Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

Kanarek R (1997) Psychological effects of snacks and
altered meal frequency. British Journal of Nutrition 77:
S105–S120.
Kennedy E and Davis C (1998) US Department of Agricul-
ture school breakfast program. American Journal of
Clinical Nutrition 67: 798S–803S.
Myers A, Sampson A, Weitzman M, Rogers B and Kayne H
(1989) School breakfast program and school perform-
ance. American Journal of Diseases of Childhood 143:
1234–1239.
Pollitt E (1990) Malnutrition and Infection in the Class-
room. Paris: UNESCO.
Pollitt E, Cueto S and Jacoby ER (1998) Fasting and cogni-
tion in well and undernourished school children: a
review of three experimental studies. American Journal
of Clinical Nutrition 67: 779S–784S.
Powell C, Walker S, Chang S and Grantham-McGregor S
(1998) Nutrition and education: a randomized trial of
the effects of breakfast in rural primary school children.
American Journal of Clinical Nutrition 68: 873–879.
Richter L, Rose C and Griesel R (1997) Cognitive and
behavioral effects of a school breakfast. South African
Medical Journal 87: 93–100.
Wyon D, Abrahamsson L, Jartelius M and Fletcher R
(1997) An experimental study of the effects of energy
intake at breakfast on the test performance of 10-year-
old children in school. International Journal of Food
Sciences and Nutrition 48: 5–12.
Breast-feeding See Infants: Nutritional Requirements; Breast- and Bottle-feeding; Weaning; Feeding
Problems; Lactation: Human Milk: Composition and Nutritional Value; Physiology
BROWNING
Contents
Nonenzymatic
Toxicology of Nonenzymatic Browning
Enzymatic – Biochemical Aspects
Enzymatic – Technical Aspects and Assays
Nonenzymatic
Jennifer M Ames, University of Reading, Reading, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The term ‘nonenzymatic browning’ refers to the
chemical reactions that result in the formation of
brown color when food is heated. In contrast to
enzymatic browning, no enzymes are involved in
nonenzymatic browning reactions. The most import-
ant nonenzymatic browning reaction is the Maillard
reaction, which encompasses the cascade of reactions
that occur when reducing sugars are heated with
compounds possessing a free amino group (e.g.,
amino acids, amines, and proteins) and which result
in thousands of reaction intermediates and products.
The Maillard reaction is named after the French
scientist, Louis Camille Maillard, who first investi-
gated reducing sugar–amino acid interactions in
1912. Other nonenzymatic browning reactions in-
clude Maillard-type reactions between amino com-
pounds and other compounds possessing a free
carbonyl group, e.g., ascorbic acid and lipid oxida-
tion products. A further type of nonenzymatic
browning is caramelization, which occurs when
sugars discolor in the absence of amino compounds.
In all types of nonenzymatic browning mentioned,
the chemistry involved is very similar. In this article
(unless stated otherwise), the terms ‘nonenzymatic
browning’ and the ‘Maillard reaction’ are used inter-
changeably and refer to reactions between reducing
sugars and amino compounds.
0002As well as giving brown colors, the Maillard reac-
tion results in various other outcomes in foods. These
include development of flavor, increase in antioxida-
tive capacity, loss of nutritional and functional prop-
erties, and formation of compounds with potential
adverse effects on human health. Since the Maillard
BROWNING/Nonenzymatic 665
reaction has so many important consequences for
food quality, it is without doubt one of the most
significant reactions that occur in food. Various
factors affect the course of the Maillard reaction.
These include the nature of the reactants, tempera-
