Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


antibiotic. It occurs in milk and is effective against
some bacteria, but not yeasts or molds, and is used in
processed cheese. (See Curing; Nisin.)
004 2 The development of low-sugar preserves necessi-
tates the addition of a preservative (often sorbic
acid) to prevent mold growth and a similar situation
exists for low-fat spreads where the usual 80% fat of
margarine can be reduced to below 30%, with cor-
responding increase in a
w
and susceptibility to mold
growth.
004 3 Table 3 indicates that propionic acid, usually in the
form of calcium propionate, is used to control mold
growth in bread. However some bakers have chosen
to replace this preservative by vinegar, a solution of
acetic acid in water. This change was due to adverse
consumer perceptions of food additives or ‘chemicals’
(which could be identified by their E-numbers) in
contrast to vinegar which is classed as an ingredient
and does not carry an E-number. However the preser-
vative action of vinegar is due to the acetic acid
present – a weak acid like propionic acid.
004 4 Enzymatic activity varies widely with pH and is
reduced by lowering the pH to below 4 but this acidic
environment (e.g., use of vinegar) must be acceptable
in terms of flavor – otherwise blanching may be
preferred. Treatment with sulfur dioxide or sulfites
is also effective – a particular application is preven-
tion of darkening of potato slices/chips during chill or
frozen storage. Such use for prevention of enzymatic
browning of apple slices is restricted due to flavor
taints but acid dipping (e.g., citric acid, lemon juice)
is effective. Peroxidases in general cause oxidative
changes to color and flavor and their control by heat
treatment, pH control, or sulfites must be adopted to
the particular food system. (See Enzymes: Functions
and Characteristics.)
004 5 Chemical changes limiting storage stability are
principally lipid oxidation and nonenzymatic (Mail-
lard) browning. The latter is particularly relevant for
dried milk, dried egg white, and dried fruits. Oxida-
tive rancidity of lipids is a radical oxidation process
and phenolic antioxidants (e.g., tocopherols, butyl-
ated hydroxyanisole (BHA), butylated hydroxy-
toluene (BHT) ) are effective at concentrations below
200 p.p.m. In practice combinations of a phenolic
antioxidant and ascorbic acid are often used since
they have a synergistic effect. (See Antioxidants: Nat-
ural Antioxidants; Synthetic Antioxidants; Browning:
Nonenzymatic; Oxidation of Food Components.)
004 6 The maintenance of the correct physical state of a
food can include maintaining the free-flow properties
of a powder (e.g., table salt or icing sugar) and
the stabilization of oil–water emulsions (e.g., salad
cream), whilst humectants (sugar, glycerol) help to
prevent moisture loss and hence dry textures. The
texture of a food often depends on it being a colloidal
system (emulsion, gel, foam) and additives are neces-
sary to stabilize such systems, especially if long
storage times at low temperatures are involved.
(See Stabilizers: Types and Function; Applications.)
0047Emulsifiers, like the naturally occurring lecithin in
egg yolk, help to form stable mixtures (emulsions) of
oil and water which are found in salad creams,
mayonnaise, margarine, icecream – the last has air
incorporated whilst freezing to form a solid foam.
To aid emulsion stabilization, e.g., in salad creams,
thickening agents (referred to as stabilizers) are used
to increase viscosity and so hinder oil and water
separation. Such stabilizers are natural polysacchar-
ides like starch, seaweed extracts such as alginates
and carrageenans, plant extracts such as gum arabic,
carob gum (locust bean gum) and pectins, and micro-
bially produced xanthan gum. Blends of these
so-called hydrocolloids are widely used to create
and to maintain texture in chilled and frozen pro-
cessed foods, especially dairy products ranging from
icecream to gelled milk products and yogurt. (See
Emulsifiers: Organic Emulsifiers; Phosphates as
Meat Emulsion Stabilizers; Uses in Processed Foods.)
0048Less obvious applications are the use of the emulsi-
fiers to retard bread staling, the use of a suspending
agent, often carboxymethyl cellulose, to maintain the
water-insoluble carotene pigment as a fine dispersion
in orange squashes (and so prevent the ‘orange scum’
at the top of the bottle!), and the use of glyceryl
monostearate in dried potato mash to insure rehydra-
tion without stickiness developing.
0049Additives can usefully be incorporated into prod-
ucts to minimize textural changes during their frozen
storage. The use of hydrocolloids in this connection
has been referred to above, and they are often referred
to as cryoprotectants in this connection. Thus the
addition of cryoprotective alginates to icecream
helps to prevent ice-crystal formation (and hence
‘grittiness’) during storage. The manufacture of sur-
imi (frozen, stabilized washed fish mince) depends on
the incorporation of a cryoprotectant mixture con-
sisting of 4% sucrose, 4% sorbitol, and 0.3% poly-
phosphate. This prevents protein denaturation during
frozen storage, and so maintains the heat gelation
properties of the fish proteins required for the pro-
duction of products such as shellfish analogs (crab
sticks), fish sausage, noodles, and burgers. Recently
so-called ‘antifreeze proteins’ have been isolated from
cold-water (e.g., Antarctic) fish and shown strongly
to inhibit ice-crystal formation in icecream products
and to reduce ice-crystal formation in frozen meat,
with resultant reduced drip loss on thawing. Presently
the high cost of these cryoprotectants precludes their
commercial application.
STORAGE STABILITY/Parameters Affecting Storage Stability 5617

Shelf-Life Extension
005 0 It is clear from the examples mentioned that methods
adopted to insure shelf-life extension must consider
the state of the food at the point of storage and select
the best combinations of the relevant parameters.
0051 Organoleptic reasons and concepts of naturalness
are increasingly important factors in determining the
methods used to insure the required storage stability.
