Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

clean sweet taste with no unpleasant aftertaste. It is
blended with other sweeteners such as, saccharin,
cyclamate, and acesulfame-K to maximize the quality
of sweetness.
0008 Alitame offers several benefits such as stability at
high temperatures and a broader pH range. For in-
stance, it is stable for over a year at pH 6–8 and room
temperature and withstands pasteurization. How-
ever, prolonged storage of acidic solutions at high
temperatures or in combination with certain ingredi-
ents (hydrogen peroxide or sodium bisulfite) may
produce off-flavors. In the presence of high levels of
reducing sugars, alitame can undergo Maillard reac-
tions.
0009 Alitame is noncariogenic. From an oral intake,
7–22% is unabsorbed and excreted in the feces.
The remainder is hydrolyzed to aspartic acid and
alanine amide. The aspartic acid is normally
metabolized, and the alanine amide is excreted
in the urine as a sulfoxide isomer, sulfone, or con-
jugated with glucuronic acid. The incomplete ab-
sorption and metabolism result in a core value of
1.4 kcal g
1
.
0010The Joint Expert Committee on Food Additives
(JECFA) concluded that alitame was not carcinogenic
and did not show reproductive toxicity. In 1996, an
acceptable daily intake (ADI) of 0–1 mg per kilogram
of body weight was allocated. It is approved for use in
Australia, New Zealand, Mexico, and China. A food
additive petition was submitted to the US Food and
Drug Administration (FDA) in 1986, and approval is
awaited. In the petition, the estimated daily intake
is 0.34 mg per kilogram of body weight, which re-
presents the amount if alitame is the only sweetener
in the diet. The level at which no observed adverse
effects occur in animals is 100 mg kg
1
. Potential uses
include baked goods, baking mixes, hot and cold
beverages, dry beverage mixes, table-top sweet-
eners, chewing gum, candies, frozen desserts, and
pharmaceuticals.
Sucralose
0011Sucralose, 1,6-dichloro-1, 6-dideoxy-b-d-fructofura-
nosyl 4-chloro-4-deoxy-a-d-galacto-pyranoside or
4,1
0
,6
0
-trichloro-4,1
0
,6
0
-trideoxy-galacto-sucrose
(Figure 2), is a chlorinated derivative of sucrose, dis-
covered in 1976 by carbohydrate research chemists at
Queen Elizabeth College and Tate and Lyle, UK. It is
derived from a patented multistep process, involving
selective chlorination of sugar at the 4, 1
0
, and 6
0
positions substituting three hydroxyl groups on the
sucrose molecule. It is the result of a study on a large
number of related compounds, carefully synthesized
and evaluated to determine the spatial structure
and molecular configuration required for sweetness
perception.
0012Ingredients and tabletop forms of sucralose are
being marketed under the brand name Splenda
1
. Its
chemical formula is C
12
H
19
O
8
Cl
3
(MW 397.35).
Sucralose is a white, odorless crystalline powder and
is readily dispersible and soluble in water, methanol,
and ethanol. At 20
C, a 280 g l
1
solution of sucra-
lose in water is possible. Sucralose presents Newton-
ian viscosity characteristics, a negligible lowering of
surface tension, and no pH effects, and its solubility
increases with increasing temperature. In ethanol, the
solubility ranges from approximately 110 g l
1
at
20
C to 220 g l
1
at 60
C, and its solubility in etha-
nol facilitates in formulating alcoholic beverages and
flavor systems.
OH
HO
R
3
O
OR
2
OR
1
O
O
O
CH
2
fig0005 Figure 5 Chemical structure of the sweet glycoside of Stevia
rebaudiana Bert.
HO
O
O
O
O
H
3
C
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
H
H
H
O
OH
HO
HO
HO
COOH
COOH
COOH
fig0004 Figure 4 Chemical structure of glycyrrhizin.
SWEETENERS/Others 5697

tbl0001 Table 1 Properties of some intense sweeteners
Sweetener (INS)
a
Sweetness
b
Sweetness characteristics Synergism
c
Solubilityin water Stability ADI
d
(mgper kilogram of
body weight)
In solution During heating
Alitame 2000 Clean, no unpleasant aftertaste sac, cyc, aces Good, 130 g l
1
, pH 5.6 Good, pH 6–8 Very good 0–1 (1996)
Sucralose (955) 400–800 slow onset, clean sweet sugar-
like, prolonged sweetness
cyc, aces, nhdc Good, 280 g l
1
,20
C Good, pH > 3, loss of
sweetness, pH < 3
Very good 0–15 (1990)
Neohesperidin
dihydrochalcon (959)
250–2000 delayed onset, lingering
licorice–menthol-like
aftertaste
sac, asp, aces,
cyc, sucral,
sugar alcohols
Low, 0.5 g l
1
,20
C,
650 g l
1
,80
C
Stable, pH 2–6 Good Not evaluated by JECFA,
0–5 (1987–SCF
d
, EC)
Glycyrrhizin (958) 50–100 Slow onset, long aftertaste,
licorice flavor
stev, thau, asp Good Stable, pH > 4.5 Good Not evaluated by JECFA
Stevioside 100–300 Slow onset, menthol at high
levels, bitter
asp, cyc, aces,
glyc
Low, 0.8 g l
1
Stable, pH 3–10 Good Not evaluated by JECFA
Thaumatin–Talin (957) 2000–3000 Slow onset, persistent, licorice-
like
sac, aces, asp,
cyc, stev, glyc
Good, 600 g l
1
Stable, pH 2.7–6.0 Stable, neutral
to low pH
Not specified (1985)
a
INS, International Numbering System.
b
In relation to sucrose.
c
sac, saccharin; cyc, cyclamate; aces, acesulfame-K; nhdc, neohesperidin dihydrochalcone; asp, aspartame; sucral, sucralose; stev, stevioside; thau, thaumatin; glyc, glycyrrhizin.
d
SCF, Scientific Committee for Food.
From Glo
´
ria MBA (2000) Intense sweeteners and synthetic colorants. In: Nollet LML (ed.) Food Analysis by HPLC, pp. 523–573. New York: Marcel Dekker.

