Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

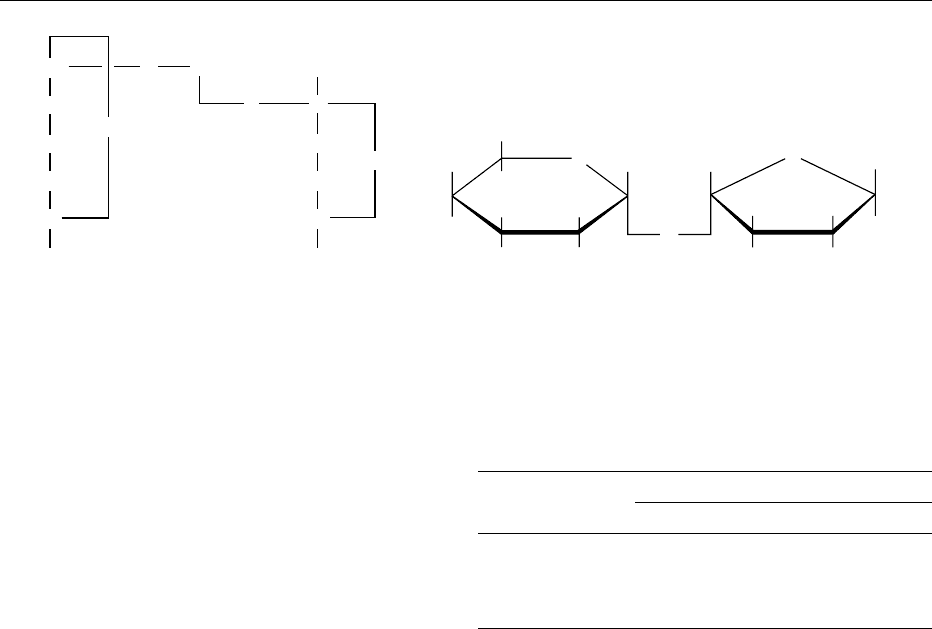
Physical Properties
0009 Crystalline sucrose Sucrose in a pure state crystal-
lizes in a monoclinic system, forming transparent,
colorless, and odorless sphenoidal prismatic crystals,
the form of which may be strongly affected by impur-
ities (needle-like shapes).
0010 Sucrose has a characteristic sweet taste without any
other flavor or aftertaste; it is the standard reference
for sweetness. If sucrose is given a sweetness rating of
100, the relative sweetness of fructose is between 105
and 125 and that of glucose between 65 and 75,
depending on the conditions (acidity, pH, tempera-
ture, etc.).
0011 The crystal density (D
15
4
) is 1.5879, but the appar-
ent specific gravity of high-grade white sugar varies
between 0.72 and 0.88 kg dm
3
, depending on the
crystal size and humidity, the storage duration, and
the thickness of the layer in the storage silo.
0012 Sucrose decomposes at about 186–188
Cto
form brown products (caramel) and, finally, chars,
but impurities and the products of thermal decom-
position significantly lower the temperature of de-
composition.
0013 The heat of combustion is 1351.3 kcal mol
1
or
3.95 kcal g
1
. Sucrose is thus less caloric than fat
(9.3 kcal g
1
) and protein (4.1 kcal g
1
).
0014 Sucrose solutions Sucrose is readily soluble in water,
and its solubility increases with temperature. By
cooling or evaporating a saturated sucrose solution,
a metastable supersaturated solution is obtained.
Sucrose is sparingly soluble in ethanol and practically
insoluble in ether.
0015 The solubility of sucrose has been calculated at
different temperatures. In 1978, the International
Commission for Uniform Methods of Sugar Analysis
(ICUMSA) officially adopted the tables of Vavrinecz
and of Charles. Table 1 gives example solubilities at
various temperatures, which have been taken from
the Charles tables.
0016Sucrose in aqueous solution has the property of
rotating the plane of linear polarized light, and the
angle of rotation is proportional to the concentration
and to the path length of the solution traversed by
the light beam. This angle is measured by means of
polarimeters. In the sugar industry, special polarim-
eters, called ‘saccharimeters,’ are directly calibrated
in ‘degrees sugar’ (
Z). The ICUMSA adopted a
new definition of the international sugar scale as
from 1 July 1988. The basis of the 100
Z point of
the international sugar scale is the optical rotation
of a ‘normal sugar solution’ at the wavelength (l)
of the green line of the mercury isotope
198
Hg
(l ¼546.2271 nm in vacuo) measured at 20.000
C
in a 200.000-mm polarimeter tube. The ‘normal
sugar solution’ corresponds to a concentration of
26.000 g sucrose weighed with brass weights in air
under normal conditions (1013 hPa pressure, 20
C,
50% relative humidity) in 100.000 cm
3
of solution at
20
C. On this new international sugar scale, the op-
tical rotation of the 100
Z point is 40.777 + 0.001
.