Thus the increased use of MAP allied with the use of
chill rather than frozen storage is related to its ‘fresh’
appeal, in contrast to frozen food which has a ‘pro-
cessed’ image. Heat sterilization and sealing under
reduced air content, as typified by traditional canning,
or frozen storage at 20
C or below allows safe
storage for many months but flavor/texture changes
may arise.
0052 The sous-vide or nouvelle carte processes have been
promoted as maximizing flavor, texture, and nutrient
content,aswellasbeingbacteria-free.These processes
involve vacuum packing in heat-resistant packaging,
cooking the vacuum-packed product under vacuum
in a moist steam oven or pressure cooker at less than
110
C, rapidly cooling, and storing at 1–4
C for up
to 3 weeks. This represents the combination of chill
storage, relevant packaging, and exclusion of oxygen
to extend shelf-life. Where low-acid foods are
concerned, the additional use of preservatives is
suggested and overall a maximum storage time of 2
weeks is recommended.
0053 When chill storage is used, the shelf-life is meas-
ured in days and there is less of a margin for storage
abuse than with frozen storage. For foods which are
chill-stored and which are not reheated (above 70
C)
before consumption, the recommended chill storage
temperature and time must be carefully observed.
This places more emphasis on accurate sell- and use-
by dates by the processor, careful stock control by the
retailer, and increased awareness by the consumer.
Seealso: Antioxidants:NaturalAntioxidants;Synthetic
Antioxidants; Biotechnology in Food Production;
Canning:QualityChangesDuringCanning; Chilled
Storage:UseofModified-atmospherePackaging;
Curing; Drying:TheoryofAir-drying; Freeze-drying:The
BasicProcess; Freezing:Principles; Irradiation of
Foods:BasicPrinciples; Oxidation of Food
Components; Preservation of Food; Spoilage:
BacterialSpoilage;MoldsinSpoilage; Stabilizers:Types
andFunction;Applications; Sterilization of Foods;
Storage Stability:MechanismsofDegradation
Further Reading
Ahuenainen R (1996) New approaches to improving the
shelf life of minimally processed fruit and vegetables.
Trends in Food Science and Technology 7: 179–187.
Coultate TP (1996) Food – The Chemistry of its Com-
ponents, 3rd edn. London: Royal Society of Chemistry.
Debeaufort F, Quezada-Gallo JA and Voilley A (1998)
Edible films and coatings: tomorrow’s packaging: a
review. Critical Reviews in Food Science 38: 299–313.
Feeney RE and Yeh Y (1998) Antifreeze proteins, current
status and possible food uses. Trends in Food Science
and Technology 9: 102–106.
Fennema OR (ed.) (1996) Food Chemistry, 3rd edn. New
York: Marcel Dekker.
Institute of Food Science and Technology (UK) (1999) Pos-
ition statement: the use of irradiation for food quality
and safety. Food Science and Technology Today 13:
177–179.
IFST (UK) Current Hot Topics (1999) Position Statement:
Genetic Modification of Food. http//ifst.org/hottop
10.htm.
Jeremiah LE (1996) Freezing Effects on Food Quality. New
York: Marcel Dekker.
King GA and O’Donaghue EM (1995) Unravelling senes-
cence: new opportunities for delaying the inevitable in
harvested fruit and vegetables. Trends in Food Science
and Technology 6: 385–389.
Rockland LB and Beuchat LR (1987) Water Activity:
Theory and Application to Food. New York: Marcel
Dekker.
Taub IA and Singh RP (1998) Food Storage Stability. Boca
Raton, Florida: CRC Press.
Vesmeiren L, Deulieghere F, van Beest M, de Kruijf N and
Debeuere J (1999) Development in active packaging
of foods. Trends in Food Science and Technology 10:
77–86.
Shelf-life Testing
M Hole, University of Lincolnshire and Humberside,
Lincoln, Grimsby, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001The shelf-life of a food is perhaps the most important
detail a consumer needs to know in relation to their
purchase and home storage of foods. This informa-
tion is embodied in the ‘best before’ and ‘use by’ dates
indicated for the food, and which are required by law
to be clearly shown on the food packaging. The ‘use
by’ date is particularly critical in relation to the safe
consumption of a purchased food. Such foods have
relatively short shelf-lives, often in terms of days, and
include fresh and chilled meats and dairy products. In
contrast, ‘best before’ dates are used for foods where
quality, rather than safety, is the practical concern –
thus, a potato crisp, which has absorbed moisture so
that it has lost its crispness, is of undesirable quality
but does not pose a food safety issue.
5618 STORAGE STABILITY/Shelf-life Testing
0002 If the manufacturer should propose an unrealistic
shelf-life, leading to a ‘use by’ date that is too long,
then the likely consumer complaints due to adverse
health and/or quality aspects, possibly requiring
product recalls, would do great damage to the manu-
facturer’s reputation. The subsequent commercial
consequences for the food manufacturer would be
serious, and it is obvious that the correct determin-
ation of the shelf-life is most important – for both the
manufacturer and the consumer. The consumer can
obtain very useful information on the purpose of ‘use
by’ dates from the Foodsense leaflets provided by the
Ministry of Agriculture, Fisheries and Food.
0003 In unprocessed and unpreserved foods, the factors
that limit the shelf-life are mostly related to the
growth of spoilage microorganisms, whilst, in non-
perishables, chemical and physical factors become
more important, e.g., development of off-odors and
off-flavors due to rancidity in fried snack foods, mois-
ture migration in biscuits, and staling of bread and
cakes. The factors that limit the shelf-life of a selec-
tion of foods are indicated in Table 1.
0004 In order to give an indication of the shelf-life of
a product, some type of test must be available to
the manufacturer. Storage studies would normally
be performed as new products are developed, with
regular testing for the shelf-life limiting factors.
However, with some long-term preservation methods,
it may be more convenient to have accelerated shelf-
life studies. This would enable production to com-
mence quite rapidly after the initial development of
the product.