0013 Sucralose is 400–800 times sweeter than sucrose
(Table 1). Although its sweetness varies with pH,
sucralose has a clean sugar-like taste and a time–
intensity profile like that of sucrose, albeit more per-
sistent. It has an excellent taste profile and no bitter
or objectionable aftertaste. It is a flavor enhancer and
shows sweetness synergism with cyclamate, acesul-
fame-K, and neohesperidin dihydrochalcone.
0014 Sucralose offers a broad pH, aqueous, thermal
processing, and shelf stability. It does not interact
with food ingredients and is stable in the dry form
(4 years at 20
C). It withstands high temperatures,
thus making it well suited for use in pasteurized,
aseptic processing, sterilized, cooked, and baked
foods. However, under extreme conditions of pH,
temperature, and time, sucralose may be hydrolyzed,
producing 4-chloro-deoxy-d-galactose and 1,6-
dideoxy-1,6-dichloro-d-fructose, or degraded with
elimination of hydrogen chloride in basic medium.
0015 Sucralose is noncariogenic. It resists hydrolysis in
the human digestive tract, being excreted unchanged
in the feces, and the very small portion absorbed is
rapidly eliminated in the urine. Therefore, it produces
no glycemic response and is virtually noncaloric.
Following safety testing and toxicological studies in
humans and animals, the FDA concluded that sucra-
lose does not pose any carcinogenic, reproductive, or
neurological risk. The JECFA reviewed it favorably
and in 1990 recommended an ADI of 0–15 mg per
kilogram of body weight. Sucralose is approved for
use in a wide range of food products in Canada, USA,
Australia, Mexico, Russia, Romania, China, the
European Union, and Mercosur. It has been used as
a table-top sweetener and in carbonated, still, and
alcoholic beverages, frozen desserts, confectionery,
bakery products, canned fruits and vegetables, fruit
spreads, chewing gum, dry-mix products, dairy prod-
ucts, condiments, dressings, and breakfast cereals.
Neohesperidin Dihydrochalcone
0016 Citrus fruits contain bitter flavanone glycosides, all
derivatives of the disaccharide 2-O-a-l-rhamnopyra-
nosyl-b-d-glucopyranose, neohesperidose. In 1963,
Horowitz and Gentili found that catalytic hydrogen-
ation of the chalcone form gave dihydrochalcone
neohesperidosides, several of which were intensely
sweet. Numerous dihydrochalcone derivatives were
synthesized for taste and toxicity trials, from which
neohesperidin dihydrochalcone (NHDC) emerged as
a promising sweetener. It is prepared by alkaline hy-
drogenation of the biflavanoid neohesperidin present
in Seville (bitter) oranges (Citrus aurantium).
0017 NHDC is a semisynthetic nonnutritive intense
sweetener. Chemically, it is 1-[4-[[2-O-(6-deoxy-a-l-
mannopyranosyl)-b-d-glucopyranosyl]oxy]-2,6-
dihydroxyphenyl]-3-(3-hydroxy-4-methoxyphenyl)-
1-propanone with the molecular formula C
28
H
36
O
15
and a molecular weight of 612.60 (Figure 3). NHDC
shows a slow buildup of sweetness, rising from 250 to
2000 times that of a 50 g l
1
sucrose solution, but
more persistent (Table 1). It has a pleasant taste,
flavor-modifying properties, ability to improve the
sweetness quality and profile, and remarkable syner-
gistic effects. Its flavor enhancement has been per-
ceived in several products, specially fruit flavors.
NHDC has the ability to decrease the perception of
bitterness, saltiness, sharp, and spicy attributes. The
sweetness intensity of NHDC depends on many
factors such as concentration, pH, and the product
to which it is added. As the concentration increases,
the sweetness of NHDC decreases relative to the level
of sucrose. Caffeine significantly enhances the sweet-
ness of NHDC in certain soft drinks. At higher con-
centrations, NHDC has a lingering menthol or
licorice-like aftertaste and a cooling sensation,
which distinguishes it from other sweeteners. How-
ever, modifications of the sensorial properties of
NHDC are possible by the admixture of bulk sweet-
eners, certain flavors, and other taste-modifying food
additives such as gluconates, amino acids, or nucleo-
tides. It also shows synergism with saccharin, aspar-
tame, cyclamate, sucralose, acesulfame-K, and sugar
alcohol.
0018NHDC is a nonhygroscopic colorless crystalline
solid. It is sparingly soluble in water (0.50 g l
1
)at
20
C but is highly soluble at 80
C (650 g l
1
). It is
also soluble in alcohol and aqueous alkali, but a
higher solubility is achieved in ethanol–water mix-
tures than in water or ethanol alone. Where a higher
solubility is required, monobasic salts may be used
that are freely soluble in water and exhibit a shorter
duration of sweetness than the parent compound. The
solubility of NHDC may also be enhanced by dissolv-
ing it in glycerol and propylene glycol, as well as by
using it in mixtures with readily water-soluble polyols
such as sorbitol. Interestingly, these bulk sweeteners
also act as taste modifiers of NHDC by reducing its
menthol-like aftertaste.
0019NHDC presents high stability at pH 2–6. It is
stable under most food-processing and storage condi-
tions and withstands pasteurization, UHT processes,
and the normal shelf-life of soft drinks. It is stable
during fermentation of yogurt, but undergoes hy-
drolysis at high acidity and elevated temperatures,
yielding hesperetin dihydrochalcone, hesperetin
dihydrochalcone-4
0
-b-d-glucoside, rhamnose, and
glucose.
0020NHDC is noncariogenic and has a caloric value
of 2 kcal g
1
. Little of the compound is absorbed
SWEETENERS/Others 5699

unchanged from the small intestine. After cleavage of
the glycosidic side-chain by intestinal mucosal or bac-
terial glycosidases, the residual primary metabolites
are partly excreted unchanged in the bile and partly
metabolized further. Standard toxicity tests have sug-
gested its safety. In 1987, the Scientific Committee for
Food of the Commission of the European Commu-
nities allocated an ADI of 0–5 mg per kilogram of
body weight. It is currently approved for use in the
European Communities, Sweden, Switzerland, Mo-
rocco, and Tunisia.