0017The density of sucrose solutions is a function of the
mass concentration and of the temperature. The dens-
ity values universally used are those of Plato (first
published in 1900) giving D
20
4
for the solutions
tbl0001Table 1 Solubility of sucrose at four example temperatures
according to the ICUMSA
T(
C) Solubility of sucrose (g) in
1g of water 100 g of solution
10 1.884 65.32
20 1.994 66.60
50 2.576 72.04
90 4.262 81.00
HC
HC
HCOH
HCOH
CH
2
OH
HOCH
CH
2
OH
HC
C
HCOH
CH
2
OH
HOCH
O
(a) (b)
O
O
a
b
H
OH
OH
H
CH
2
OH
H
H
O
H
H
OH
OH
O
H
HOCH
2
H
CH
2
OH
OH
O
fig0001 Figure 1 Structural representations of sucrose: (a) Fisher projection; (b) Haworth-type structure. Reproduced from Sucrose:
Properties and Determination, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ
(eds), 1993, Academic Press.
SUCROSE/Properties and Determination 5637

between 0 and 95 degrees Brix (grams per 100 g of
solution).
0018 The refractive index of sucrose solutions is a func-
tion of the amount of dissolved material and also of
the temperature. The ICUMSA has published refract-
ive index tables for sucrose solutions from 0 to 85
degrees Brix at 20 and 27
C, as well as temperature
correction tables.
0019 There are many other properties that have been
measured, such as viscosity, osmotic pressure, specific
heat, boiling point elevation, and equilibrium relative
humidity, the knowledge of which is very useful in
food technology.
Structural Properties
0020 In addition to its use in food processing as a sweetener
and an energy source, sucrose has other functions.
Thus, it is not sufficient to replace sucrose by artificial
noncaloric sweeteners and expect simply to obtain
similar products with the same sweetness but without
the calories. (See Sweeteners: Intensive.)
0021 Sucrose acts as a preservative by its ability to
reduce water activity and to increase osmotic pressure
to a level where the growth of even the most osmo-
philic microorganisms is no longer possible (as in
jams, jellies, fruit syrups, concentrated milk).
0022 Sucrose acts as a bulking agent and a texturizer in
confectionery, baked goods, and soft drinks. Owing to
its high solubility and viscosity, it gives body and
mouth-feel to various preparations. It influences the
pore size distribution, softness, and structure of pastry.
0023 Sucrose also has properties of humectancy, i.e., it
assists the ability of products to withstand changes
in moisture content, and so extends the shelf-life of
goods such as cakes.
0024 Sucrose is an antioxidant, preventing the oxidation
of flavors in fruit preserves. (See Antioxidants: Nat-
ural Antioxidants.)
0025 Sucrose can fulfill the role of a diluent or carrier for
flavoring or coloring additives and, at the same time,
act as enhancer for natural food flavors, balancing
sweetness, sourness, and bitterness. Sucrose alone can
also give a caramel flavor and a browning of the
products in baking, as a result of partial degradation.
Inversion on Storage and Processing
0026 Sucrose, in a solid crystallized form, is one of the most
chemically pure food products available in the world
with a purity of 99.97 + 0.05%. It can be stored
unchanged almost indefinitely under ideal conditions.
To avoid caking or, conversely, attraction of moisture
or even liquefaction in the silo, the sugar produced,
prior to storage, must be dry and cold and must have
undergone a conditioning process.
0027Freshly produced crystals are surrounded by a thin
film of supersaturated mother liquor and possibly
contain syrup inclusions. It takes a few days to
achieve crystallization and to release free moisture,
and, if this water is not allowed to escape, serious
storage problems can occur, such as chemical or
microbiological inversion, and, with the lowering of
the pH, an accelerated degradation with formation
of colored products. It is thus useful to store sugar
initially in a holding bin aerated with cold dry air
before storage in the final silo.
Fermentation of Sucrose
0028Sucrose may be readily fermented by several micro-
organisms and is a good starting material for the
microbial production of many chemical products
such as ethanol, butanol, glycerol, citric acid, levuli-
nic acid, dextran, and many others. Molasses, a
byproduct of the sugar industry that no longer con-
tains crystallizable sucrose, is often used as a cheap
source for these processes.