Experimental Design
0005With any experimental procedure, results will only be
valid if the initial sampling and testing stages are
carefully planned and controlled. If any statistical
analysis of the result is required, then the testing
procedure should be designed to be appropriate to
the statistical method being applied. The samples
taken should be representative of the whole batch
and, in careful shelf-life studies, samples should in-
clude any extremes of product storage conditions. It is
also important to test at regular intervals during pro-
duction, especially after any change in ingredients or
methods of production. The design of any procedure
for testing shelf-life should include consideration of
the following factors:
.
0006elucidation of the major types of quality loss for the
product (see Table 1 for examples);
.
0007knowledge of the factors that control the initial
quality during manufacture, e.g., use of food addi-
tives;
.
0008the environmental conditions of storage, e.g., tem-
perature, equilibrium relative humidity (ERH), and
light;
.
0009the type and properties of the packaging to be used,
e.g., permeability to oxygen, light and moisture;
.
0010the kinetics of the reaction or reactions leading to
loss of quality in the actual food (stored in the usual
way).
Acceptability Criteria
0011The acceptance of a food product depends to some
extent on the individual preferences of the consumer;
however, all consumers have a right to expect whole-
some foods that will not cause disease, are of attract-
ive appearance, and are of a good (unchanged)
nutritional value. The major attributes of acceptabil-
ity can be described as: absence of pathogenic micro-
organisms; low levels of spoilage organisms; absence
of off-flavors; no deterioration in color or overall
appearance; no loss of desired texture; and high (un-
changed) nutritional value. It is the function of the
manufacturer to decide upon suitable acceptability
limits for each type of product, and this should form
part of the specification of that product.
tbl0001 Table 1 Factors that limit the shelf-life of foods
Type of food Acceptability criteria
Bread Mold growth
Loss of moisture, staling
Breakfast cereals Rancidity development
Moisture gain/loss of crispness
Vitamin loss
Dried pasta Moisture gain (or loss)
Color loss
Adsorption of undesirable flavors
Fried snack foods Oxidative and hydrolytic rancidity
Fresh poultry Pathogen growth/microbial decay
Bruising
Frozen poultry Sensory quality changes
Color changes/rancidity
Syneresis, moisture loss
Fresh meat Bacterial growth
Loss of color
Fresh fish Bacterial decomposition
Frozen fish Lipid oxidation
Protein denaturation (toughening)
Frozen vegetables Color
Dairy products and milk Bacterial growth
Lipid hydrolysis/flavor changes
Ice cream Textural changes
Oxidation of lipid
Evaporated milk Loss of vitamins
Salad dressing Breakdown of emulsion
Color/flavor changes
Canned foods Taste/flavor changes
Chilled foods Bacterial growth
Flavor changes
STORAGE STABILITY/Shelf-life Testing 5619

Methods of Testing
0012 It is vital that the tests carried out need to relate
specifically to the safety aspect concerned or, in
quality terms, relate to the limiting quality criteria.
0013 The main methods of testing the shelf-life of a food
include chemical/physical tests, microbiological enu-
merations and sensory evaluations. Due to the com-
plex nature of most composite foods, many chemical
tests, e.g., to establish nutrient levels, are very lengthy,
often involving complex extraction procedures and
use of sophisticated and expensive equipment. Micro-
biological and sensory tests are generally regarded as
easier to perform and are more adaptable for use in
nonspecialized laboratories.
0014 Although the ultimate test of a product’s accept-
ability or attractiveness lies with the consumer, the
consumer cannot necessarily judge whether foods are
safe.
Microorganisms
0015 The first criterion of any testing is to insure that the
product is safe – both from food-poisoning micro-
organisms and from any toxins. Food-poisoning
organisms, e.g., Salmonella typhimurium, may be
detected by growth of the organism on selected
media, but growth is usually relatively slow, and a
result would not be obtained until 3–4 days after the
test had been initiated. Rapid methods of measuring
microbial concentrations are now available, and a
range of automated instrumental methods can be
used, e.g., the ‘Bactometer’.(See Food Poisoning:
Tracing Origins and Testing; Microbiology: Detec-
tion of Foodborne Pathogens and their Toxins.)
Chemical Toxins
0016 A variety of tests can be utilized to test for the range of
toxins found in foods. For example, the presence of
toxic metals, e.g., mercury or lead, may be deter-
mined by the use of atomic absorption spectrometry,
and the highly toxic aflatoxins, produced as second-
ary metabolites from the growth of the mold Asper-
gillus flavus on cereals, may be detected by using
the fluorescent properties of the compounds, e.g.,
use of high-performance liquid chromatography
with fluorescence detection. (See Heavy Metal Toxi-
cology; Mycotoxins: Occurrence and Determination.)
Flavor Changes
0017 From Table 1, it is apparent that the shelf-life of
‘microbiologically safe’ foods is often limited by
either changes in flavor or development of off-odors
and off-flavors, e.g., production of volatile carbonyl
compounds from lipid oxidation leading to rancid
flavors. These changes in flavor may be monitored
by sensory means or from utilization of analytical
techniques such as headspace gas chromatography.
With this technique, the volatiles arising from a food-
stuff are swept into a chromatography column con-
tained in a thermostatically controlled oven. A carrier
gas moves the flavor compounds through the column
where selective separation occurs, depending on the
polarity of the packing material (stationary phase) of
the column andthe molecular structure of the volatiles.
Eventually, the separated compounds are detected and
are registered as a peak on a trace. The identity of the
peaks may be established by comparison of their
retention times with standards or from coupling the
gas chromatography with a mass spectrometer. The
contribution of a specific volatile to any off- or
undesirable odor may be found by smelling the indi-
vidual components as they emerge from the column.
