysteine,
either free or bound in small peptides (e.g., glu-
tathione) or in large proteins, generates colorless
compounds. However, due to their o-diphenolic
structures, these compounds can be either oxidized
by laccase or react with excess o-quinones (by a
coupled oxidation mechanism) and form intensely
colored products. The same kind of addition reac-
tions occur with amino groups (primary or secondary
amines), although a little less readily ( (reactions (5–7)
of Figure 3). Furthermore, substitutions with other
thiol or amino groups of proteins may occur, leading
to intra- and intermolecular cross-links. Lastly, water
slowly adds to the o-quinones to form triphenols
which are readily oxidized by excess o-quinone
(by a coupled reaction mechanism), leading to the
p-quinones (reaction (8) of Figure 3). (See Amines;
Ascorbic Acid: Properties and Determination.)
tbl0001 Table 1 Michaelis constant (K
m
: mmol l
1
) andV
m
(maximal rate) values (expressed as a percentage of the V
m
of chlorogenic acid) of
polyphenol oxidases from different sources for three common natural substrates
Apple Grape Pear Peach Apricot Potato
V
m
a
K
m
a
V
m
b
K
m
c
V
m
b
K
m
c
V
m
b
K
m
c
K
m
c
Chlorogenic acid 100 4.2 100 2.5 100 16.1 100 1.2 1.4
(þ)-Catechin 58 6.2 64 1 60 2.1 373 0.74
Caffeic acid 8.1 0.14 69 5.5 43 61 0.5 2.4–2.9
Adapted from:
a
Janovitz-Klapp A, Richard F, Goupy P, Nicolas J (1990) Kinetic studies on apple polyphenol oxidase. Journal of Agricultural and Food Chemistry 38:
1437–1441.
b
Macheix JJ, Fleuriet A, Billot J (1990) Fruit Phenolics. Boca Raton: CRC Press.
c
Vamos-Vigyazo L (1981) Polyphenol oxidase and peroxidase in fruits and vegetables. CRC Critical Reviews in Food Science and Nutrition 15: 49–127.
tbl0002Table 2 Molar extinction coefficients of quinones from different
o-diphenolic substrates of polyphenol oxidases
Substrate Wavelength (nm) Extinction
coefficient
Pyrocatechol
a
390 1417
3,4-Dihydroxyphenylacetic acid
a
390 1311
4-t-Butylcatechol
a
400 1150
4-Methylcatechol
a
400 1400
L-DOPA
a
480 3388
Hydrocaffeic acid
a
412 1124
Chlorogenic acid
b
420 2000
(þ)-Catechin
b
380 1200
Adapted from:
a
Waite JH (1976) Calculating extinction coefficients for enzymically
produced o-quinones. Analytical Biochemistry 75: 211–218.
b
Rouet-Mayer MA, Ralambosoa J, Philippon J (1990) Roles of o-quinones
and their polymers in the enzymic browning of apples. Phytochemistry 29:
435–440.
682 BROWNING/Enzymatic – Biochemical Aspects
0024 The reactivity (or in other words, stability) of the
o-quinones in these different cases is very variable. It
depends strongly on the substituted nature of the
parent phenol, and on the medium (composition,
pH, temperature, etc.). Thus, in the same conditions,
the o-quinones derived from 4-methylcatechol are
more stable than those from chlorogenic acid which,
in turn, are more stable than those from catechins.
Obviously, the presence of reactive molecules, with
amino or thiol groups, in the medium can greatly
affect the stability of the o-quinones. Moreover, the
reactivity with reducing compounds, namely those
entering in coupled oxidation reactions, is under the
control of the redox potentials of the systems
involved. Thus, the o-quinones of chlorogenic acid
are able to cooxidize catechins into o-quinones of
catechin and regenerate chlorogenic acid, whereas
the reverse is not true.
Inhibition of Enzymatic Browning
0025 Enzymatic browning is often an undesirable reac-
tion and its prevention is a major concern for food
scientists. Therefore, a large part of the many studies
devoted to enzymatic browning concern its control.
This section gives only the physicochemical basis for
the control of the reaction. The more applied view of
the problem in food technology will be described in
the next article. The different ways of browning con-
trol can be divided into three classes, depending on
whether they affect enzymes, substrates, or reaction
products. However, some inhibitors are able to act
simultaneously on more than one of these factors.
Action on Enzymes
0026First, heat treatment is often employed, and usually
short exposure to temperatures between 70 and 90
C
is sufficient to inactivate PPOs. High-pressure treat-
ments have also been proposed for the denaturation of
PPO. Very high pressures (400–500 MPa) are needed
to inactivate the enzyme at 25
C. However, PPO
appears to be more vulnerable to high-pressure CO
2
treatment (close to 6 MPa) or to a combined treatment
of heat and ultrasonic waves under moderate pressure
(manothermosonication). Since the optimum pH of
most PPOs lies between pH 4 and 7 (see earlier) on
OH
O
O
R
R
OH
OH
OH
R
OH
OH
H
2
O
NHPr
Ox
R
OH
OH
R
R
O
O
R
O
O
R
O
O
R
O
O
OH
OH
R
OH
OH
R
Ox
OH
OH
R
OH
OH
R
OH
Ox
SPr
NHAA
Pr NH
2
H
2
O
HO
O
R
O
O
R
O
OH
R
R
DHA
SO
2−
SO
−
Asc A
(8)
(7)
(3)
(4)
(5)
(6)
(1) (2)
OH
OH
OH
R
Laccase
Brown
polymers
OH
OH
and
R
OH
O
2
OH
Pr SH
RSH
Pro NH
Pro N
Pro N
NH
2
AA
S
R
S
or
R
3
3
fig0003 Figure 3 Reactions of o-quinones with nonphenolic compounds. (All reactions are nonenzymatic except those with laccase.