0029The most important fermentation process known
from time immemorial is probably alcoholic fermen-
tation by yeasts used to produce beverages such as
beer and wine. The first stage of alcoholic fermenta-
tion is an enzymatic hydrolysis of sucrose to glucose
and fructose by invertase.
Analysis, Extraction, and Isolation
0030The term ‘sucrose analysis’ can include numerous
determinations depending on the purpose of the
analysis.
Sucrose in the Solid State (Commercial Sugars)
0031The purity of sucrose can be determined by polarim-
etry or, indirectly, by determining the amount of non-
sucrose material present, i.e., water content and other
compounds (organic or inorganic).
0032The water content can be determined by direct
methods, such as the Karl–Fischer titration or the
conventional ‘loss on drying’ method.
0033Inorganic compounds can be evaluated gravimetri-
cally by incineration of the sugar. The result is the
sum of the water-soluble and water-insoluble residues
or ‘ash’ in the form of ‘carbonate ash’ or ‘sulfated
ash,’ according to the method chosen. Ionized soluble
salts (‘conductivity ash’) can be determined con-
ductimetrically. This method is easier and less time-
consuming than incineration, but does not have
precisely the same significance as ‘gravimetric ash.’
0034Among the so-called organic nonsugars, there
may be small amounts of ‘invert sugar,’ a mixture of
reducing sugars. There are numerous methods to
5638 SUCROSE/Properties and Determination

determine reducing sugars in the presence of sucrose,
based on the reduction of a copper(ii) complex with
tartaric acid in alkaline solutions, e.g., Lane and
Eynon titration, in addition to the Emmerich method
for the determination of traces of reducing sugars in
pure sucrose.
0035 It is also possible, when required, to determine
individual ions such as copper, lead, iron, calcium,
chloride, arsenic, sulfite, and organic nonsugars such
as aminonitrogen, betaine, lactic acid, citric acid, and
so on.
0036 In addition to the above purity evaluations, buyers
require other quality criteria according to the specific
uses intended for the sucrose. These may include
particle size and uniformity, visual appearance,
color and turbidity in aqueous solution or in alcoholic
solution, insoluble matter or the presence of visible
extraneous impurities, filterability, foaming pro-
perties, and floc formation in soft drinks. Some
industries have special requirements concerning
microbiological quality. For instance, the canning in-
dustry has a procedure for determining spore-forming
thermophilic bacteria, and in the soft drinks industry,
procedures are carried out for the detection of meso-
philic bacteria, yeasts, and molds.
Sucrose Solutions and Technical Sugar Juices
0037 Polarimetry may be used to determine the sucrose
content of pure sucrose solutions and of intermediate
liquid sugar products. In the latter case, it will often
be necessary to eliminate many impurities to obtain a
clear solution to allow passage of light through the
polarimeter tube. This clarification is normally
achieved with basic lead acetate solution, but the
present trend is to replace this toxic reagent with
nontoxic clarifying agents such as aluminum salts.
0038 It is also possible to measure the densities or
refractive indices of pure sucrose solutions to deter-
mine the sucrose content, since these values are func-
tions of the sucrose concentration. For impure sugar
juices, the influence of the soluble nonsucrose com-
pounds on these parameters is almost the same as that
of the sucrose. Consequently, the measurement repre-
sents the total soluble dry matter of the solution and
allows the determination of the degree Brix (or degree
Balling), i.e., the percentage of dry matter in the
solution. The ratio of sucrose content (Pol) to total
soluble dry matter (Brix) is called the quotient of
purity (Q):
Q ¼
Pol
Brix
100 ð2Þ
The density can be measured by pycnometers
or hydrometers, possibly calibrated in degrees
Brix (formerly in degrees Baume
´
). However, these
densitometric methods require many precautions
and are time-consuming. It is much easier to measure
the refractive index, but because the influence of the
nonsugars is not the same, a distinction must be made
between densitometric Brix and refractometric Brix,
giving a slightly different purity quotient. (See Rheo-
logical Properties of Food Materials.)
Sugar-containing Products (Sweet Foods,
Molassed Feeds)
0039Considering the high solubility of sucrose in water,
the first step in analysis will often be an aqueous
extraction followed possibly by clarification (or
sometimes an aqueous alcoholic extraction). As glu-
cose or invert sugar will also be frequently present
in this extract, a double polarization or a double
reducing sugars determination should be made, one
before and one after inversion of the sucrose present
in the extract. By calculation, the total sugars and
sucrose content of the product can be determined.
0040In the sugar industry, the sugar content of beet or
cane has to be determined, as this is the basis of
payment for the raw material and allows calculation
of the amount of sugar entering the factory. For this
purpose, there are conventional methods specific to
the sugar industry.