Increasing use is made of ‘electronic noses’ (e.g.,
‘Aromascan’) for the instrumental characterization
of aroma. In contrast to gas chromatography, these
electronic noses detect the overall aroma, giving
rise to an aroma fingerprint that can be used to indi-
cate the shelf life limit. (See Sensory Evaluation:
Aroma.)
0018It is well established that a wide range of analytical
tools are available to the food analyst for determin-
ation of the (molecular) components of foods that
may be responsible for any shelf-life deterioration,
e.g., nutrients (essential amino acids and vitamins).
In some foods, determination of the factors that
actually limit shelf-life may involve very complex
analyses; thus an associated factor, which changes at
the same rate but does not affect shelf life directly,
may be used. The reader is referred to the bibliog-
raphy for a more thorough review of methods used in
the chemical analysis of foods.
Color
0019Changes in the color of foods are often major factors
limiting shelf-life. Instrumental testing of surface
color, or of the color of a well-homogenized sample,
may be achieved with a colorimeter, which gives
results in terms of the Hunter L, a and b values.
That is, a measure of the degree of ‘whiteness,’‘red-
ness,’ and ‘blueness’ of a sample. Otherwise, com-
parisons of the food color with acceptable colors are
useful, but this is essentially a sensory method of
analysis. (See Colorants (Colourants): Properties and
Determination of Natural Pigments; Properties and
Determinants of Synthetic Pigments; Sensory Evalu-
ation: Appearance.)
5620 STORAGE STABILITY/Shelf-life Testing
Texture
0020 Sensory methods are also used to provide information
on the texture of foods. Attributes such as firmness,
crispness, juiciness, and chewiness are fairly typical of
those used. In addition, the texture of certain foods,
e.g., biscuits and apples may be measured using phys-
ical (instrumental) tests and equipment such as the
Instron. This particular piece of equipment may be
modified to undertake a variety of penetration, exten-
sibility, or shearing tests. (See Sensory Evaluation:
Texture.)
Sensory Methods
0021 A wide variety of sensory evaluation tests are avail-
able for use in testing the acceptability of foods. The
tests may be classified into those that are affective
(i.e., ask for an opinion or preference) and those
that are nonaffective (i.e., require a score to be
assigned for a particular attribute). However, for
shelf-life studies, it is usually more appropriate to
use scoring tests. For the results to be meaningful, it
is important to have a suitably designed taste panel/
sensory test giving results that can be statistically
validated. This also requires the establishment of a
null hypothesis, e.g., no difference between the
samples presented, in order to establish the signifi-
cance of the results. It is usually recommended that at
least 10 persons are required to act as panellists if the
results obtained are to be valid, the actual number
depending on their level of training (if untrained, then
more panellists will be required). Sensory panels are
sometimes regarded as time-consuming and expen-
sive to set up, but they do have an advantage over
instrumental methods in that several attributes of the
food can be measured in one session. (See Sensory
Evaluation: Sensory Characteristics of Human
Foods; Food Acceptability and Sensory Evaluation;
Practical Considerations; Descriptive Analysis.)
Accelerated Shelf-life Testing
0022 Ideally, a food would be tested under the conditions
of intended storage in order to measure the length of
time over which it is acceptable, whether from a
safety or quality aspect. For a food with a shelf-life
of a few days or weeks (e.g., chilled meat), this is
no problem, but for foods with shelf-lives of months,
or years, the manufacturer would like a quicker
method, i.e., one that gives a result in a shorter
time than the shelf-life under the intended storage
conditions.
0023 Since storage temperature is usually the main
factor influencing the shelf-life, most accelerated
methods simply use a higher temperature than the
intended storage temperature and hope to relate the
shorter shelf-life at the higher temperature to the
shelf-life at the intended storage temperature. The
theoretical basis is related to the Arrhenius relation-