0021 Owing to its highly intense and long-lasting sweet-
ness, NHDC is normally used at concentrations of
less than 100 mg kg
1
. Only in chewing gum are
higher levels required because of its slow release
from the gum base. For use in soft drinks, NHDC
has been recommended in combination with other
sweeteners at a concentration of 20 mg kg
1
. Owing
to its ability to reduce bitterness and to its flavor
enhancing properties, NHDC is an ideal sweetener
for grapefruit or orange juice. Promising results
have been reported from its use in fruit-flavored
yogurts. In table-top products, the addition of small
amounts of NHDC may result in significant savings
because of its synergistic, sweetness enhancing effect.
It has been used in juice, soft drinks, dairy products,
desserts, confectionery, spreads, jams, chewing gum,
chocolate based products, and icecream.
Glycyrrhizin
0022 Glycyrrhizin is found in licorice root of a small leg-
uminous shrub, Glycyrrhiza glabra L. from Europe
and Central Asia. Glycyrrhizin or 20-b-carboxy-11-
oxo-30-norolean-12-en-3b-yl-2-O-b-d-glucopyranu-
rosyl-a-d-glucopyranosiduronic acid (C
42
H
62
O
16
,
MW 822.92), is a triterpenoide glycoside (saponin)
with glycyrrhetinic acid, which is condensed with
O-b-d-glucuronosyl-(1
0
!2)-b-d-glucuronic acid
(Figure 4). After harvest, the roots are dried to 10%
moisture, shredded, extracted with aqueous ammo-
nia, concentrated in vacuum evaporators, precipi-
tated with sulfuric acid, and crystallized with 95%
alcohol providing a crude ammonium glycyrrhizin
(AG). Further treatment yields a white, crystalline
mono-ammonium glycyrrhizin (MAG). Both deriva-
tives have the same sweetness but differ in solubility
and sensitivity to pH. AG is relatively stable and
highly soluble in hot or cold water and in alcohol. It
withstands temperatures above 105
C for a short
period of time, and precipitates at pH values below
4.5. MAG is used in applications where low pH and
color rule out AG.
0023 Glycyrrhizin is 50–100 times sweeter than sucrose
and has a slow onset of sweetness followed by a
lingering licorice-like aftertaste (Table 1). It exhibits
a sweet woody flavor, which limits its use as a pure
sweetener. Glycyrrhizin enhances food flavors, masks
bitter flavors, and increases the perceived sweetness
level of sucrose. It has the potential for providing
functional characteristics, including foaming, viscos-
ity control, gel formation, and possibly antioxidant
characteristics.
0024Studies have focused on the pharmacological
effects of glycyrrhizin as antiulcer, antiinflammatory,
antiviral, anticariogenic, and antispasmodic. It also
has corticoid activity, influencing steroid metabolism
to maintain blood pressure and volume and to regu-
late glucose/glycogen balance. Glycyrrhizin can be
hydrolyzed by human intestinal microflora to 18-b-
glycyrrhetinic acid and two molecules of glucuronic
acid. After release of the acids, the compound binds
to plasma protein, enters the enterohepatic circula-
tion, and is almost completely metabolized. However,
side-effects, typically involving cardiac dysfunction,
edema, and hypertension have been reported among
subjects receiving high doses of glycyrrhizin-based
pharmaceuticals or consuming large amounts of lic-
orice-containing confectionery or health products
over a prolonged period.
0025The ammonium salt of glycyrrhizin is approved as
a flavoring and flavor enhancer in the USA. It is on
the FDA ‘generally recognized as safe’ (GRAS) list.
The use of glycyrrhizin is permitted in Japan and
Taiwan. In Japan, it is used as a flavoring for hydro-
lyzed vegetable protein, soy sauce, and bean paste to
control saltiness. At levels of 30–300 mg kg
1
, it en-
hances the flavor of cocoa and chocolate-flavored
products, flavors and sweetens candy, confectionery,
and beverages, and masks the bitter taste of pharma-
ceuticals. Because of its pharmacological action, it
should be used in moderate amounts as a sweetener.
Stevia Sweeteners
0026Stevia rebaudiana Bertoni (Compositae) is a herb
native to Paraguay but cultivated in South-east Asia,
Japan, Paraguay, Brazil, Israel, and USA. Stevia
sweeteners are extracted from the dried leaves, clari-
fied and crystallized. The sweet constituents of stevia
include eight diterpene glycosides: stevioside, steviol-
bioside, rebaudiosides A, B, C, D, and E, and dulco-
side A, which collectively are 100–300 times sweeter
than sucrose. They are similar in structure in that a
steviol aglycon is connected at C-4 and C-13 to
mono-, di-, or trisaccharides consisting of glucose
and/or rhamnose residues, as shown in Figure 5.
Stevioside is the major constituent, whereas dulcoside
A and rebaudiosides A and C are the other main
components. Stevioside is 300 times sweeter than
5700 SWEETENERS/Others

sucrose (Table 1). It shows a sweetness profile similar
to that of sucrose, except that it has an unpleasant,
persistent, menthol, and bitter aftertaste. However,
the development of new cultivars, derivatization and
incorporation of cyclodextrin, l-histidine, potassium
phosphate, glucono-d-lactone and maltose in formu-
lations has eliminated undesirable aftertastes. Rebau-
dioside A is more stable, sweeter, and has a better
taste profile than stevioside. The remaining diterpene
glycosides are not as sweet as stevioside.
0027 Stevioside is a white powder, highly soluble in
water, ethanol, and methanol, and is nonfermentable.
When heated at 100
C for 1 h, solutions of stevioside
at pH 3–9 show little loss in sweetness and no change
at 22
C for 5 months. However, considerable decom-
position occurs at pH 10. Some degradation of stevio-
side and rebaudioside has been observed in
carbonated beverages acidified with phosphoric and
citric acids during storage at 37
C. Heating at 60
C
for 6 days has resulted in 0–6% loss of sweetness.
Exposure to 1 week of sunlight does not affect stevio-
side, but results in 20% loss of rebaudioside A. The
high stability of stevioside makes it a suitable sweet-
ener for cooked and baked foods and for beverages.
0028 Stevioside suppresses the growth of oral micro-
organisms, and both stevioside and rebaudioside A
provide very few calories. In Paraguay, S. rebaudiana
is used for the treatment of diabetes because of its
hypoglycemic activity. Studies have suggested that
stevioside is not toxic, mutagenic, or teratogenic in a
number of animal species. In addition, the product
has been used for more than 10 years in South
America and Japan, but there are contradictory
reports on the in-vivo metabolism to steviol, which
is mutagenic for Salmonella typhimurium TM677.