Traces of Sugars
0041It can be important to detect traces of sugar in some
products, such as the presence of sugar in the feed
water of boilers as a result of the use of condensed
water from the sugar juice evaporation, and to
control the losses of sugar in the waste water. This is
carried out by qualitative or semiquantitative
methods (a-naphthol with sulfuric acid) or by auto-
matic apparatus based on chemical reactions or phys-
ical properties (conductivity or fluorescence).
Methods of Analysis
0042Polarization This method is based on measurement
of the rotation of polarized light of a solution con-
taining the normal weight (i.e., 26 g in 100 cm
3
)ofa
sugar sample or of the unknown sugar solution to be
examined (after clarification if necessary) by means of
a saccharimeter calibrated to give 100
Z with pure
sucrose. Calibration of the saccharimeter is carried
out with standard quartz plates. The result is given as
the percentage of sucrose in the sugar sample or of the
unknown sugar solution to be analyzed.
0043Loss on drying Instead of speaking of water content
in this indirect method, it is more correct to speak of
‘loss on drying.’ The principle of this method is the
determination of the loss in weight of a sugar sample
placed in an oven at 105
C for 3 h and cooled
SUCROSE/Properties and Determination 5639

afterwards in a desiccator under uniform conditions.
The loss in weight for a high-grade white sugar is
normally between 0.02 and 0.05%.
0044 Conductivity ash The specific conductivity of a so-
lution containing 28 g of sugar per 100 g is measured
at 20
C. The water used will have a conductivity of
less than 2 mScm
1
. The concentration of 28 degrees
Brix has been chosen because the conductivity curve
shows a flattened peak in this concentration region.
The measured conductivity corrected for the water
used is converted by a conventional factor into the
percentage of conductivity ash in the sugar.
0045 Visual appearance The visual grade of crystalline
white sugar is assessed by comparing the sample
with standard color types numbered from 0 to 6,
made up of sugar artificially colored and of a defined
particle size. The comparison is made by eye under
well-defined conditions. Some instruments are now
on the market that are intended to replace the eye
and so yield a better reproducibility. (See Sensory
Evaluation: Appearance.)
0046 Color and turbidity in solution A solution of 50 g of
sugar in 50 g of a buffer solution at pH 7 is filtered
through a 0.45-mm membrane filter, and the absor-
bancy of the deaerated filtered solution is measured
by means of a spectrophotometer at a wavelength of
420 nm. The final result is expressed in ICUMSA
color units as a percentage of the dry sample.
0047 Turbidity is measured by means of a turbidimeter
calibrated with a formazin turbidity standard. The
result is expressed in nephelometric turbidity units.
0048 Floc test The soft-drink industry uses various tests
to check the appearance of a floc during the shelf-life
of a bottled beverage. This floc formation, in acid
conditions, may be due to the presence of polysac-
charides (gums).
0049 Sulfur dioxide The level of sulfur dioxide in sugar is
often limited by national legislation. A method of
determining low levels of sulfur dioxide in white
sugar is based on a colorimetric determination of
the sulfite/rosaniline complex, as measured by spec-
trophotometry at a wavelength of 560 nm after
reaction with formaldehyde.
0050 Particle size The particle size of crystalline sugar
can be characterized by the mean aperture (MA)
and coefficient of variation (CV). The MA is the
theoretical mesh aperture, in millimeters, of a sieve
that retains (and also lets through) 50% of the sugar.
The CV is the standard deviation expressed as a
percentage of the MA. It is an evaluation of the dis-
persion of the crystal size around the MA.
0051Chromatographic methods High-performance liq-
uid chromatography (HPLC) and gas–liquid chroma-
tography (GLC) techniques for the analysis of
commercial sugar products have been developed in
recentyears.(SeeChromatography:High-performance
Liquid Chromatography; Gas Chromatography.)
0052Sucrose in beet and cane molasses can be deter-
mined by GLC. Molasses samples together with tre-
halose as an internal standard are silylated using a
trimethylsilyl (TMS) reagent to convert the sugars
into a form in which they are volatile. The TMS
ethers of sucrose and trehalose are separated from
each other and from other volatile components
using a low- to medium-polarity column. Detection
is carried out using a flame ionization detector, and
accurate estimation of the sucrose content can be
achieved by measurement of the peak areas with the
use of an electronic integration system. Sucrose, glu-
cose, and fructose in cane molasses can be determined
by GLC according to the method described above by
introducing an oxime formation step prior to the
silylation.