ship, one form of which is:
ln k ¼ cst:
Ea
RT
,
where cst., Ea (the activation energy), and R (uni-
versal gas constant) can be taken as constants.
Hence:
ln k /
1
T
,
where T is the temperature (degrees Kelvin), and k
is the rate constant, which, in this context, can be
related to the shelf-life time, i.e.:
lnðshelf-lifeÞ/
1
T
:
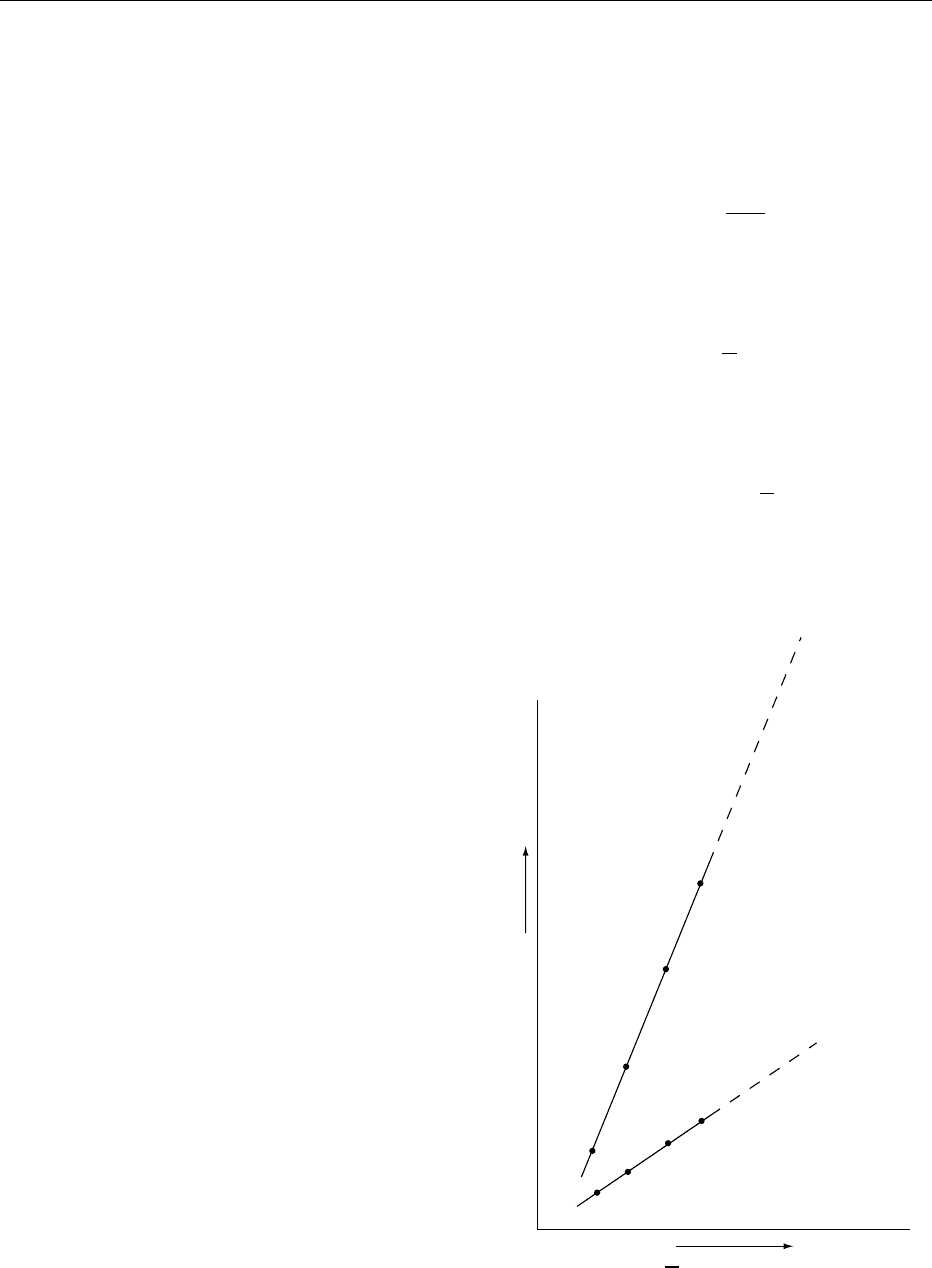
A graph of ln(shelf-life) against 1/Twill give a straight
line (see Figure 1). Thus, if the shelf-life is measured
(Kelvin)
1
T
In (shelf-life)
fig0001Figure 1 Arrhenius plot showing extrapolated shelf-lives for
two foods with different rates of quality deterioration.
STORAGE STABILITY/Shelf-life Testing 5621

for a series of temperatures higher than the intended
storage temperature, extrapolation will give the shelf-
life at the lower temperature. The method has limita-
tions and can be costly, but this approach is often
used. Some examples are given below; other examples
will be found in the bibliography. Frozen foods often
have shelf-lives of many months, or years, and accel-
erated shelf-life testing can be usefully applied. How-
ever, the elevated temperature in this case must be
realistic – a temperature at which the food was not
frozen would not be realistic. Thus, the shelf-life at an
intended storage temperature of 30
C might be
usefully indicated from shelf-life measurements
made at up to 5
C. This would ensure that the
food matrix was similar (i.e., frozen) at the elevated
temperatures. This illustrates an important point; the
nearer the elevated temperatures are to the intended
storage temperature, the more realistic the extra-
polated shelf-life will be.
0024 Where foods are being reformulated and the effect
of different formulations on shelf-life is needed,
accelerated methods are very useful in indicating
relative shelf-lives. This allows a new shelf-life to be
proposed from a knowledge of the original shelf-life.
It has been stressed that the shelf-life determinations
are only relevant if based on measurements of the
actual limiting factor, for example, odor. This aspect
can become critical when accelerated shelf-life testing
is carried out, if the limiting factor changes at the
higher temperature. An example (discussed by
Labuza – see Further Reading) is provided by the
storage of dehydrated mashed potato. At ambient
temperatures, the limiting factor is off-odors from
lipid oxidation, but above about 40
C, the limiting
factor for organoleptic acceptance is discoloration due
to Maillard browning (See Browning: Nonenzymatic).
Hence, if accelerated shelf-life testing is carried out
above 40
C, sensory analysis will give results that
extrapolate to an incorrect shelf-life for ambient tem-
perature storage. More specifically, results obtained
above 40
C extrapolate to a shelf-life above 1000
days at 20
C, whilst measurements made at 20
C
(where odor is the limiting factor) indicate a shelf-
life of about 500 days.
0025 Thus, the food system must be carefully studied to
insure that the accelerated shelf-life testing is done as
realistically as possible. The sensitivity of a food to
temperature changes is indicated by the slope of the
‘Arrhenius graph’ (See Figure 1) – the more sensitive
food having the larger gradient. An alternative
approach is the use of the Q
10
value, where
Q
10
¼
rate at ðTþ10Þ
C
rate at T
C
or
Q
10
¼
shelf-life at T
C
shelf-life at ðTþ10Þ
C
:
The more sensitive the food is to temperature change,
the higher the Q
10
value, as illustrated in Table 2.
For an initial shelf-life of 100 days at T
C, a food
with Q
10
¼2 would have a half-life of 50 days at
(Tþ10)
C, whilst if Q
10
¼4, the half-life would be
25 days at (T þ10)
.
Prediction of Shelf-life
0026The use of accelerated shelf-life testing avoids the
need for prolonged storage studies – and in reality is
a predictive method based on measurements made at
higher temperatures than the actual storage tempera-
ture. The possible problems with accelerated shelf-life
testing can be minimized if predictive methods using
data from the actual storage conditions can be used.
Thus, if the rate of deterioration (i.e., the kinetics) is
known (at the actual storage temperature), initial
values of the loss of quality, for example, can be
used to indicate the quality loss, and hence shelf-life,
for extended storage times.