ture, and time of heating, the pH and water activity
(a
w
) of the food and the presence of reaction inhibi-
tors, e.g., sulfite. Due to the importance of the
reaction, its control is important for food quality
and kinetic approaches to modeling and control are
showing considerable promise.
Chemistry
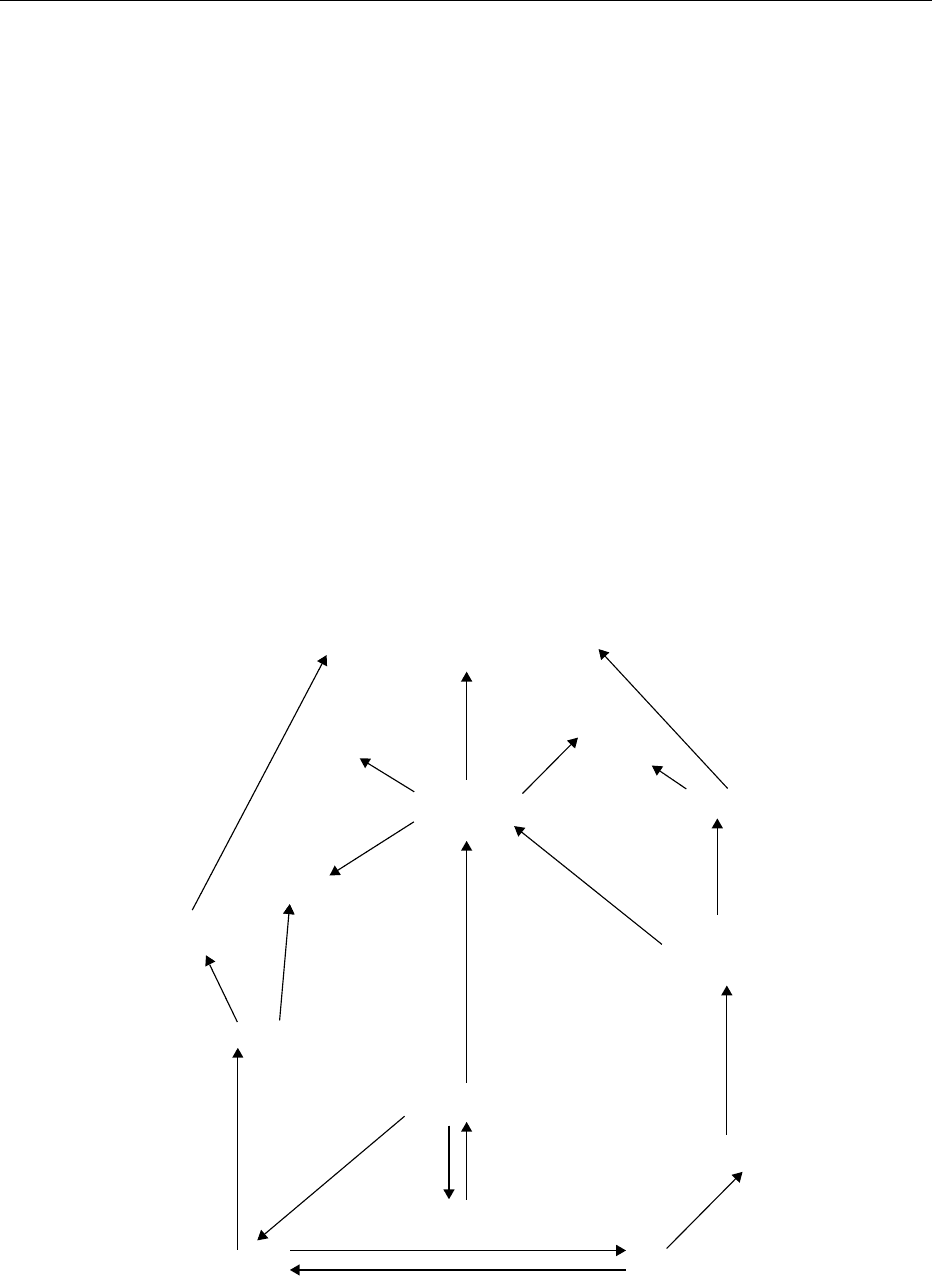
0003 A summary of the chemical pathways comprising the
Maillard reaction is given in Figure 1. The early stage
of the reaction is well understood. It involves the
condensation of the reducing sugar with the amino
compound to give an N-substituted glycosylamine
which rearranges to the 1-amino-1-deoxy-2-ketose,
also known as the Amadori rearrangement product
(ARP). The relative importance of the subsequent
pathways largely depends on the pH of the food. At
a pH below 5, the route involving 3-deoxyosone as
the intermediate prevails but, if the pH is above 7,
the pathways involving the 1- and 4-deoxyosones
and the 1-amino-1,4-dideoxyosone are more import-
ant. Since most foods are in the pH range 4–6,
the 3-deoxyosone route usually predominates. The
3-deoxyosone reacts with amino acids to give mela-
noidins (the final products of the Maillard reaction
which are macromolecular and colored), Strecker al-
dehydes via the Strecker degradation (Figure 2) and
pyrroles and furfurals and their derivatives. Enami-
nols, which are products of the Strecker degradation,
subsequently condense to give pyrazines (Figure 2).
The other deoxyosones are much less stable than the
3-deoxyosone. They are converted into reductones
which may react with other intermediates to give
melanoidins. In addition, these deoxyosones (as
well as other Maillard intermediates, e.g., the N-
substituted glycosylamine) readily fragment via retro-
aldolization. The low-molecular-weight products
that result are highly reactive and may participate
Fragmentation
products
Low-molecular-weight colored compounds
and melanoidins
Reductones
Cyclic flavor
compounds
1-Deoxyosone,
4-deoxyosone,
1-amino-1,4-dideoxyosone
Strecker
aldehydes
3-Deoxyosone
Furfurals
Pyrroles
N-substituted
glycosylamine
Reducing sugars +
amino compound
1,2-Eneaminol
Amadori
rearrangement product
(ARP)
2,3-Enediol
Heterocyclic
amines (HAs)
fig0001 Figure 1 Outline of the chemical pathways of the Maillard reaction.
666 BROWNING/Nonenzymatic
in the Strecker degradation, condensation reactions
resulting in cyclic products, and reactions leading to
melanoidins.
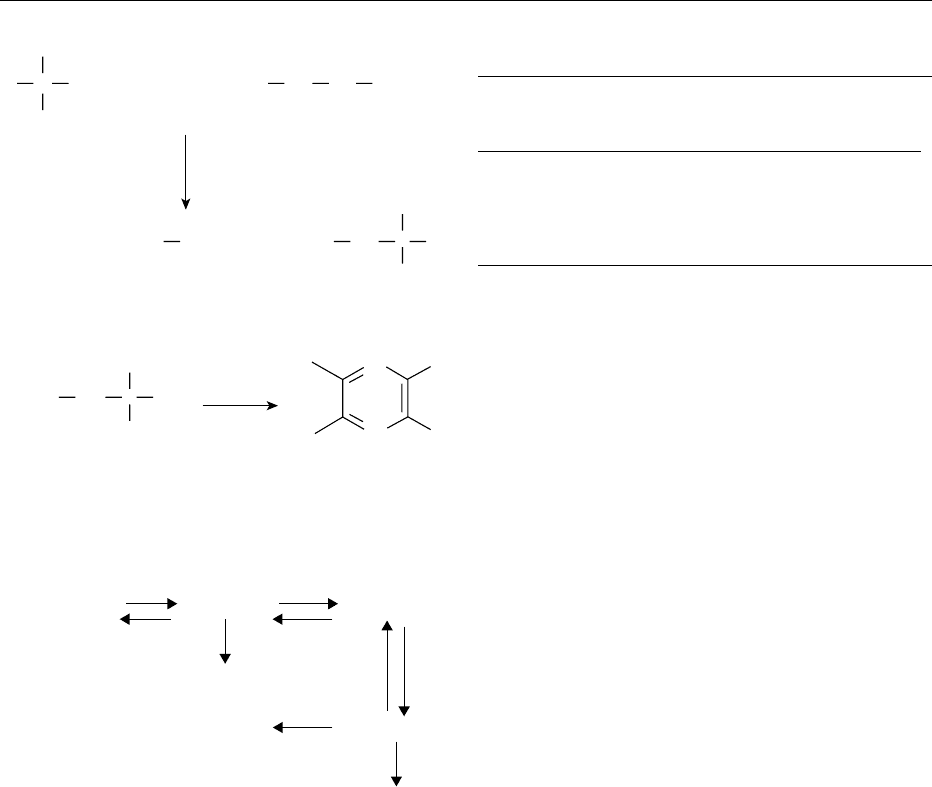
0004 At temperatures above 130
C or at pH values of
below 3 or above 8, sugars degrade in the absence
of amino acids to give key intermediates that also
form in the Maillard reaction (Figure 1), as shown
in Figure 3.
Flavor
0005 Hundreds of flavor compounds formed by the Mail-
lard reaction have been identified. The compounds
concerned are largely those that contribute to aroma
(i.e., they are volatile and are thus perceived by the
nose), although a few components that possess
specific tastes (and are therefore perceived by the
tongue) have also been reported. Roasted coffee,
cooked meat, bread, cookies, breakfast cereals,
roasted nuts, and baked potatoes are among the
foods that owe their particular flavor to the Maillard
reaction. Also, the Maillard reaction is employed by
the flavor industry for the production of process or
reaction flavors. In addition to the development of
desirable flavors, the Maillard reaction is responsible
for off-flavors in certain foods, particularly in com-
modities in which the original fresh flavor of the
product is desired. Thus, it can lead to cooked or
stale flavors in processed orange juice or dried milk
powder. (See Flavor (Flavour) Compounds: Struc-
tures and Characteristics.)