0053Sucrose in beet and cane molasses can also be
determined by HPLC. The separation column is a
cation-exchange resin converted to a metal ion
form. The diluted sample is filtered prior to injection
on the column. The hydroxyl groups of the different
sugars interact with the cations to varying extents,
resulting in different elution times. Detection is
achieved by differential refractometry of the column
eluant. The peak area or height for sucrose is
obtained by an electronic integration system, which
is compared to that obtained for standards.
White Sugar or Refined Sugar
0054Irrespective of whether a sugar is a product of the
‘first strike’ or has actually been refined by remelt,
decoloration, and recrystallization, the EC has de-
fined a number of criteria to which a sugar must
correspond, to be called ‘white sugar’ or ‘refined
sugar.’ The criteria are based on the results of some
of the analyses described above – polarization, loss on
drying, conductivity ash, visual appearance, color in
solution, invert sugar, and sulfur dioxide content.
See also: Antioxidants: Natural Antioxidants;
Carbohydrates: Classification and Properties;
Chromatography: High-performance Liquid
Chromatography; Gas Chromatography; Date Palms;
Fructose; Rheological Properties of Food Materials;
Sensory Evaluation: Appearance; Sorghum; Sugar:
Sugarcane; Sweeteners: Intensive
5640 SUCROSE/Properties and Determination

Further Reading
Birch GG and Parker KJ (1982) Nutritive Sweeteners.
London: Applied Science.
Clarke MA and Godschall MA (1988) Chemistry and pro-
cessing of sugarbeet and sugarcane. In: Sugar Series, vol.
9. Amsterdam: Elsevier.
International Commission for Uniform Methods of Sugar
Analysis (ICUMSA) (1978) Report of the Proceedings of
the 17th Session, Montreal.
International Commission for Uniform Methods of Sugar
Analysis (ICUMSA) (1982) Report of the Proceedings of
the 18th Session, Dublin.
International Commission for Uniform Methods of Sugar
Analysis (ICUMSA) (1986) Report of the Proceedings of
the 19th Session, Cannes.
International Commission for Uniform Methods of Sugar
Analysis (ICUMSA) (1990) Report of the Proceedings of
the 20th Session, Colorado Springs.
Mauch W (1971) The chemical properties of sucrose. Sugar
Technology Reviews 1(3): 239–290.
Mauch W and Farhoudi E (1980) Quality factors in com-
mercial white granulated sugar. Sugar Technology
Reviews 7(2): 87–171.
Norrish RS (1967) Selected Tables of Physical Properties of
Sugar Solutions. Scientific and Technical Surveys, No.
51. Leatherhead: The British Food Manufacturing In-
dustries Research Association.
Schneider F (1979) Sugar Analysis, ICUMSA Methods.
Peterborough: International Commission for Uniform
Methods of Sugar Analysis.
Dietary Importance
G H Anderson, University of Toronto, Toronto, Ontario,
Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 For centuries, sucrose has played an important role
both in nutrition and in making foods palatable. Su-
crose, in many natural and refined forms, has been
widely used in the diet since its first recorded use in
India in 500 bc, when a rough sugar was produced
from boiled cane. This product was called gur, mean-
ing ‘nice, sweet, sticky stuff.’ Sugar began to be used
in the Middle East in 600–700 ad and reached wide-
spread usage in Europe by 1000 ad. From the eight-
eenth century onward, relatively inexpensive brown
sugar was available for the average family. The Indus-
trial Revolution and large-scale food processing
brought refined white sugar to everyone’s table, and
consumption increased rapidly. Since the mid-1920s
sugar consumption has remained relatively constant,
except during the Second World War, when rationing
led to a decrease in consumption.
0002Sucrose consumption has declined since the
1970s when nutritive sweeteners from corn became
available. Corn sweeteners are available as corn
syrups, other saccharides, or as high-fructose
corn syrup, which is produced by enzymatic conver-
sion of some of the dextrose to fructose. To avoid
confusion in this presentation, ‘sucrose’ will be used
when sugar from cane and beet sugar is being dis-
cussed and the word ‘sugars’ will be used when
all added free sugars, including corn sweeteners, are
discussed.
0003The facts about sugars today are often distorted,
producing incorrect public perceptions; therefore a
discussion of the metabolism, health implications,
and current consumption levels of dietary sugars
follows.
Digestion and Metabolism of Sucrose
0004Sucrose is hydrolyzed by the enzyme sucrase, an
a-glucosidase in the human small intestine, to its
component monosaccharides fructose and glucose.
About 10–25% of the fructose is converted to glucose
in the brush border of the upper gastrointestinal tract.