0029 Stevioside is available in three purity ranges: crude
extract, and 50% and 90% purity. Since 1970, stevia
sweeteners have been used in a wide range of food
and beverage applications in Japan, including soft
drinks, candies, chocolate, chewing gum, icecream,
yogurt, jam, pudding, and table-top sweeteners. It is
commonly used in combination with sucrose and
fructose and also with other sweeteners such as aspar-
tame, cyclamate, and acesulfame-K, but not with
saccharin. It is currently approved for use in Japan,
Taiwan, and Mercosur. An acceptable daily intake of
stevioside for humans of 7.94 mg per kilogram of
body weight has been suggested.
Thaumatin
0030 Thaumatin is a mixture of sweet proteins originally
isolated from the fruit of the West African plant
katemfe (Thaumatococcus danielli Benth.). There
are at least five thaumatins, which are obtained by
extraction with water, concentration, and ultrafiltra-
tion. Tate and Lyle Ltd. manufactures a mixture of
two as talin. These are proteins with isoeletric points
of 11.5–12.5. Thaumatin consists of a single chain of
207 normal amino acid residues with eight disulfide
bonds and has a molecular weight of about 22 000. It
is very soluble in water (600 g l
1
) and is stable at pH
2.7–6.0 and under pasteurization conditions (Table
1). At higher pH values, the protein becomes more
heat-labile, despite its stability at pH values up to 8
under ambient conditions. The stability of solubilized
thaumatin requires careful monitoring of pH, time,
temperature, and other processing parameters. In
addition, protection of the protein against yeasts
and molds in solutions is also critical for stability.
Thaumatin can associate with negatively charged
compounds such as synthetic colors and acidic gums
like xanthan, pectin, carrageenan, alginate, and car-
boxymethylcellulose, resulting in a loss of sweetness.
Association with synthetic colorants may also cause
color loss.
0031Thaumatin is 1600–3000 times sweeter than su-
crose. However, it has unusual taste profile, slow in
onset, followed by intensification to lingering sweet-
ness with a licorice-type aftertaste. It masks metallic
or bitter tastes. To achieve a taste closer to that of
sucrose, thaumatin must be blended with other in-
tense sweeteners or with sugars. Synergism has been
observed with saccharin, acesulfame-K, and stevio-
side, but not with aspartame and cyclamate. By com-
bining thaumatin with alanine and organic acids,
there is a doubling in sweetness and a reduction in
aftertaste and in the delay in sweetness. At subsweet-
ness levels, thaumatin functions as a flavor enhances.
It has the ability to enhance certain flavors and
aromas, such as those in peppermint, spearmint,
coffee, and ginger. The synergistic effect noted with
monosodium glutamate enhances the aroma and im-
proves the flavors of processed meats and fish prod-
ucts when used at levels of 0.5–2.0 g l
1
.
0032Thaumatin can be metabolized to its constituent
amino acids, contributing the same calories as pro-
tein. However, because of its high sweetness, it has a
low-calorie value per unit of sweetness, less than
0.002 kcal. It is noncariogenic and has undergone
safety tests indicating that it is not allergenic, muta-
genic, or teratogenic. Furthermore, it has a long his-
tory of use without any adverse effects. In 1985, a
‘not specified’ ADI was allocated by the JECFA. It has
been approved for use in Japan, the UK, Australia,
Canada, South Africa, Mexico, the European Union,
Switzerland, Taiwan, Morocco, and Tunisia. In
the USA and Switzerland, it is permitted as a flavor
enhancer in chewing gum, and in the USA, it has been
classified as GRAS.
SWEETENERS/Others 5701

0033 The major uses include chewing gum, beverages,
coffee, savory flavor, dairy products, dental and
pharmaceuticals, animal and pet foods. However, be-
cause of its high cost, it is used on a small scale.
Biotechnological alternatives to eliminate uncertain-
ties and variability associated with agricultural pro-
duction and to lower costs are being investigated.
Blends of Sweeteners
0034 Today, there is an increasing tendency to explore
the use of blends of sweeteners. When two or more
sweeteners are combined, blends with an increased
stability, longer shelf-life, lowered production costs,
improved taste and flavor, and decreased side- and
aftertastes result, as well as a positive impact on taste
quality, flavor-enhancing, and flavor-modifying prop-
erties. Also, mixtures of sweeteners can exhibit addi-
tive and synergistic effects, and since lower amounts
of each sweetener can be used, the average daily
intake of each sweetener is low, thus minimizing the
health risk from any one sweetener.
0035 Since food laws in most countries regulate the use
of intense sweeteners, analytical control for the pres-
ence and levels of sweeteners in food is essential.
According to the JECFA, it is also important to
know the level of additives in food products in order
to estimate the actual consumption by the popula-
tion. This information shows the average intake in
relation to the ADI over a period of time. Based on
this knowledge, regulatory authorities can propose
regulations to insure intakes below the ADI. Further-
more, some sweeteners undergo decomposition
during processing and storage of food products,
forming a variety of degradation products that may
have sensory and toxicological significance.
0036 Several types of methods have been described for
the analysis of intense sweeteners, among them, spec-
trophotometric, enzymatic, titrimetric, capillary iso-
tachophoretic, thin-layer chromatography, liquid and
gas chromatographic methods. High-performance
liquid chromatography (HPLC) is by far the most
frequently used technique. However, with the in-
creased number of sweeteners available and their
use being approved in specified food products and
beverages by different countries, methods capable of
separating several sweeteners simultaneously are still
required.
See also: Sweeteners: Intensive
Further Reading
Borrego F, Canales I and Lindley MG (1995) Neohesperidin
dihydrochalcone: state of knowledge review. Zeitschrift
fu
¨
r Lehensmitte/untersuchung und -forschung 200:
32–37.
Duffy VB and Anderson GH (1998) Position of the Ameri-
can Dietetic Association: use of nutritive and nonnutri-
tive sweeteners. Journal of the American Dietetic
Association 98(5): 580–587.
Dwivedi BK (1993) Artificially sweetened low-calorie foods
other than drinks. In: Altschul AM (ed.) Low-calorie
Foods Handbook, pp. 331–342. New York: Marcel
Dekker.