0006The volatile Maillard flavor compounds may be
divided into three groups: those formed from the
sugar, those formed from the amino acid, and those
requiring both the sugar and the amino acid for their
formation (Table 1). Strictly speaking, it is only the
compounds in the latter group that are formed by the
Maillard reaction, i.e., reducing sugar–amino com-
pound interactions. Members of a further category
of aroma compounds are formed by reactions involv-
ing intermediates of both the Maillard reaction and
lipid degradation. These components often possess
fried odors and contribute fried aromas to foods
such as potato chips. Much less is known about the
products of the Maillard reaction that may contribute
to taste. However, it has been established that some
pyrrole derivatives contribute to the bitter taste of
roasted coffee and malt. The structures of some spe-
cific compounds, together with their odor (or taste)
threshold values and contributions to food flavor, are
given in Table 2.
Color
0007The compounds formed early on in the Maillard re-
action are colorless and absorb in the ultraviolet (UV)
region of the electromagnetic spectrum. As the reac-
tion progresses, absorption in the UV region intensi-
fies and begins to tail into the visible region, resulting
in the appearance of yellow, orange, and brown
H
R C COOH + R' CO CO R"
NH
2
CO
2
+ R CHO + R' CO
C
R"
Amino acid
Dicarbonyl, e.g.,
3-deoxyosone
H
NH
2
Strecker
aldehyde
Enaminol
A pyrazine
2 R' CO C R"
H
NH
2
−2H
2
O
−2H
R'
R'
N
N
R"
R"
fig0002 Figure 2 Strecker degradation and pyrazine formation.
Aldose
reducing sugars
1,2-Enediol
Ketose
reducing sugar
2,3-Enediol1-Deoxyosone
3-Deoxyosone
4-Deoxyosone
fig0003 Figure 3 Degradation of reducing sugars at temperatures
above 130
C, or at pH values below 3 or above 8, and in the
absence of amino compounds.
tbl0001Table 1 Volatile compounds derived from the sugar, the amino
acid, and sugar/amino acid interactions
Compounds derived
from the sugar
Compounds derived
from sugar^amino
acidinteractions
Compounds derived
from the amino acid
Furans Pyrroles Sulfur compounds
Pyrones Pyridines Aldehydes
Cyclopentenes Pyrazines Thiazoles
Carbonyls Oxazoles Cyclic polysulfur
compoundsAcids Thiophenes
BROWNING/Nonenzymatic 667
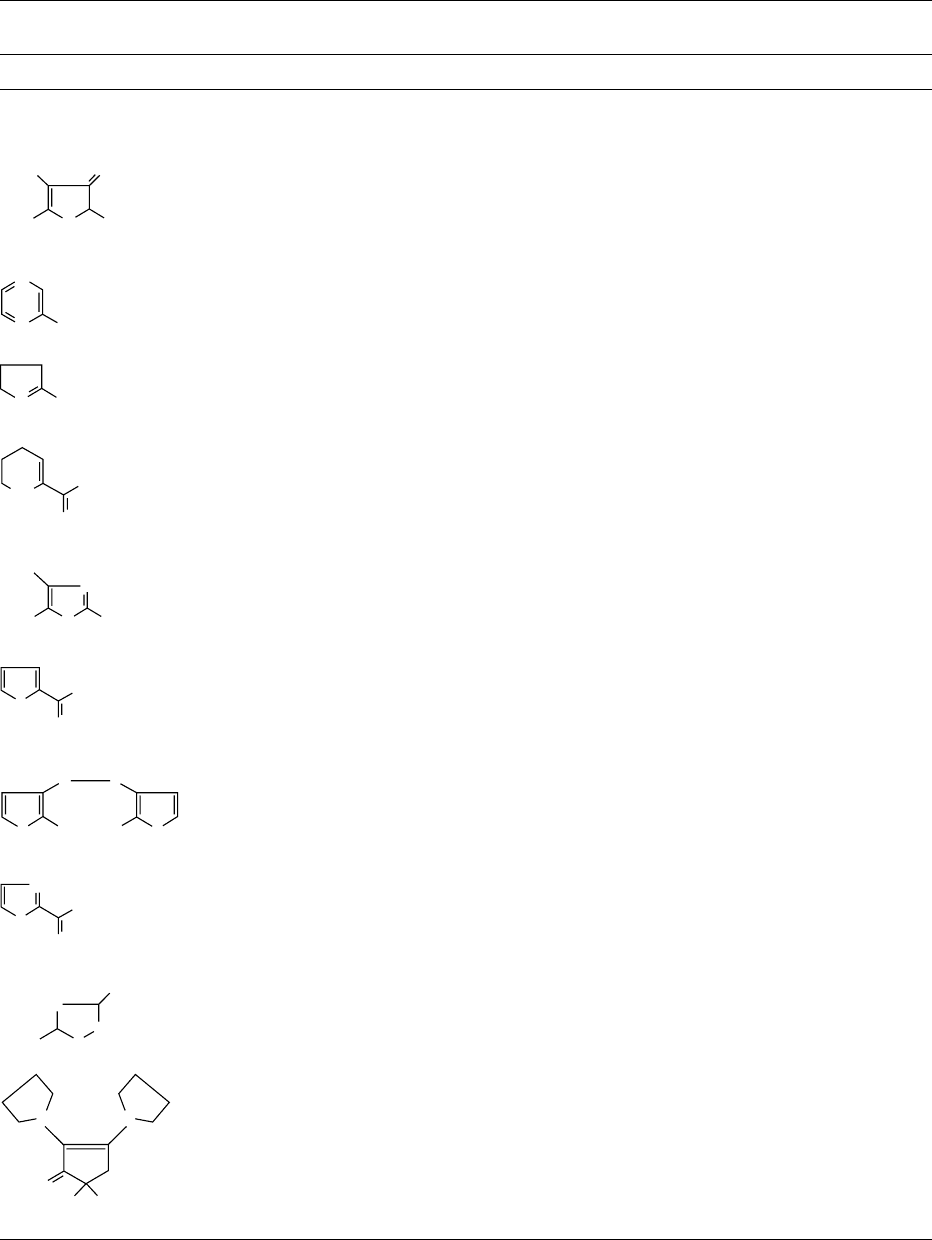
tbl0002 Table 2 Properties of selected flavor compounds
Compound Odor threshold value (mgkg
1
)Odor
Butanedione (diacetyl) CH
3
COCOCH
3
7 Buttery
3-(Methylthio)propanal (methional) CH
3
SCH
2
CH
2
CHO 200 Cooked potatoes
2,5-Dimethyl-4-hydroxy-3(2H)furanone (furaneol) 0.04 Caramel, burnt pineapple, strawberries
Methylpyrazine 6 10
4
Nutty
2-Acetyl-1-pyrroline 0.1 Freshly baked wheat bread crust
2-Acetyl-1,4,5,6-tetrahydropyridine 1.6 Crackers
Trimethyloxazole H
3
C 5 Green, nutty, sweet
2-Acetylthiophene 0.1 Onion-link, mustard-like
bis(2-Methyl-3-furyl) disulfide 2 10
5
Cooked meat
2-Actylthiazole 10 Nutty, popcorn
3-Methyl-5-pentyl-1,2,4-trithiolane Fried chicken
5–10 10
3a
Bitter
a
Taste threshold.