The monosaccharides are absorbed and transported
to the liver via the portal vein and subsequently trans-
ported to all tissues.
0005Within the liver fructose may be converted to glu-
cose; it may enter the glycolysis pathway, or provide
acetate units for the synthesis of fatty acids. Glucose
can be stored in the liver as the glucose polymer
glycogen; it can be used in glycolysis to produce
energy; or it can provide acetate units for the synthe-
sis of fatty acids.
0006Glucose is made available to other body tissues via
the blood stream and is the primary form of carbohy-
drate used as energy by the tissues. Under circum-
stances reflected by normal consumption of sucrose
or high-fructose corn syrups, little fructose appears in
the blood because it is rapidly metabolized by the
liver for synthesis of glucose and primarily fatty
acids and triglycerides, and possibly by metabolism
in the kidney. The utilization of fructose by peripheral
tissues seems to be negligible, perhaps because glu-
cose, which is always present in the blood, inhibits
both its uptake and entry into the metabolic pathway
of glycolysis.
0007As a result of insulin release in response to sucrose
ingestion, blood glucose is maintained within a
closely regulated range, a requirement for normal
functioning of the central nervous system. Sugar
does not cause abnormal insulin and blood glucose
responses. In fact, the effect of ingesting sucrose on
SUCROSE/Dietary Importance 5641
insulin release and blood glucose is less than when an
equivalent amount of carbohydrate is consumed from
bread. The excess of glucose above the energy re-
quirements of the tissues can be stored to some extent
as glycogen in muscle. The remainder is used to syn-
thesize fatty acids and glycerol, which allow efficient
storage of energy in the form of triglycerides in
adipose tissue.
0008 The effect of sugars on blood glucose can be cap-
tured by measuring the total glycemic response over
time. To compare the response produced by different
carbohydrates, the glycemic index (GI) was de-
veloped. The GI compares the incremental area
under the blood glucose curve produced by 50 g of a
carbohydrate test food to that produced by 50 g of
a standard food (white bread or glucose). The
glycemic indices of sugars depends on their compos-
ition. For example, sucrose has a GI which is only
87% of the white bread standard. This glycemic re-
sponse is explained by its components glucose and
fructose. Glucose alone has a GI relative to white
bread of 138%, whereas that of fructose is only
32%. Relative to many common starchy foods, both
sucrose and fructose have lower GI values (Table 1).
Nutritional Properties of Sucrose
0009 Sucrose has two important nutritional properties. It
provides energy at 3.8 kcal g
1
and, through its sweet
taste, it increases the palatability of foods.
0010Sucrose, in the form that it reaches the table of the
consumer, is the purest of common foods and free of
contaminating chemical residues. As a result, it pro-
vides only one nutrient, carbohydrate, which is read-
ily converted to energy by the body. It is this aspect of
sucrose that has led to the concept of ‘empty calories.’
Clearly, sucrose can be used to manufacture foods
that are low in essential nutrients and, if consumed
in excess, may lead to nutrient-deficiency syndrome.
However, there is no objective evidence that sugar
intake leads to nutrient deficiencies at current con-
sumption levels, nor that its widespread use has com-
promised the food supply so that nutritionally
adequate meals and diets cannot be obtained from
the marketplace.
0011Diets containing too much sugar are theoretically
possible, and examples based on readily available
foods can be easily constructed. For the majority of
people, however, consumption of sugar falls well
within a safe range of intake.
0012The probable reason why sugar does not lead to
nutrient-imbalanced diets is because it is used to en-
hance the intake of many nutrient-rich foods which
may not otherwise be readily eaten. For example,
small amounts of sugar are added to many fruits,
vegetables, and cereals. Increasing the palatability of
these foods with sugar makes them more likely to be
consumed.
0013Many reviews have examined the role of sugar in
health and indicate that, other than contributing to
dental caries, which is a consideration for all carbo-
hydrates, the amounts consumed in the average diet
produce no deleterious health effects. However, per-
ceptions that sugar is the cause of hypertriglycerid-
emia, diabetes, and hyperactivity in children still
persist.
0014Hypertriglyceridemia is the elevation of triglycer-
ides in the blood and is believed to play a role in heart
disease. It has been recognized for years that large
amounts of dietary sugars, particularly fructose, can
raise triglycerides. Thus, sugars that contain fructose,
such as sucrose and high-fructose corn syrups, also
have this potential. The potential effect of fructose
consumption on blood lipids is explained by its
unique metabolic pathway in the liver and evidence
that it is a better substrate than glucose for lipid
synthesis. Therefore, it should not be a surprise
to find an elevation of blood triglyceride con-
centrations in some subjects given excessive quan-
tities of fructose or sucrose. However in studies in
which amounts of sugars typical of the western diet
were provided, such responses are not usually
observed.