Fenwick GR, Lutomski J and Nieman C (1995) Liquorice,
Glycyrrhiza glabra L. – composition, uses and analysis.
Food Chemistry 38(2): 119–143.
Giese JH (1993) Alternative sweeteners and bulking agents:
an overview of their properties, function and regulatory
status. Food Technology 47(1): 114–126.
Glo
´
ria MBA (2000) Intense sweeteners and synthetic color-
ants. In: Nollet LML (ed.) Food Analysis by HPLC,
pp. 523–573. New York: Marcel Dekker.
Grenby TH (1991) Intense sweeteners for the food industry:
an overview. Trends in Food Science and Technology
2(1): 2–6.
Hollingsworth P (1998) Sucralose approval sweetens low-
cal market. Food Technology 52(5): 34.
Hough L (1993) High-intensity, low calorie sweeteners. In:
Khan R (ed.) Low Calorie Foods and Food Ingredients,
pp. 138–164. New York: Blackie.
Liu J, Ong CP and Li SFY (1997) Subcritical fluid extrac-
tion of stevia sweeteners from Stevia rebaudiana. Jour-
nal of Chromatographic Science 35(9): 446–450.
Renwick AG (1990) Acceptable daily intake and the
regulation of intense sweeteners. Food Additives and
Contaminants 7(4): 463–475.
Sardesai VM and Waldshan TH (1991) Natural and
synthetic intense sweeteners. Journal of Nutritional
Biochemistry 2(5): 236–244.
Schaller I (1997) Is Codex the answer? Food Ingredients
Analysis International 19(3): 24–28.
Staff Report (1996) Thaumatin – the sweetest substance
known to man has a wide range of food applications.
Food Technology 50(1): 74–75.
Verdi RJ and Hood LL (1993) Advantages of alternative
sweeteners blends. Food Technology 47(6): 94–101.
Wallis KJ (1993) Sucralose: features and benefits. Food
Australia 45(12): 578–580.
5702 SWEETENERS/Others

SWEETS AND CANDIES
Sugar Confectionery
W P Edwards, Bardfield Consultants, Gt. Bardfield, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The confectionery industry is not a science-based
industry: it is an industry that has been built on
the confectioner’s craft. Confectionery is normally
divided into three classes: flour confectionery, choc-
olate confectionery, and sugar confectionery. Flour
confectionery covers products that are made from
flour and are baked. Chocolate confectionery consists
of chocolate. Sugar confectionery covers the rest of
confectionery.
0002 Confectionery making does share some technolo-
gies with the pharmaceutical industry, specifically
tabletting, panning, and lozenge making. Most of
the knowledge available to early confectioners was
empirical rather than scientific.
0003 Sugar confectionery making is an international
industry. Some of the terms in use are clearly French
in origin such as fondant (from fondre to dissolve),
dragees, and pastilles. Unlike chocolate confection-
ery, sugar confectionery has few legal definitions.
In some jurisdictions, caramel has a higher milk
content than toffee, but in others, the two terms are
used interchangeably. The types of product known
as gums were undoubtedly originally made from
the gum from Acacia senegal but are now made
from any suitable ingredient. Because sugar confec-
tionery has a long shelf-life and does not need con-
trolled temperature storage, an international trade
in confectionery had developed by the nineteenth
century.
0004 In general, the important concepts in sugar confec-
tionery are water activity, colligative properties, solu-
bility, and the need to use a mixture of sugars. Toffees
depend on the Maillard reaction. High boilings re-
quire a sugar glass to form. Gums, jellies, and licorice
need the right rheology. Panned coatings depend on
crystallization. Chewing gum is a chewable piece of
polymer chemistry. Aerated products are based on
stabilizing and setting a foam.
Stability
0005 Sugar confectionery products keep well compared
with most food products. The long life of confection-
ery products occurs because spoilage organisms are
unable to multiply rather than because the product is
kept sterile.
Water Activity
0006The relevant parameter is not only the water content
but the water activity. Water activity is a thermo-
dynamic concept that was invented to explain why
materials with different levels of water content did
not behave in the same way chemically or biologic-
ally. The water activity reflects the ability of the water
to be used in chemical or biological reactions. It is the
concentration corrected for the differences in the abil-
ity of the water to undertake chemical reactions. If a
nonvolatile solute were dissolved in water, the vapor
pressure should decrease in a certain way for a perfect
mixture. A thermodynamically ideal substance would
always have a water activity of 1. If a product is held
at its own water activity, it will neither gain nor lose
weight.
Equilibrium Relative Humidity (ERH)
0007This term is normally abbreviated to ERH. The
ERH is the relative humidity that matches the water
activity of the product. The ERH has practical im-
portance, since it is an indication of the conditions
under which the product can be stored without de-
terioration.
Colligative Properties
Boiling Points
0008Colligative properties are defined as those properties
that depend on the number of particles present rather
than the nature of the particles. In sugar confection-
ery, the most important of these is the elevation of the
boiling point. Because sugars are very soluble, very
large boiling point elevations are produced, e.g., as
large as 50
C. Remembering that the elevation of the
boiling point is proportional to the concentration of
the solute, it is not surprising that the boiling point is
used as a measure of the concentration and hence as a
process control.
0009The boiling point of a liquid is the temperature at
which the vapor pressure is equal to the atmospheric
pressure. If the pressure is increased, the boiling point
will increase, whereas reducing the pressure will
reduce the boiling point. Most sugar confectionery
is made by boiling a mixture of sugars to concentrate
them. The use of a vacuum has several advantages.