HO
H
3
C
O
O
CH
3
CH
3
N
N
N
COCH
3
NH
O
CH
3
O
N
CH
3
H
3
C
H
3
C
O
S
CH
3
O
O
S
S
CH
3
H
3
C
O
N
S
CH
3
NN
O
OH
H
3
C
S
S
S
CH
3
H
11
C
5
668 BROWNING/Nonenzymatic
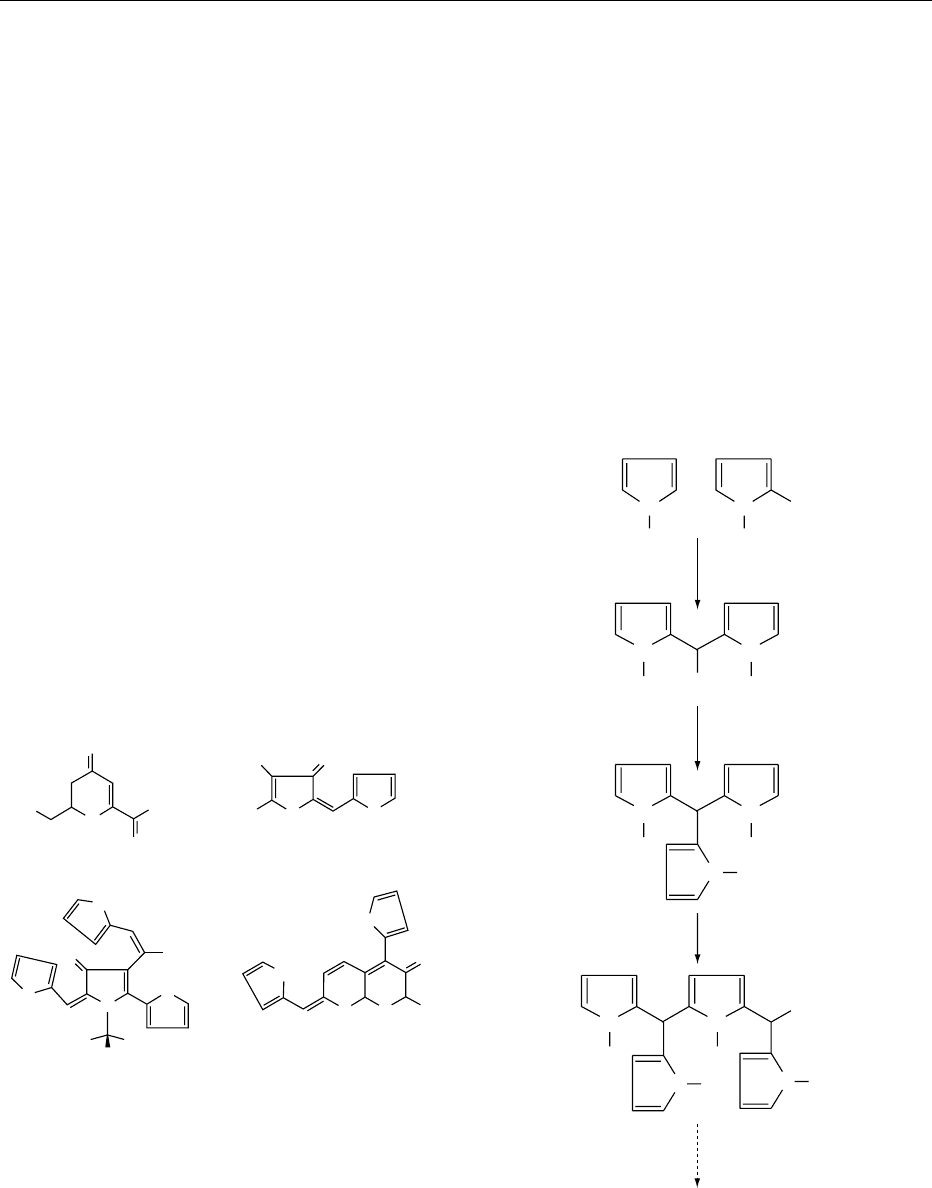
colors. The Maillard reaction is responsible for the
desirable yellow or brown colors in foods, including
bread crust, potato chips, popcorn, cookies, and
other flour confectionery, but it can also lead to
discoloration, e.g., in heated fruit juices and milk
powder.
0008 The colored Maillard reaction products may be
divided into two groups, i.e., the low-molecular-
weight compounds that possess up to about five
linked rings, and the macromolecular melanoidins.
Most work on colored Maillard reaction products
has concerned the low-molecular-weight compounds
isolated from model food systems. A selection of
compounds isolated from aqueous sugar–amino acid
systems, heated with or without additional furfural,
is shown in Figure 4. Progress in the field of low-
molecular-weight colored Maillard products has
advanced rapidly over the last 5 years. Nevertheless,
much remains to be done.
0009 No homogeneous melanoidin has been isolated
from a food or model food (sugar–amino acid) system
to date and no structures for melanoidins isolated
from food are available. Progress concerning the
structures of high-molecular-weight melanoidin-like
structures has been made by investigating the
polymers produced on polymerization of first,
2-hydroxymethyl-N-methylpyrrole in trichloro-
methane and, second, N-methylpyrrole with 2-for-
myl-N-methylpyrrole (or furfural) in methanol.
Figure 5 gives an example of the structures identified
by
1
H/
13
C nuclear magnetic resonance spectroscopy,
fast atom bombardment–mass spectrometry and
matrix-assisted desorption ionization–time of flight–
mass spectrometry. Although the formation of such
regular homopolymers in food systems is unlikely, it
is reasonable to expect that the type of structure
depicted in Figure 5 represents a domain of a food
melanoidin.