0015Diabetes mellitus is a condition in which an insulin
deficiency or decreased insulin sensitivity results in
tbl0001 Table 1 Glycemic index of selected foods
a
Foods Glycemicindex (GI)
White bread 100
All Bran 60
Cornflakes 119
Oat bran 78
Apple 52
Banana 83
Cornmeal 98
Rice, white 81
Rice, brown 79
Rice, instant 128
Rice, parboiled 68
Baked potato 121
Boiled white potato 80
Milk, whole 39
Spaghetti, white 59
Chocolate (various) 84
Sucrose 87
Fructose 32
Glucose 138
Soya beans 23
a
Selected from FAO/WHO (1997) Carbohydrates in Human Nutrition.
FAO/WHO Expert Consultation on Carbohydrates in Human Nutrition. Rome,
Italy: FAO/WHO.
5642 SUCROSE/Dietary Importance

hyperglycemia. The most prevalent form of diabetes
is noninsulin-dependent diabetes mellitus (NIDDM),
which most often has its onset in adulthood and is
associated with obesity. All reviews on the subject
have concluded that there is no evidence that sugar
intake is related to the incidence of diabetes or
mortality. In fact, as with obesity, an inverse relation-
ship between sugar intake and diabetes has been
found.
0016 The belief that sugar causes hyperactivity in chil-
dren is one of the most persistent and widespread
myths about sugar. However, the link between sugar
and hyperactivity has been based largely on subjective
observations made by both parents and teachers.
Recent well-controlled challenge studies clearly indi-
cate that sugar does not affect hyperactivity, attention
span, or cognition in children. In fact, studies have
shown that children are calmer and more sedate
following sugar preloads.
Sugar Consumption versus Sugar
Disappearance Data
0017 A great deal of confusion surrounds sugar consump-
tion statistics. This is because there are two types of
consumption being interchanged in discussion of the
statistics. One consumption statistic is based on
economic concepts and refers to national usage or
availability of sugar for use in the food supply,
whereas the other consumption statistic is an estimate
of the amount eaten by humans and is a physiological
concept.
0018 Estimates of the total amount of nutritive sweeten-
ers available for use within a country are assembled
on an annual basis by statistics or economic branches
of the government. The figures are reported as per
capita consumption, but they do not represent
per capita intake by individuals. They indicate avail-
ability of added sugars to the marketplace, after
consideration of production, imports, and exports,
and beginning and ending stocks. No allowance is
made for losses or wastage in food manufacturing or
in other aspects of usage. Thus, while these consump-
tion statistics have proved useful in describing trends
in national availability of added sugars, they have
proved to be misleading in predicting actual intake
by humans. For example, in 1980–85, total caloric
sweeteners available for consumption in the USA
averaged 155 g (620 kcal) per person per day and
17% of the energy content of the available food
supply. However, much of the sugar available is des-
troyed in production processes such as making bread,
pickles and alcoholic beverages, or wasted during
distribution, storage, and usage, or consumed by
animals.
0019Added sugar consumption in the USA was esti-
mated to average only 53 g day
1
, or 30% of
estimated availability (155 g) derived from disappear-
ance data in 1977–78. Consumption data for sugars
added to the food supply were derived from the
1977–78 (US Department of Agriculture (USDA))
Nationwide Food Consumption Survey. Average
daily intake of sugars for this population sample of
30 677 subjects was 53 g day
1
, or 11% of the daily
intake of calories. Younger age groups had a higher
intake than older age groups. The highest consumers,
those in the 90th percentile of daily intake of added
sugar, ranged from 1.5 to 2.5 times the mean value for
the 14 age/sex groups. For the total population the
90th percentile of daily intake of added sugar was
104 g day
1
or 20% of calories.
0020More recent (1999) economic estimates of per
capita consumption show that availability of caloric
sweeteners averages 196 g (745 kcal) per person per
day. This increase of 29%, since the early 1980s, is
somewhat less than the increased per capita availabil-
ity of total flour and cereal products (36%) and is
greater than the increase in total fat availability
(19%). When total added sugars availability is related
to the availability of calories in the food supply it now
approximates 20%, slightly up from 17% in the early
1980s. In contrast, more recent dietary surveys esti-
mate consumption of sweeteners to be close to 80 g
day
1
or nearly 18% of the energy consumed. These
data suggest that the amount of added sugars eaten
has risen by almost 50% from 20 years ago and is
42% of the added sugar availability. However the
majority of this reported increase in intake can be
accounted for by a change in the approach and defin-
ition of sugars used by USDA in reporting dietary
survey data.