SWEETS AND CANDIES/Sugar Confectionery 5703
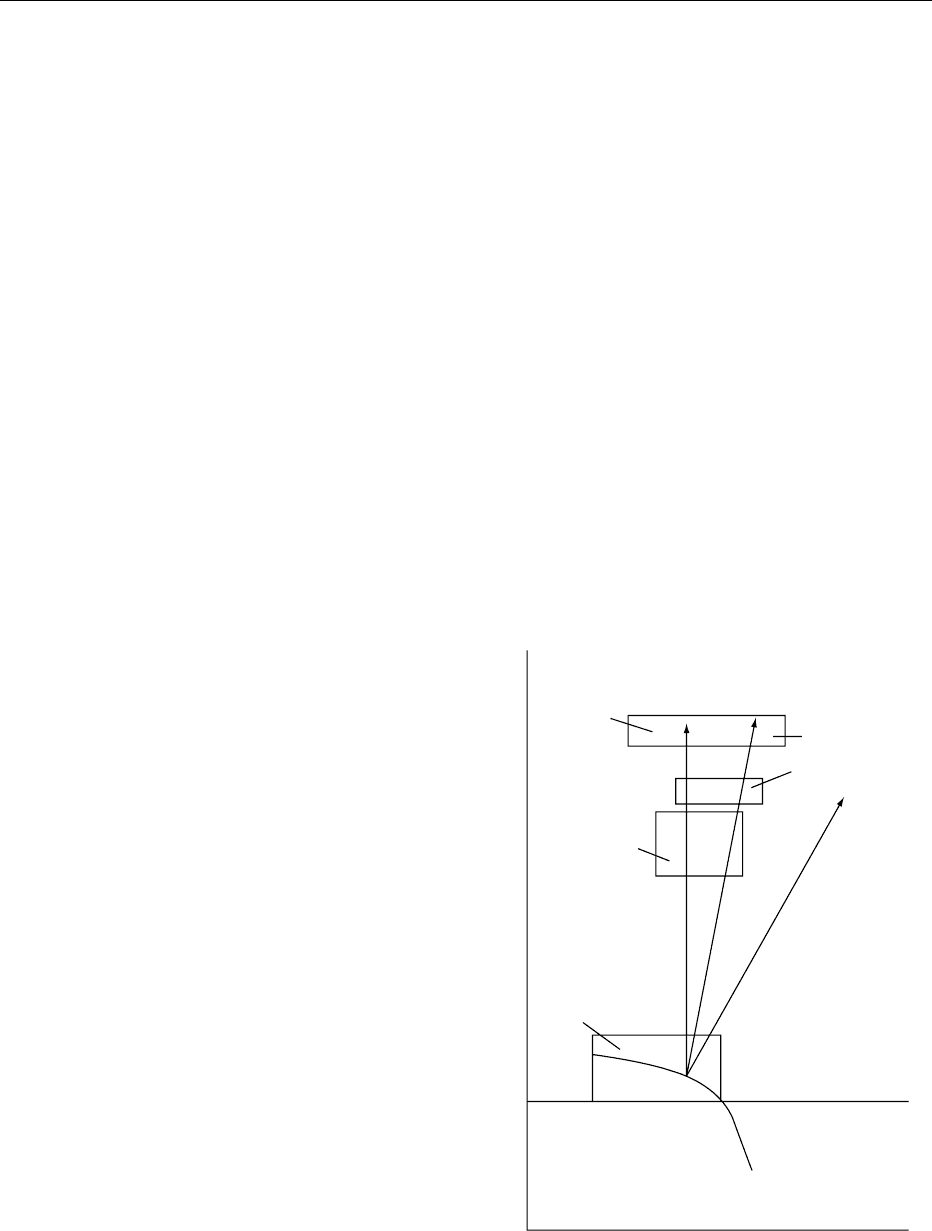
The energy consumption is reduced, browning is
reduced, and the process is speeded up. A common
practice is to boil a mixture of sugars under atmos-
pheric pressure to a given boiling point. A vacuum is
then applied. This causes the mixture to boil under
reduced pressure. This not only concentrates the
mixture while the latent heat of evaporation cools it
rapidly but also speeds up the production process,
since the product will ultimately have to be cooled
to ambient temperature.
Solubility
0010 Sucrose is insufficiently soluble, at ambient tempera-
tures, to lower the water activity sufficiently to give a
microbiologically stable solution. A stable product
can only be made by mixing the sucrose with other
sugars. The ingredient that is most used as a source of
nonsucrose sugars is the starch hydrolysate known
variously as glucose syrup or corn syrup. Neither
name is entirely accurate as the major ingredient is
normally maltose rather than dextrose, but the
product can be, and is, manufactured from wheat or
potato starch as an alternative to maize starch. The
degree of hydrolysis is characterized by the dextrose
equivalent (DE), which is a measure of the equiva-
lence of the solids in the syrup to dextrose in the
Fehlings titration. The type of syrup normally used
is referred to as confectioners’ glucose and has a DE
of around 40.
0011 In this work to avoid confusion between glucose
syrup and chemical dextrose, glucose will only be
used to refer to the syrup, and the pure sugar will be
referred to as dextrose.
0012 Originally, the starch was hydrolyzed by using
acid, which is a random process. Now, hydrolysis
can be carried out by enzymes or a mixture of
enzymes and acid. Using enzymes, it is possible
to control which bonds are broken to allow the com-
position of the product to be varied as needed. In
principle, the starch could be hydrolyzed to produce
any combination of dextrose oligomers from dex-
trose through maltose and maltotriose to higher
oligomers.
0013 The proportion of glucose syrup used in different
types of sugar confectionery varies for a number of
reasons. Commercial pressure would encourage the
use of a maximum amount of glucose syrup solids,
but technical considerations restrict the proportion
to be used. As glucose syrup inhibits crystallization,
the highest proportion is used where crystallization
is undesirable, e.g., in boiled sweets, and the lowest
where crystallization is desirable, e.g., fondant.
Figure 1 shows the ratio of sugar to glucose used for
different products.
Maillard Reaction
0014A major feature of sugar confectionery manufacture
is ensuring that the Maillard reactions occur where
they are wanted in products like toffees and do not
occur in products like high boilings.
0015Maillard reactions are nonenzymic browning reac-
tions. In practice, any browning in foods is a Maillard
reaction except where it is enzymic, e.g., the
browning of a cut apple is enzymic; hence, it is not
a Maillard reaction. The Maillard reaction is not a
name reaction where all the details can be found in a
text book. The term covers a whole range of reactions
that occur in systems ranging from food to life
sciences. The name of the reaction goes back to
Louis Camille Maillard, who heated amino acids in
a solution with high levels of dextrose. The chemistry
of the Maillard reaction is easily described as com-
plex. It is complex not only because the reaction can
give complex products but because the starting ma-
terials are themselves complex. Most model systems
involve studies of one reducing sugar being heated
1S:3G 1S:2G 1S:1G 2S:1G 3S:1G 4S:1G 5S:1G
Ratio of sugar to glucose syrup solids
Saturated solution
Syrup solids too low
Gums
and
pastilles
Jellies and mallows
Fondant
pastes
Toffees
fudge
Grained
Ungrained
Boilings Edinburgh rock (graining)
95
90
80
70
% soluble solids
Nougat
fig0001Figure 1 Sugar and glucose composition of confectionery.