Reactions (1), (2), (3), (6), and (8) are able to regenerate the original phenol.) Products with differing color intensities are indicated
by asterisks. Ox, further oxidation reactions by oxygen or o-quinone; Pr-SH and Pr-NH
2
, proteins; Pro-NH, proline; AA-NH
2
, amino
acids; Asc A, ascorbic acid; DHA, dehydroascorbic acid; R
00
SH, small thiol compounds (e.g., cysteine and glutathione). Reproduced
from Browning: Enzymatic-Biochemical Aspects. Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
BROWNING/Enzymatic – Biochemical Aspects 683

the one hand, and the phenols are more readily oxi-
dized when the pH increases on the other, acidifica-
tion below pH 4 is often proposed, although PPOs are
still active in some cases (grape, apple, etc.).
0027 Besides these general treatments, more specific
chemical inhibitors are known. PPOs having copper
as a prosthetic group are inhibited by many metal-
chelating agents such as cyanide, azide, diethyldithio-
carbamate, and ethylxanthate, although inhibition
depends on the source of the PPO. Inhibition by halide
ions observed in numerous cases is pH-dependent,
increasing when the pH decreases; it may be caused
by the formation of a complex between the halide ion
and copper which is enhanced by low pH values. Due
to their structural resemblance to phenolic substrates,
aromatic carboxylic compounds are usually found to
be competitive inhibitors. As has been shown for
apple PPOs, the inhibitory properties are highly de-
pendent on structure (Table 3). Thus, for a particular
substituent, inhibition decreases in the order cin-
namic, benzoic, phenylpropionic, and phenylacetic
acid. In each series, inhibition is slightly enhanced
by p-hydroxy substitution and greatly reduced by
o-methoxy substitution. Moreover, the presence of a
benzene nucleus is not an absolute requirement for
the inhibitory effect since sorbic acid, an aliphatic
carboxylic acid with two conjugated double bonds,
is almost as effective as benzoic acid. In all cases,
inhibition increases as pH decreases and it was
shown that the un-ionized form of the carboxylic
group is mainly responsible for inhibition.
0028 More recently, substituted resorcinols have been
recognized as PPO inhibitors. Although resorcinol is
a poor PPO inhibitor, the 4-substituted resorcinols
exhibit the highest inhibition properties. Among
them, the 4-hexylresorcinol has been proposed as a
valuable sulfite alternative for use in the food industry
in order to prevent enzymatic browning. It has been
shown that 4-hexylresorcinol inhibits PPO in a non-
classical mechanism involving the rapid formation
of an enzyme–inhibitor complex which undergoes a
slow reversible reaction (slow-binding competitive
inhibitor).
Action on Substrates
0029Inhibition of enzymatic browning can also be
obtained by removal of one of the two substrates
(oxygen and phenolic compounds) from the reaction
medium. Complete oxygen removal is the most
satisfactory way to control the phenolic oxidation
catalyzed by PPOs. However, although this method
may be applied to dead tissues, either by creating a
physical barrier to oxygen diffusion or by vacuum
infiltration (a more detailed description on this sub-
ject will be given in the next chapter), it is inapplic-
able to living tissues due to the risk of metabolism
deviations caused by anaerobic conditions. Concern-
ing the phenolic substrates, two methods are avail-
able. The first one is the physical elimination by
ultrafiltration or by specific adsorbents. In the latter
case, the most widely used, polyvinylpyrrolidone
(PVP) and its insoluble form (PVPP), are very effective
in controlling enzymatic browning. Thus, PVP has
also been shown to be a competitive inhibitor of
PPOs. Other complexing agents of phenols can be
used, such as polyethylene glycol or polyamide. In
the same way, borate acts by complexing the
o-dihydroxy groups of phenolic substrates. Lastly,
the inhibitory properties of b-cyclodextrins, observed
in apple juice browning, are related to their abilities
to form inclusion complexes with PPO substrates. A
similar mechanism of browning inhibition could be
ascribed to some polysaccharides such as carragenan,
amylose sulfate, xylan, and chitosan.
0030The second way of removing phenolic compounds
is by their modification. This can be performed by
two kinds of enzymes. The first modification is
methylation of the o-diphenolic substrates of PPOs
by an O-methyltransferase (e.g., caffeic acid is con-
verted to ferulic acid). Unfortunately, this elegant
tbl0003 Table 3 Inhibition constants of sodium halides and some carboxylic acids for apple polyphenol oxidases at pH 4.5. All are
competitive inhibitors, with the exception of sodium chloride, which is noncompetitive
Inhibitor K
i
(inhibition constant) (mmoll
1
)Inhibitor K
i
(mmoll
1
)
Sodium iodide 117 Sodium bromide 106
Sodium chloride 20 Sodium fluoride 0.07
Benzoic acid 0.64 p-Hydroxybenzoic acid 0.57
Vanillic acid 10 Syringic acid 34.5
Cinnamic acid 0.092 p-Coumaric acid 0.04
Ferulic acid 0.29 Sinapic acid 15
Phenylacetic acid 13 Phenylpropionic acid 1.4
p-Hydroxyphenylpropionic acid 1.1 Sorbic acid 0.51
Adapted from Janovitz-Klapp A, Richard F, Goupy P, Nicolas J (1990) Inhibition studies on apple polyphenol oxidase. Journal of Agricultural and Food
Chemistry 38: 926–931.
684 BROWNING/Enzymatic – Biochemical Aspects