0027The loss of quality for most foods can be repre-
sented by:
dA
dt
¼ kA
n
,
where A is the quality factor measured, e.g., extent of
nonenzymatic browning, t is the time, k is a constant
that depends on the temperature and water activity,
n is a power factor giving the order of the reaction
and dA/dt is the rate of change of A with time (nega-
tive, loss of A; positive, production of undesirable end
products). In practice, zero-order (n ¼0) or first-
order (n ¼1) kinetics are relevant for quality changes
in foods.
Zero-order Kinetics
0028The order of the reaction, n, defines whether the rate
is dependent on the value of A. Many food systems
tbl0002Table 2 Q
10
values for selected foods
Type of food Q
10
value Criteriaforendofshelf-life
Fresh cod 4.4 Bacterial growth
Sterilized milk 1.71 Flavor change
Pasteurized milk 2.64 >10
6
CFU
a
ml
1
Pasteurized egg 5.37 Flavor change
Spray-dried egg 1.21 60% loss of vitamin A
Margarine 1.91 25% loss of vitamin A
Canned kidney beans 1.7 20% loss of thiamin
a
CFU, colony-forming units.
5622 STORAGE STABILITY/Shelf-life Testing

are assumed to behave as zero-order reactions (n ¼0).
Thus, the rate of loss is constant under constant
storage conditions. Thus:
dA
dt
¼ k,
which may be integrated to give
A ¼ A
o
kt or A
e
¼ A
o
kt
s
,
where A
o
is the initial quality value at time t, A
e
is the
value of A at the end of the shelf-life and t
s
is the shelf-
life in days, months, or years.
0029 A may be defined analytically or by taste-panel
evaluation. If A
o
is assumed to be 100% quality and
A
e
just acceptable quality, then:
k ¼
A
o
A
e
t
s
¼
100%
t
s
,
Thus, based on this knowledge, it is possible to pre-
dict the shelf-life of a food at a single given tempera-
ture, if the value of the quality change at any given
time is known.
0030 For example, if it is known that a certain food has
lost 25% of its quality in 50 days when held under
constant conditions, then the rate of loss:
k ¼
A
o
A
t
¼
100 75
50
¼ 0:5% per day:
The amount of shelf-life left as a function of time may
also be represented by a graphical plot (Figure 2).
0031 Reaction types in foods that are thought to give
zero-order kinetics include enzymatic degradation,
nonenzymatic browning, and lipid oxidation (rancid-
ity). Loss of quality of frozen foods in general follows
zero-order kinetics.
First-order Kinetics
0032Many foods deteriorate by first-order kinetics (n ¼1),
which results in an exponential decrease in the rate of
change as the quality decreases. Thus, the rate of
quality loss is directly dependent on the amount left:
dA
dt
¼ kA:
Integration gives:
ln ðA
c
=A
o
Þ¼kt
s
:
A semi-logarithmic plot of A
c
/A
o
against time (t) gives
a straight line with slope k.
0033The types of deterioration that follow first-order
kinetics include microbial growth (fresh meat and
fish), microbial production of off-flavors, vitamin
losses (canned and dried foods), and loss of protein
quality (dried foods). (See Canning: Quality Changes
During Canning; Fish: Spoilage of Seafood; Meat:
Preservation.)
0034A knowledge of the kinetics of food deterioration
has been incorporated into computer packages,
which can be applied to rapidly predict shelf-lives
for more complex situations.
0035Such computer-based predictive models were de-
veloped initially to predict the growth of pathogens
in foods in order to predict shelf-lives where the food
would be safe to eat. The ‘Food Micromodel’, de-
veloped by the Ministry of Agriculture, Fisheries
and Food in the early 1990s is such a model and can
be applied to predict the growth of a wide range
of microorganisms, both pathogenic and spoilage
organisms, under a wide range of storage conditions.
The approach is particularly relevant for chilled
foods, and the ‘Forecast’ service provided by Camp-
den and Chorleywood Food Research Association
(CCFRA) allows the prediction of the growth of
common spoilage bacteria for a range of storage con-
ditions (pH, temperature, salt content, etc.). Another
example of a computer-based predictive model (also
from CCFRA) is the prediction of mold-free shelf-life
using the ERH CALC
TM
package. This model allows
rapid calculation of the ERH (or water activity) and
hence the growth of molds from a knowledge of
the ingredients and storage conditions of bakery
products.
Time–Temperature Indicators (TTIs)
0036Since temperature is such an important factor in de-
termining shelf-life, it would be useful to know the
full thermal history of a food after processing. Any
thermal abuse during distribution and storage would
alter the shelf-life, and hence the ‘use by’ date of the
food.
0 50 100 150 200
0
20
40
60
80
100
120
Time (days)
Quality changes (%)
fig0002 Figure 2 Zero-order reaction plot showing residual shelf-life
against time (k ¼ 0.5% per day).
STORAGE STABILITY/Shelf-life Testing 5623
0037 Time–temperature indicators are available that
give a full thermal history of the food, and such
sensors attached directly to the packaged food would
be most useful in this connection. If they were linked
to the computer-based models for shelf-life predic-
tion, any temperature abuse would result in a new
shelf-life prediction.
Home Storage
0038 There is an obvious need for the manufacturer’s stor-
age instructions to be followed in order that the ‘use
by’ date is valid. The food industry can arrange the
correct storage conditions up to the point of sale, but
thereafter, it is necessary for the consumer to insure
correct home storage, especially for chilled foods. The
possible lack of correct temperature control during
home storage indicates the need for some margin of
error to be incorporated, especially into the ‘use by’
date.