0010It has been demonstrated that heating food pro-
teins (casein) in the presence of glucose results in
protein cross-linking and a parallel increase in color
O
HO
N
I
III IV
II
O
O
CHO
O
O
O
O
OO
O
OH
O
N
H COOH
CH
3
CH
3
H
3
C
HO
O
OO
fig0004 Figure 4 Selected low-molecular-weight colored compounds.
I, from xylose and glycine; II, from xylose and glycine or xylose
and lysine; III and IV, from xylose, alanine and furfural. (I:2-
Acetyl-6-(hydroxymethyl)-5,6-dihydro-4H-pyridinone; II: 2-furfury-
lidene-4-hydroxy-5-methyl-3(2H)-furanone; III: 4-[1-formyl-2-(2-
furyl)ethenyl) ]-5-(2-furyl)-2-[(2-furyl)methylidene]-2,3-dihydro-a-
methyl-3-oxo-1H-pyrrole-1-acetic acid; IV: 4-(2-furyl)-7-[(2-furyl)-
methylidene]-2-hydroxy-2H,7H,8aH-pyrano[2,3b]pyran-3-one.)
N
R
N
R
CHO
+
N
R
N
R
OH
VVI
NR
N
R
N
R
N
R
N
R
OH
N
R
N
R
+V, +VI
Up to about 26 units
+ V
+ VI
fig0005Figure 5 Structure of the polymer prepared from N-methylpyr-
role and 2-formyl-N- methylpyrrole.
BROWNING/Nonenzymatic 669
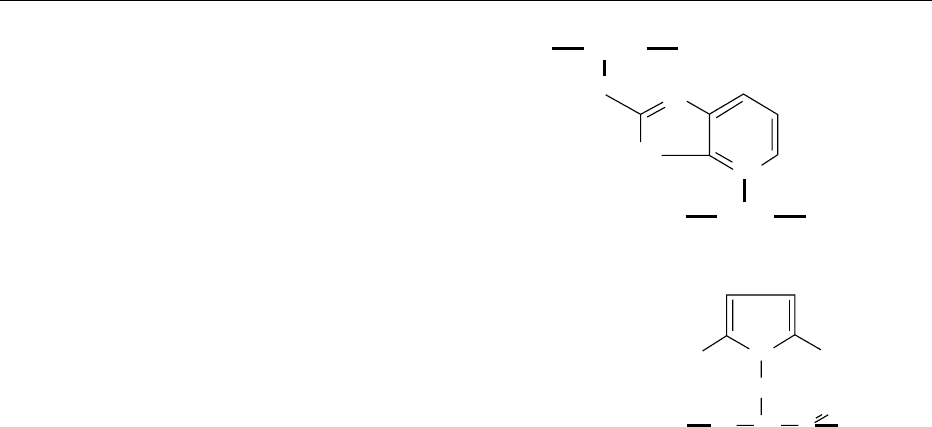
intensity of the reaction products. This color develop-
ment is due to the incorporation of colored Maillard
reaction products into the cross-linked protein.
Therefore it is likely that, in foods, a portion of
the brown color formed on heating is due to low-
molecular-weight colored Maillard products binding
to colorless protein backbones giving protein cross-
linking and browning.
Antioxidant Activity
0011 The Maillard reaction may influence the oxidative
stability of foods because some Maillard reaction
products are able to retard lipid oxidation. An in-
crease in stability may be achieved by modifying the
heat process applied, e.g., to milk, adding Maillard
reaction precursors, e.g., to cookie dough, prior to
heat treatment, or addition of Maillard reaction
products to susceptible foods, e.g., margarine.
0012 An increase in pH and the amino compound–
sugar molar ratio enhances the formation of nondia-
lyzable melanoidins and the inclusion of nitrogen in
the Maillard reaction products. There is a positive
correlation between all these factors and antioxi-
dant activity. Nondialyzable melanoidins can scav-
enge hydroxyl radicals and other active oxygen
species. Antioxidant activity increases with formation
of melanoidins up to a maximum and then decreases
with further heating and browning. Reducing activity
and metal chelating ability are factors that may
account for antioxidant activity. A strong candidate
for such activity is the reductone group. However,
enediol-like reductones and amino reductones
(examples of the latter being those formed by the
Amadori rearrangement) may act as antioxidants or
prooxidants, depending on the reaction conditions,
and the structures responsible for antioxidant activity
remain uncertain. (See Antioxidants: Natural Anti-
oxidants; Role of Antioxidant Nutrients in Defense
Systems.)
Nutritional Effects
0013 The Maillard reaction can result in serious loss of
availability of essential amino acids, both in the free
form and in peptide chains. Loss of the available
amino acid occurs as soon as the ARP is formed. In
most foods, lysine destruction is most significant, due
to its e-amino group. Ascorbic acid and also vitamins
possessing a free amino group, e.g., thiamine, can
participate in Maillard-type reactions leading to loss
in activity of the vitamin concerned. Various Maillard
reaction products may complex nutritionally im-
portant metals, e.g., copper, zinc, iron, thus making
them unavailable. ARPs or more advanced reaction
products fed orally to humans increase zinc excretion
via urine. The effect is more marked in patients fed
intravenously.
0014The posttranslational modification of proteins and
the development of protein cross-links as a conse-
quence of the Maillard reaction reduce protein digest-
ibility (as well as functionality). Pyrraline (identified
in heated milks) and pentosidine (identified in roasted
coffee) are examples of such modifications and their
structures are shown in Figure 6. Reduction in protein
digestibility may be due to decreased access by digest-
ive enzymes to the modified protein or by the direct
inhibition of the enzymes. The main effect is to block
normal tryptic hydrolysis on the carboxyl side of
lysine.
Mutagen Formation
0015More than 20 heterocyclic amines (HAs) that are
products of the Maillard reaction have been identified
at the p.p.b. level in cooked beef, pork, lamb, chicken,
and fish muscle. Amino acids, sugars, and bio-
chemicals present in muscle tissue, e.g., creatinine
and creatine, are all required for their formation.
The structures of a selection of HAs are shown in
Figure 7. The amounts produced increase with tem-
perature and time of cooking; temperatures encoun-
tered during, for example, grilling and frying favor
their formation. Most HAs cause mutations in bac-
teria, mammalian cells and cancer in animals. They
are the most potent mutagens examined by the Ames
test and are able to produce frameshift mutations in
arginine
N
N
N
+
lysine
HN
Pentosidine
HOH
2
C
N
(CH
2
)
4
CHO
NH CH C
O
Pyrraline
fig0006Figure 6 Structures of pyrraline and pentosidine. The peptide
backbone is represented by a solid line.