5704 SWEETS AND CANDIES/Sugar Confectionery
with one amino acid. A typical confectionery system
like a toffee would involve heating a mixture of
proteins, usually from milk with a mixture of redu-
cing sugars and fats. In sugar confectionery, the con-
ditions of the reaction are likely to be a high
temperature but a low water activity. In the early
stages of the reaction, the free amino group of an
amino acid, usually in a protein, condenses with the
carbonyl group of a reducing sugar. The resulting
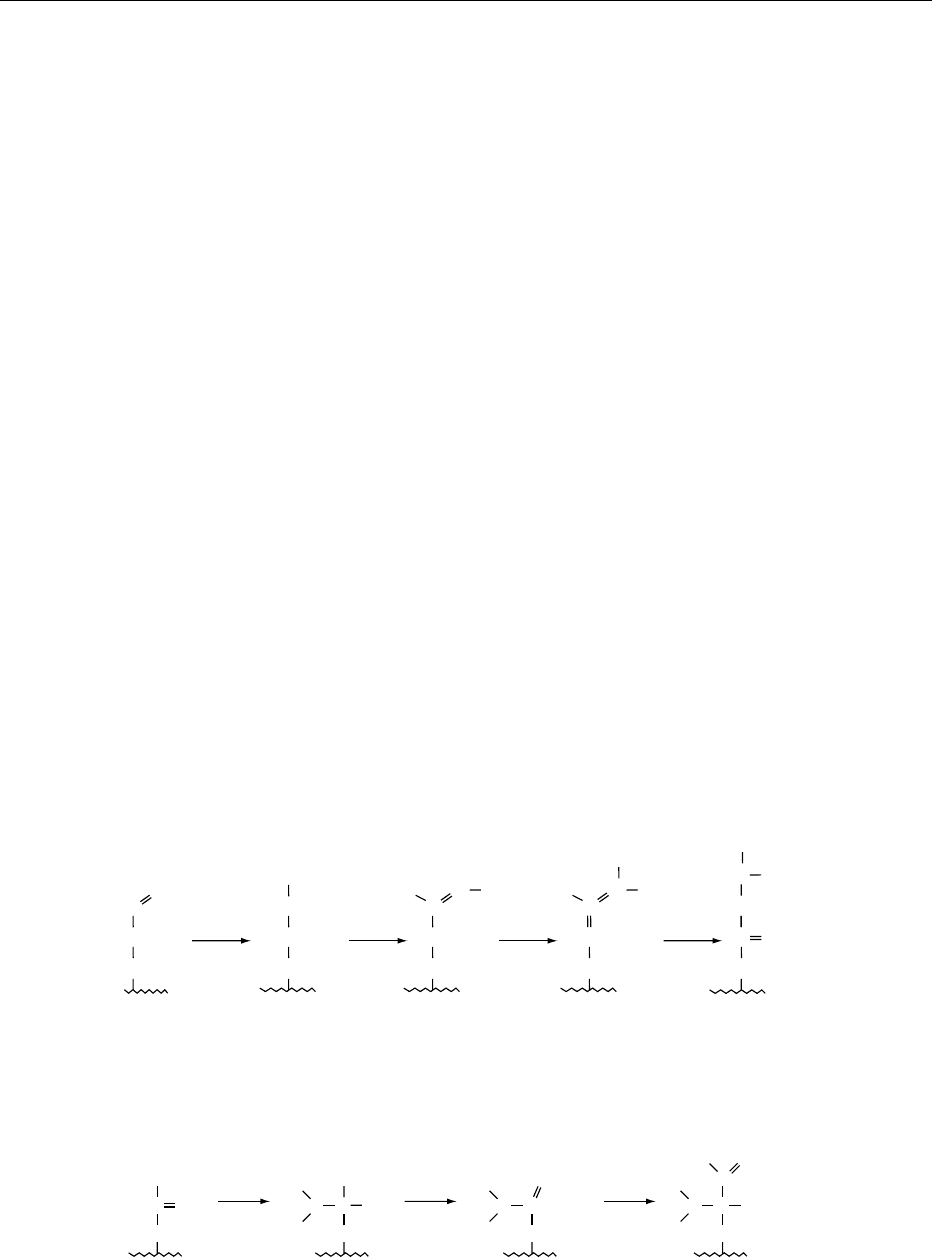
Schiff bases can rearrange by Amadori (Figure 2)or
Heyns rearrangement (Figure 3), the products being
an N-substituted glycosylamine (if dextrose is the
sugar) or an N-substituted fructosylamine for a
ketose such as fructose. The N-glycosylamine can
degrade to fission products by a free-radical mechan-
ism. In the advanced stages of the reaction, the Ama-
dori and Heyns rearrangement products degrade by
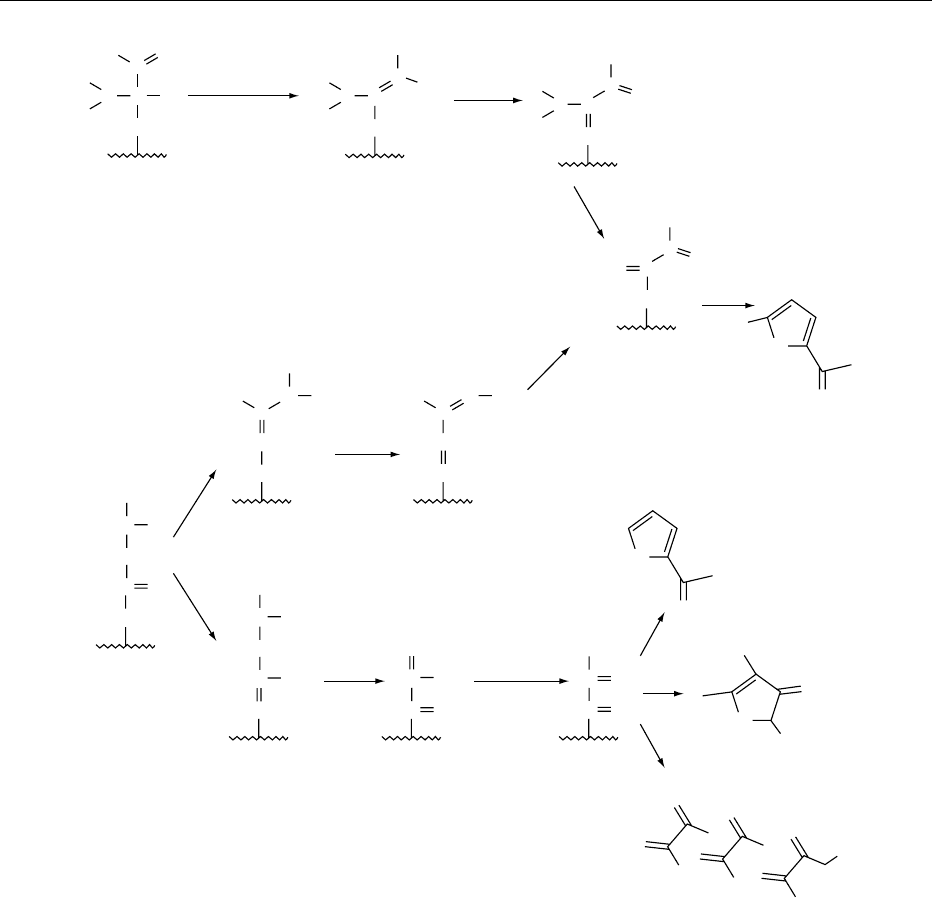
one of three possible routes. They break down via
deoxysones, fission, or Strecker degradation (Figures
4 and 5). The 1-deoxyglycosones and 3-deoxyglyco-
sones can form reactive a-dicarbonyl compounds
such as pyruvaldehyde and diacetyl by a retro-aldiza-
tion reaction. These reactive intermediates are then
available to react with ammonia and hydrogen
sulfide.
0016 At the end of the reaction, brown nitrogen-
ous polymers and copolymers form. It is known
that protein gels form when proteins are heated
with carbonyl compounds. This could occur in
toffees.
Sulfur-containing Amino Acids
0017Whereas sulfur free amino acids are broken down to
amines via decarboxylation, the sulfur containing
amino acids such as cysteine can undergo more
complex reactions.
Products from Proline
0018Various schemes have been proposed to explain the
production of nitrogen-containing heterocyclic com-
pounds such as pyrrolidines and piperidines from
proline. Nitrogen heterocyclic compounds are com-
monly found to be potent flavoring chemicals.
Strecker Aldehydes
0019These chemicals are produced by the Strecker degrad-
ation of the initial Schiff base (Figure 5). An a-amino
carbonyl compound and Strecker aldehyde are
generated by rearrangement, decarboxylation, and
hydrolysis.
Sugar Glasses: The Chemistry of Boiled
Sweets
0020In these products, which are variously called high
boilings, hard boilings, hard candy, or boiled sweets,
the sucrose is in the glassy state. The glassy state
of matter is not a thermodynamic phase but a