See also: Fish: Spoilage of Seafood; Food Poisoning:
Classification; Tracing Origins and Testing; Heavy Metal
Toxicology; Meat: Preservation; Microbiology:
Detection of Foodborne Pathogens and their Toxins;
Mycotoxins: Occurrence and Determination;
Preservation of Food; Water Activity: Effect on Food
Stability; Sensory Evaluation: Sensory Characteristics
of Human Foods; Food Acceptability and Sensory
Evaluation
Further Reading
Campden and Chorleywood Food Research Association
(CCFRA) (1997) Evaluation of Shelf Life for Chilled
Foods, Technical Manual No. 28. Chipping Campden,
UK: CCFRA.
Campden and Chorleywood Food Research Association
(CCFRA) (1999) ERH CALC
TM
– Version 2. Chipping
Campden UK: CCFRA.
Fennema OR (ed.) (1996) Food Chemistry, 3rd edn. New
York: Marcel Dekker.
Institute of Food Science and Technology (IFST) (1993)
Shelf Life of Food – Guidelines for its Determination
and Prediction. London: IFST.
Labuza TP (1984) Application of chemical kinetics to
deterioration of foods. Journal of Chemical Education
61: 348–358.
Ministry of Agriculture, Fisheries and Food (MAFF) (1998)
Understanding Food Labels. London: Foodsense, Food
Safety Directorate.
Man CMD and Jones AA (1994) Shelf Life Evaluation of
Foods. London: Blackie Academic and Professional.
Nielson SS (1998) Food Analysis, 2nd edn. Gaithersburg,
MD: Aspen.
Singhal RS, Kulkarni PR and Rege DV (1997) Handbook of
Indices of Food Quality and Authenticity. Cambridge,
UK: Woodhead.
Smith G (1993) Encyclopaedia of Food Science, Food Tech-
nology and Nutrition. London: Academic Press.
Thorne S (1992) Mathematical Modelling of Food Process-
ing Operations. London: Elsevier Applied Science.
Stouts See Beers: History and Types; Wort Production; Raw Materials; Chemistry of Brewing; Biochemistry of
Fermentation; Microbreweries
STRAWBERRIES
R B Smith and L J Skog, University of Guelph,
Vineland Station, Ontario, Canada
A Dale, University of Guelph, Simcoe, Ontario, Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The strawberry belongs to the genus Fragaria, and is a
member of the rose family, Rosaceae. The cultivated
strawberry, F. ananassa L. Duchesne, is a hybrid
between F. chiloensis Duch and F. virginiana Duch.
The ananassa designation is due to the resemblance to
the pineapple in flavor, odor, and shape. F. chiloensis
was introduced into Europe from Chile and F. virgini-
ana from eastern North America. The fruit of the
strawberry is grown and enjoyed worldwide. This
article discusses the global distribution, varieties,
commercial importance, morphology, nutritional
composition, handling, storage, and industrial uses
of this crop.
5624 STRAWBERRIES

Global Distribution
0002 The strawberry plant is highly adapted to a wide
range of climatic and soil conditions. Commercial
plantings of strawberries are found on every con-
tinent (except Antarctica) and from the subarctic
(Finland) to the tropics (Ecuador). However, most of
the world’s production is in the northern hemisphere
(Table 1). Strawberries are planted on soil types
ranging from desert sands requiring extensive irriga-
tion to heavy clay soils requiring underdrainage and
the addition of organic matter. In subarctic areas, the
plants may be exposed to temperatures as low as
50
C in winter and as high as 40
C in summer. In
desert areas, temperatures may be above 50
C in the
day and near 0
C at night. Where harsh winter con-
ditions prevail, plants may be protected from extreme
temperatures by covering with straw or other organic
mulch. Mist irrigation is sometimes used to cool the
fruit and leaves under conditions of high summer heat.
000 3 The geographical areas with the highest total pro-
duction and the highest production per hectare are
located where there is a Mediterranean-type climate.
These areas include California, Spain, southern Italy,
and southern France. In these areas strawberries can
be produced virtually all year and day-neutral var-
ieties, i.e., those in which fruit bud initiation is inde-
pendent of day length, are grown more extensively.
The June-bearing varieties, which predominate in
most other growing areas, have a requirement for a
short day (some as little as 8 h).
Varieties
0004The commercial strawberry is a heterozygous octo-
ploid with characteristics inherited mainly from two
genetically variable wild species. The first crosses
between F. chiloensis and F. virginiana were made in
Europe in the early 1760s. Breeding work with other
species has not been successful, primarily owing to
genetic incompatibility.
0005Variability in the wild species has been used by
breeders who use parents with desired traits when
crosses are made. When seeds from a cross are
planted, the resultant plant is a clone. Thus, breeders
select individual plants with desired characteristics
rather than selecting a population of plants.
0006The general objectives of strawberry-breeding pro-
grams are as follows: (1) to enhance yields; (2) to
improve fruit quality, which includes factors such as
flavor, firmness, keeping quality or other characteris-
tics; (3) to produce plants adapted to specific environ-
mental or cultural conditions; (4) to develop varieties
resistant to diseases and insects; and (5) to develop
varieties with specific requirements, such as suitabil-
ity for mechanical harvesting and processing, or extra
firmness for long-distance shipping. New strawberry
varieties are continuously being introduced, thus
worldwide they number in the hundreds. Some of
the more important varieties are listed in Table 2.
Commercial Importance
0007The Food and Agriculture Organization (FAO) lists
strawberry production statistics for 64 countries.
Seven countries – Spain, Japan, Korea, Turkey,
Poland, Mexico, and the USA – account for more
than 66% of the world’s 2.7 10
9
kg annual produc-
tion (Table 1). This worldwide production would
have a farm-gate value of over $5.4 10
9
(US) based
on their value in North America. This return would
represent $2 kg
1
, averaged for fresh-market berries.