670 BROWNING/Nonenzymatic
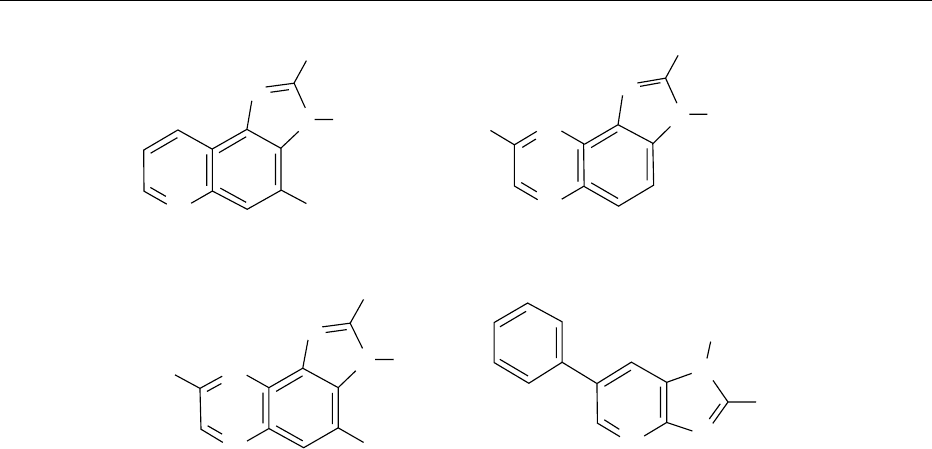
Salmonella and base pair substitutions in hamster
ovary cells. The risk to humans of consuming foods
containing measurable amounts of HAs is difficult to
estimate and requires epidemiological studies. Esti-
mates based on animal cancer data and surveys of
human food intake suggest that the average exposure
to cooked food containing HAs gives a 10
4
risk of
lifetime cancer. This average value is subject to sub-
stantial variation due to differences in diet, cooking
methods, and genetic factors.
0016 Although HAs are the mutagens formed during
the Maillard reaction that have received most
attention, other Maillard products are also reported
to be mutagenic, including methylglyoxal and
5-hydroxymethylfurfural (HMF). (See Mutagens.)
Factors Affecting the Maillard Reaction
0017 The extent and course of the Maillard reaction are
affected by the profile of components in the food
capable of acting as reaction precursors, temperature
and time of heating, pH, and a
w
. The reaction is also
affected by metals, oxygen, and the presence of in-
hibitory agents, e.g., sulfite. When attempting to con-
trol the Maillard reaction, the aim may be to stop it or
to steer it towards a particular outcome in terms of
flavor or antioxidant activity. In any case, the reac-
tion is difficult to stop once it has started.
0018 The Maillard reaction may be minimized by re-
moving one of the required reaction partners, i.e.,
the reducing sugars for protein-rich foods or the
amino compounds for carbohydrate-rich foods. In
jam-making, limiting the cooking time after addition
of sucrose reduces its inversion to glucose and fruc-
tose in the acidic medium. An alternative to removing
the reducing sugars or amino compounds is to process
separately the carbohydrate and protein components
of a food, e.g., cream-style soups.
0019Pentose sugars, e.g., ribose, react faster than hexose
sugars, e.g., glucose and fructose, which in turn react
faster than reducing sugars that are disaccharides,
e.g., maltose and lactose. Amino acids also react at
different rates. Of the free amino acids, when color
formation is measured, lysine and glycine are among
the fastest while cysteine is the slowest. In peptide
chains, the amino acid residues with reactive
side chains, e.g., lysine and arginine, react the fastest.
The amino acid has a large effect on the flavor
formed. Cysteine gives meaty notes, methionine
results in a potato aroma, and cracker and bready
odors are produced by proline.
0020The Maillard reaction increases with temperature.
Q
10
values range from 2 to 8 according to the system
and parameter being measured. Therefore, if the aim
is to stop the reaction, the temperature should be
reduced to as low a level as possible. The profile of
reaction products formed varies according to the tem-
perature and time of heating and it is not possible to
produce the profile obtained at a high temperature by
heating at a lower temperature for a longer time. This
NH
2
NH
2
CH
3
NH
2
NH
2
CH
3
CH
3
CH
3
CH
3
CH
3
N
N
N
N
N
N
N
N
N
N
N
N
N
N
MeIQ
MeIQx
DiMeIQx PhIP
H
3
C
H
3
C
fig0007 Figure 7 Structures of selected heterocyclic amines. MeIQ, 2-amino-3,4-dimethylimidazo[4,5-f]-quinoline; MeIQx, 2-amino-3,
8-dimethylimidazo[4,5-f]quinoxaline; DiMeIQx, 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline; PhIP, 2-amino-1-methyl-6-phenyli-
midazo[4,5-b]pyridine.
BROWNING/Nonenzymatic 671

can be illustrated by comparing the aroma of meat
resulting from boiling at 100
C and from roasting at
200
C. The two aromas are easily distinguished be-
cause different reaction pathways, and thus different
aroma compounds, are favored by the different
cooking methods.
0021 The pH of the system plays a key role in determin-
ing the relative importance of the reaction pathways
followed (see section on Chemistry, above). At pH
values below about 5, the 3-deoxyosone route is
favored while routes involving the other deoxyosones
are favored above pH 7. In fact, there is no sharp cut-
off point. All the pathways operate at all pH values of
relevance to food, the ratio of products being influ-
enced by the pH. Thus, pH is a parameter that can
be manipulated to achieve the required profile of
reaction products.
0022 Since water is a product of the Maillard reaction,
the reaction occurs less readily in high-a
w
foods.
In low-a
w
foods, the reactants are less mobile and
this also impedes the reaction. In practice, the Mail-
lard reaction is favored by intermediate a
w
values
(0.5–0.8). When the aim is to prevent the Maillard
reaction, a
w
is of great concern during the drying or
concentration of food. In such cases, the food must be
taken through the intermediate a
w
stage as quickly as
possible, especially if heat is used. The formation of
individual Maillard reaction products is favored by
different a
w
values and therefore a
w
represents
another means of optimizing the profile of reaction
products formed.
0023 Since the Maillard reaction is so difficult to stop
once it has begun, the food industry may resort to the
use of sulfite (normally a solution of sodium bisulfite
or sodium metabisulfite) to control it. The sulfite
combines with reducing sugars and Maillard inter-
mediates possessing carbonyl and dicarbonyl groups
to inhibit the reaction.