super-cooled liquid. Glassy materials are common in
a number of products, whereas both natural and
C
CHOH
O
CHOH
CHOH
CHOH
NHR
CHOH
H
2
NR
H
2
O
CHOH
C
NRH
CHOH
COH
C
NRH
H
CHOH
CO
CH
2
NR
H
CHOH
H
+
1-Amino-2-keto sugarEnolSchiff baseN-Glycosylamine
(if aldose is
g
lucose)
Aldose sugar
fig0002 Figure 2 Amadori rearrangement.
CH
2
OH
CO
CHOH
Ketose sugar
H
2
NR
CH
2
OH
COH
N
H
R
CHOH
H
2
O H
+
N-Fructosylamine
(if ketose is fructose)
C
H
CH
N
H
R
CHOH
2-Amino-1-keto sugar
C
CHOH
N
H
R
CHOH
Enol
O
fig0003 Figure 3 Heyns rearrangement.
SWEETS AND CANDIES/Sugar Confectionery 5705
man-made substances can form glasses. Confusingly,
although a glass is not a thermodynamic state of
matter, glasses do exhibit a sharp transition tempera-
ture between the glassy and the rubbery state. Many
methods have been used to determine the transition
temperature between the rubbery and the glassy state.
One popular method is differential scanning calorim-
etry (DSC). In these instruments, the sample and a
blank are subjected to a change of temperature up or
down at a controlled rate. The instrument measures
the difference energy input or energy extracted be-
tween the sample and the control. A plot of differ-
ence gives the variation of heat capacity, C
p
, with
temperature. The glass transition is associated with
a discontinuity in C
p
. Unfortunately, there are a
number of different ways in which the output from
the DSC can be analyzed. Some workers would advo-
cate integrating the result from the DSC, i.e., measur-
ing the area under the curve. This then gives the
variation of the enthalpy with temperature. The vari-
ation in several other physical parameters with tem-
perature such as the refractive index and the dielectric
constant have been used to measure glass transitions.
In general, the glass transition temperature obtained
depends on the method of measurement. In confec-
tionery, the important point is that a product that is
intended not to crystallize should be in the glassy state
at ambient temperatures. Most sugars will form a
C
C
CHOH
2-Amino-1-keto sugar
Heyns intermediate
1-Amino-2-keto sugar
Amadon intermediate
H
Enolization
R
H
H
N
O
−H
2
O
−RNH
2
−RNH
2
−RNH
2
−H
2
O
H
2
O
C
COH
CH
H
NR
C
CHOH
R
H
N
C
H
OH
C
CH
R
H
N
C
H
O
O
O
O
C
3-Deoxyosone
C
R
H
CH
2
C
COH
CHOH
H
N
H
R
C
N
H
CH
2
O
R
1,2-
Enolisation
2.3
CHOH
C
N
H
CH
2
OH
R
CHOH
C
CH
2
OH
Enolization
1-Methyl-
2,3-dicarbonyl
CO
C
CH
2
O
CO
O
O
O
O
O
HO
O
O
O
H
OH
O
O
O
fig0004 Figure 4 Formation of furans and dicarbonyls.
5706 SWEETS AND CANDIES/Sugar Confectionery