0008In countries such as Poland and Mexico, a large
portion of the crop is processed and sold for foreign
currency. In the European Community (EC), straw-
berries are an important cash crop as this trading
block produces over 7.0 10
8
kg annually. Much of
the fresh crop produced in the USA and the EC
is shipped to northern areas for fresh-market con-
sumption. Growers harvesting for fresh-market
consumption receive a higher price per unit of fruit
than those harvesting for processing, but fresh market
harvesting costs are higher.
0009Yields in the state of California are over 40 000 kg
ha
1
– a level of production two to six times that of
other areas in the USA or in other countries. Califor-
nia produces 80% of the US crop, and 65% of the
tbl0001 Table 1 Global distribution of production by geographical area,
including a listing of countries producing 5.0 10
7
kg or more
Global distribution by geographical area and countries withinany one
area producing 5.0 10
7
kg or more
World total
Africa
North and Central America
Mexico
USA
South America
Asia
Japan
Korea, Republic of Russian Federation
Turkey
Europe
France
Germany
Italy
Poland
Spain
Oceania (includes Australia and New Zealand)
From Food and Agriculture Organization of the United Nations (1998)
ProductionStatistics. FAOSTAT,Availableathttp://apps. Fao. org
(February 2000).
STRAWBERRIES 5625

North and Central American crop, making it an
important cash crop in that state.
0010 In some countries, particularly around large cities,
consumers are invited by growers to pick their own
fruit. This has become a very important means of
harvesting and marketing the strawberry crop. Pick-
your-own farm operators do not have problems find-
ing labor to harvest their berries. However, there are
other problems, such as in-field consumption, crowd
control, incomplete harvesting, and grower–public
interaction.
Morphology
0011 The strawberry is an accessory fruit with an aggregate
of achenes (the true fruits which contain the seed),
attached in an orderly fashion to the epidermis of the
receptacle (the floral axis to which the various flower
parts are attached). The large, fleshy receptacle (con-
sisting of pith, cortex, and a vascular system) is the
succulent, edible portion of the fruit. It expands in
response to hormones produced by the ovules after
fertilization has occurred.
0012 The fruit of the commercial strawberry has a red-
colored exterior and an interior color ranging from
white to dark red. Fruit shape depends on such factors
as variety, environmental conditions under which it
was grown, and planting location. Fruit size, which
varies considerably even within cultivars, depends on
environmental factors and position of the fruit within
a cluster. The primary berry is the largest and has the
most achenes. Secondary and tertiary berries become
progressively smaller and have fewer achenes. The
fruit ranges between 2 and 5 cm in length depending
on variety and other factors. Descriptors used to
identify the shapes of strawberries include globose,
globose conic, conic, long conic, oblate, necked
(a long neck), long wedge, and short wedge.
Chemical and Nutritional Composition
0013A list of some of the nutrients in strawberries is pre-
sented in Table 3, with those for apples and oranges
included for comparison. The strawberry contains the
tbl0002 Table 2 Strawberry varieties grown in quantity in different geographical areas of the world
Geographicalarea Varieties
California (some of the same varieties in Mexico) Selva, Chandler, Commander, Oso Grande, Camerosa, Seascape, Lido,
Key Largo, E26, Coronado, San Miguel, Catalina
North America (except California and Mexico) Totem, Bounty, Kent, Earliglow, Honeoye, Governor Simcoe, Glooscap, Selva,
Camerosa, Sweet Charlie, Annapolis, Jewel, Cavandish, Delmarvel, Veestar,
Redcrest, Hood, Chandler
South America Camerosa, Chandler, Pajaro, Selva, Oso Grande, Sweet Charlie, Tudla
South Asia Hokowase, Reiko, Toyonoko, Nyoho, Sachinoka, Tochiotome, Suhong, Rachel,
Douglas, Pajaro, Cruz, Senga Sengana, Chandler, Oso Grande, Selva,
Sequoia
Western Europe (excluding Spain, Italy, France) Senga Sengana, Dania, Elsanta, Elvira, Karola, Symphony, Pegasus, Honeoye,
Evita, Bolero, Tango, Selva, Zefyr, Bounty
Spain, Italy, France Pajaro, Camerosa, Chandler, Oso Grande, Tudla, Eris, Tethis, Clea, Miranda,
Marmolada, Idea, Addie, Miss, Selva, Seascape, Elsanta, Gariguette,
Dar Select, Eros
Eastern Europe/northern Asia Senga Sengana, Zarya, Gorella, Elsanta, Kokinskaja Rannaja, Festivalnaja
Romashka, Zenit, Nadezhda, Rannyaya Plotnaya, Lurck Vira, Istochnik, Desna
Oceania Chandler, Pajaro, Parker, Selva, Camerosa
Data from Hancock JF (1999) Strawberries. Wallingford, UK: CABI Publishing.
tbl0003Table 3 Nutrient composition (per 100 g fresh weight) of
strawberries compared to that of apples and oranges
Nutrients andunits Strawberry Apple Orange
Water (g) 91.57 83.93 86.75
Food energy (kcal) 30 59 47
Food energy (kJ) 127 245 197
Protein (N 6.25: g) 0.61 0.19 0.94
Total lipid (g) 0.37 0.36 0.12
Cholesterol (mg) 0 0 0
Carbohydrate (g) 7.02 15.25 11.75
Fiber (g) 0.53 0.77 0.43
Calcium (mg) 14 7 40
Magnesium (mg) 10 5 10
Phosphorus (mg) 19 7 14
Potassium (mg) 166 115 181
Ascorbic acid (mg) 56.7 5.7 53.2
Folic Acid (mg) 17.7 2.8 30.3
Vitamin A (mg) 8.1 1.5 6.3
Data from USDA (1982) Composition of Foods: Fruits and Fruit Juices, Raw,
Processed, Prepared. US Department of Agriculture, Human Nutrition
Information Services, Agriculture Hand-book, pp. 8–9. Washington, DC: US
Government Printing Office.
5626 STRAWBERRIES