Kinetics and Modeling
0024 The Maillard reaction is much more complex than
most reactions that have been modeled by physical
chemists. However, since the reaction is of such inter-
est to the food industry, attempts have been made to
describe it using kinetics and mathematical equations,
in order to make valid predictions concerning color,
flavor, and antioxidant activity for various food com-
positions processed and stored under different condi-
tions. As a result, it is possible to predict accurately
the color development (absorbance at a single wave-
length of a model system) at a particular time. Rate
constants have also been published for a limited
selection of Maillard aroma compounds. However,
since the Maillard reaction is qualitatively and quan-
titatively affected by many factors, the direct applica-
tion of models may be of very limited use. There is a
great need for further application of kinetics prin-
ciples to the Maillard reaction so that various aspects
of the reaction may be predicted and, ultimately,
controlled in foods.
See also: Antioxidants: Role of Antioxidant Nutrients in
Defense Systems; Browning: Toxicology of
Nonenzymatic Browning; Casein and Caseinates: Uses
in the Food Industry; Flavor (Flavour) Compounds:
Structures and Characteristics; pH – Principles and
Measurement
Further Reading
Ames JM (1992) The Maillard reaction. In: Hudson BJF
(ed.) Progress in Food Proteins – Biochemistry,
pp. 99–153. London: Elsevier Applied Science.
Ames JM and Hofmann T (eds) (2000) Chemistry and
Physiology of Food Colorants. American Chemical
Society Symposium Series 775. Washington, DC: ACS.
Eriksson C (ed.) (1981) Maillard Reactions in Food.
Oxford: Pergamon Press.
Finot PA, Aeschbacher HU, Hurrell RF and Liardon R (eds)
(1990) The Maillard Reaction in Food Processing,
Human Nutrition and Physiology. Basel: Birkha
¨
user.
Friedman M (1996) Food browning and its prevention: an
overview. Journal of Agricultural and Food Chemistry
44: 631–653.
Fujimaki M, Kato H and Namiki M (eds) (1986) Amino-
Carbonyl Reactions in Food and Biological Systems.
Amsterdam: Elsevier.
Hodge JE (1953) Dehydrated foods: chemistry of browning
reactions in model systems. Journal of Agricultural and
Food Chemistry 1: 928–943.
Ikan R (ed.) (1996) The Maillard Reaction. Consequences
for the Chemical and Life Sciences. Chichester: John
Wiley.
Ledl F and Schleicher E (1990) New aspects of the Maillard
reaction in foods and in the human body. Angewandte
Chemie International Edition in English 29: 565–706.
Leong LP and Wedzicha BL (2000) A critical appraisal of
the kinetic model for the Maillard browning of glucose
and glycine. Food Chemistry 68: 21–28.
Namiki M (1988) Chemistry of Maillard reactions: recent
studies on the browning reaction mechanism and the
development of antioxidants and mutagens. Advances
in Food Research 32: 115–184.
O’Brien J and Morrisey PA (1989) Nutritional and toxico-
logical aspects of the Maillard browning reaction in
foods. Critical Reviews in Food Science and Nutrition
28: 211–248.
O’Brien J Nursten HE, Crabbe MJC and Ames JM (eds)
(1998) Maillard Reaction in Foods and Medicine. Cam-
bridge: Royal Society of Chemistry.
Waller GR and Feather MS (eds) (1983) The Maillard Re-
action in Foods and Nutrition. ACS Symposium Series
215. Washington, DC: ACS.
672 BROWNING/Nonenzymatic

BROWNING
Toxicology of Nonenzymatic
Browning
P A Finot, Nestec, Nestle
´
Research Centre, Lausanne,
Switzerland
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 Reactions between reducing sugars and free amino
groups in foods, without any catalytic involvement of
enzymes, lead to nonenzymatic browning (Maillard
reaction) causing a reduction in nutritive value and
certain physiological and/or toxicological effects.
The browning reaction develops during both home
cooking and industrial processing of foods. Whilst
contributing to an improvement of the organoleptic
properties of foods, through aroma development,
browning is often an undesirable side-effect of obliga-
tory heat treatments applied for microbiological
(sterilization and drying) or nutritional (cooking)
reasons and for convenience (storage). Since the
Maillard reaction occurs so frequently and Maillard
reaction products are present in practically all meals,
an understanding of its biological implications is of
importance.
0002 Because of the multiplicity of the food systems, the
complexity of the chemical reactions, and the large
variety of heat treatments involved, any generaliza-
tion on the biological outcome of nonenzymatic
browning of foods is not easy. Nevertheless, a number
of studies of model amino acid/protein and sugar
reaction systems permits a classification of the bio-
logical outcome as: (1) nutritional; (2) physiological;
and (3) toxic (including genotoxic) effects.
Nutritional Effects
0003 The nutritional effects of the Maillard reaction in
foods are due both to the chemical modification of
essential nutrients which thereby become unavailable
(direct effects) and to the presence of Maillard prod-
ucts which reduce the bioavailability and disturb the
metabolism of other nutrients (indirect effect).
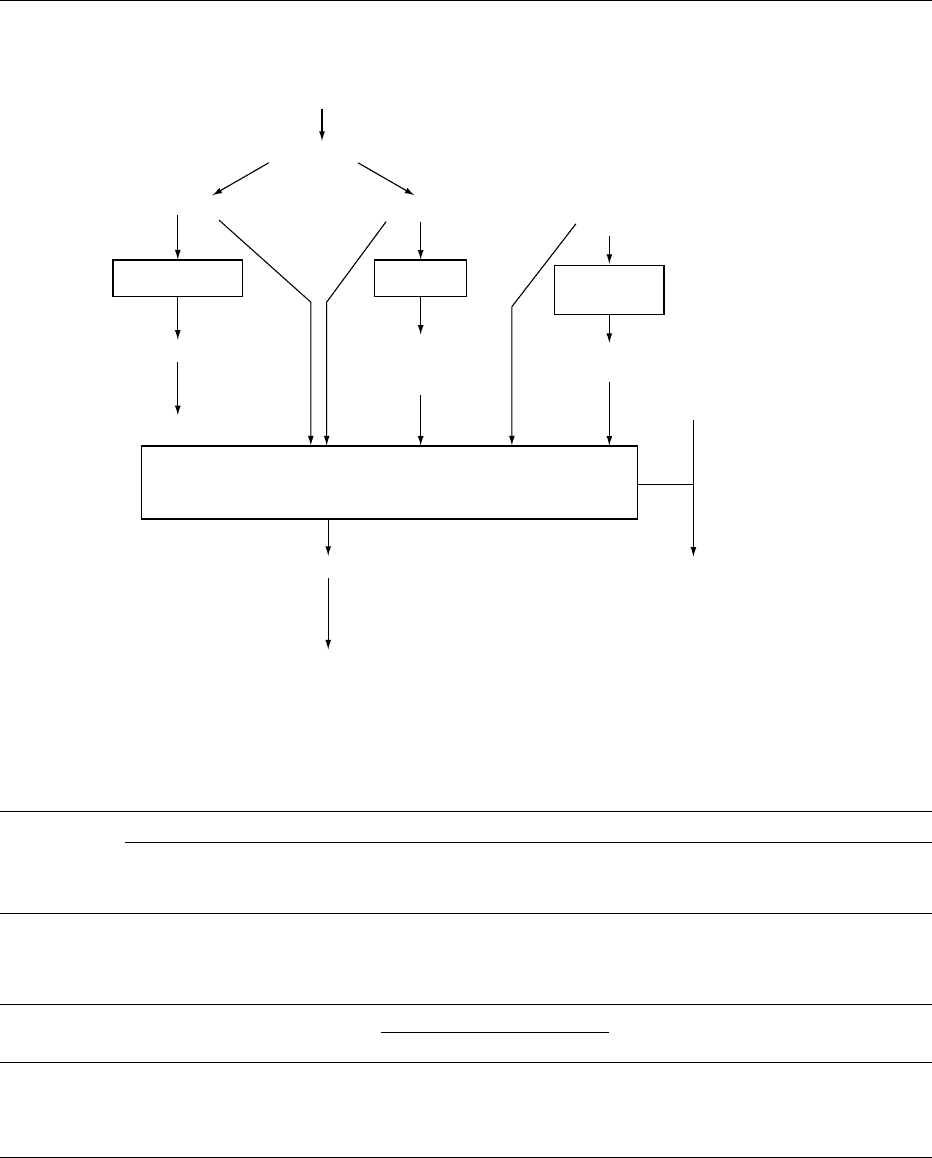
0004 Since it proceeds through many chemical routes
and produces a large number of chemical species, the
Maillard reaction has been divided for clarity and
convenience into two distinct stages, early and ad-
vanced, which are associated with different nutri-
tional and physiological consequences (Figure 1).
The Early Maillard Reaction
0005This first stage involves the reaction between a free
amino group and a reducing sugar to form, through
the Amadori rearrangement, a stable deoxyketose
addition compound, also called the Amadori com-
pound. This is the obligatory step for the continu-
ation of the reaction to the advanced stage.
0006At this Amadori stage, nutritional damage has
already been done. No enzyme in animal tissues
can split these complexes to regenerate the amino
compounds, which are nutritionally unavailable. In
the case of amino acids, rat growth and metabolic
studies have shown that the Amadori compounds are
biologically unavailable. Cecal and large intestinal
microorganisms in experimental animals can liberate
the complexed amino acids, but this occurs too late
in the digestive/absorptive process for a significant
in vivo utilization. The nutritional loss includes
those free amino acids and peptides that have reactive
a-amino groups, protein-bound lysine which has a
reactive e-amino group, and vitamins (thiamin,
pyridoxine, and folic acid).
0007In a model system representative of the early stage,
comprising milk powder stored at 60
C or lower for
several weeks, the most quantitatively important nu-
trient which is damaged is lysine. This is due to its high
level in milk protein compared to the other amino
compounds and of the high reactivity of its e-amino
group (Table 1). Milk is one of the most sensitive foods
to this reaction because of its high content of the redu-
cing sugar lactose in addition to lysine. The most im-
portant negative consequence of the early Maillard
reaction is therefore the ‘blockage’ of lysine in milk-
based products (infant formulae and weaning foods).
Lysine is essential for growth and its requirement is
high (103 mg kg
1
day
1
for babies as compared to
12 mg kg
1
day
1
for adults). The high, recommended
lysine level in baby formulae (minimum of 6.7 g per
100 g of protein, equal to the level in mother’s milk) is
reached using cows’ milk which contains an excess of
lysine of at least 20% compared to mother’s milk.
0008Industrially treated milks contain a certain amount
of such blocked lysine as its Amadori compound, the
amount varying between 0 and 15% depending on
the treatments applied (Table 2). Higher values are
reached with the roller drying process, though this
process is no longer used in the industrial-scale pro-
duction of milk formulae.
0009Lysine bioavailability is alsoaffectedby the Maillard
reaction in otherheat-treated foods likebread, biscuits,
and pastas, but the negative nutritional consequences
BROWNING/Toxicology of Nonenzymatic Browning 673
are much lower than for milk-based infant formulae
as these cereal foods are poor sources of lysine.
Quantification of Lysine Damage
0010 Many biological and chemical methods have been
developed to quantify available and reactive lysine
respectively. Available lysine (¼ reactive lysine di-
gestibility) may be evaluated after enzymic hydro-
lysis, by microbiological tests and, in animals, by
growth tests. Reactive lysine may be measured as
e-N-dinitrophenyl-lysine after derivatization with
fluorodinitrobenzene, as homoarginine after derivati-
zation with O-methylisourea, and as lysine after re-
duction of the Amadori compound with sodium
Reactions
Amadori compound
Advanced 1, 2-Dicarbonyl
Dehydration
Fission
Polymerization
Heterocyclic
amines
Melanoidins
Strecker
degradation
Creatinine
Short-chain
carbonyls and
dicarbonyls
Premelanoidins
N-heterocyclics
Aldehydes
+ NH
3
Hydroxymethylfurfural
2, 3-Dicarbonyl +
Reducing sugar
+ amino acid
Amino
acid
Biological effects
Amino acid loss
(lysine)
Vitamin loss
Mutagens
Antimutagens
Physiological
effects
Mutagens/
carcinogens
Metal
chelation
Amino acid loss
(lysine, methionine,
tryptophan)
Stages
Early
fig0001 Figure 1 Browning reaction. Chemical pathways and biological effects. Reproduced from Browning: Toxicology of Nonenzymatic
Browning, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
tbl0001 Table 1 Nutritional effects of early and advanced Maillard reactions. Model system: dried milk powder
Maillard
status treatments
Percentage of original values of untreated sample
Reactive
lysine
Lysine as
Amadori compound
Lysine in
advanced Maillard
products
Vitamin B
1
Pantothenic
acid
Vitamin B
6
Folic acid Vitamin B
12
Early
60
C, 4 weeks 74 26 0 81 90 84 29 100
Advanced
70
C, 4 weeks 17 14 69 5 7 18 3 31
Tryptophan
Reactive lysine Nitrogendigestibility Chemical analysis Bioavailability Methionine
Early
50
C, 9 weeks 79 98 100 100 100
Advanced
60
C, 4 weeks 20 75 100 92 92
674 BROWNING/Toxicology of Nonenzymatic Browning