0021By definition, sugars are mono- and dissaccharides
and this definition was used in the earlier survey
estimate of sugar intakes. In more recent surveys all
high-fructose corn syrups are included as added sugar,
which does not adjust for the 70% of these syrups
that are made up of polysaccharides. This fact, plus
the failure to consider loss of sugars in baking and
other processes, and the inclusion of high-intensity
sweeteners in the calculation, account for most of
the calculated increase in added sugar consumption.
Thus at present it is uncertain the extent to which the
modest increase in availability of added sugars as a
portion of the energy content of the available food
supply has influenced dietary intakes.
0022Since the mid-1970s there has been a consider-
able replacement of sucrose with corn sweeteners,
including high-fructose corn syrup, glucose syrups,
and dextrose. At the present time, corn sweet-
eners account for about 50% of the total added
SUCROSE/Dietary Importance 5643

sweetener that is available in the USA. Because the
most commonly used high-fructose corn syrups con-
tain 52% or less of fructose, their availability has
done little to affect fructose consumption compared
with the consumption of sucrose which is 50%
fructose.
0023 Due to changing lifestyles since the early 1900s, the
major use of sugars has shifted from direct use by
consumers in households to use by the food industry
in baked goods, processed foods, and beverages.
In 1925, consumer use accounted for about two-
thirds of total sugar disappearance, whereas the
food industry used only one-third. Today these figures
are reversed. In addition to the use of sugar as a
sweetener, it is widely used for its functional proper-
ties, such as providing bulk in baked goods and in-
hibiting spoilage microorganisms in jams and jellies.
These functional uses are the same whether they
occur in the home or the industry, and are essential
to achieving a safe and tasty food supply. Therefore
the shift to use by the food industry of a greater
proportion of sugar disappearance does not suggest
that the consumer is eating more sugar than in previ-
ous decades.
Dietary Goals and Guidelines
0024 Primarily because of concern with chronic disease,
developed countries have produced statements of
dietary goals and guidelines aimed at improving
the dietary pattern of their populations. The assump-
tion is that nutritional factors are important in the
development of chronic disease and that corrective
actions in terms of diet will prove to be beneficial.
0025 Sucrose has appeared prominently in dietary guide-
lines. Recent nutrition recommendations of the gov-
ernments of Canada and the USA have taken a more
liberal view of the nutritional role of sugar in the diet.
The American and Canadian Diabetes Associations
recommend that sugars and sugar-containing foods
do not need to be restricted by people with diabetes
and should be considered in the context of the total
amount of carbohydrates consumed. The Canadian
dietary guidelines for healthy people are silent on the
subject, based on the conclusion of the scientific
advisory committee that there was no basis on
which to advise reduction of sugar intake to the
population. Similarly, the guidelines for the USA
are no longer punitive and have shifted from ‘avoid
too much sugar’ to ‘choose beverages and foods
to moderate your intake of sugars.’ This advice
emphasizes the requirement of individuals to select
diets that are based on variety and moderation.
Avoidance of refined sugars is no guarantee of a
healthy diet.
Sucrose and Food Intake
0026Sucrose, through its sweetness and other functional
properties, makes foods more appealing and raises
the probability that they will be selected over foods
that are not as palatable. It is well known that the
hedonic value of a food is a major determinant of its
consumption. However, there is a natural response to
sweetness and palatability of foods that tends to be
self-limiting. Sensory-specific satiety, which refers to
the decreased pleasantness of a food or beverage as it
is consumed, occurs for all foods and tastes. Thus
increasing the pleasantness of a food may influence
its choice but does not necessarily lead to excess
consumption.
0027The role of a varied and palatable diet in the eti-
ology of obesity is currently of great interest because
of the high prevalence of obesity in western popula-
tions. In the past 40 years, the incidence of obesity has
increased fivefold. It is unlikely that sucrose can be
rationalized as a causative factor, for several reasons.
First, on a strictly correlative basis, the increase in
obesity does not associate with the constant intake of
sugars occurring during this same period. Second,
obese subjects do not show a disproportionate intake
of sweet foods compared with normal-weight indi-
viduals.
0028In contrast to hypotheses that sugars bypass appe-
tite-regulatory systems, experimental studies indicate
that consumption of sugars suppresses subsequent
energy intake. Both children and adults compensate
for energy provided by sucrose preloads when given
within 1 h before a test meal. Similarly, compensation
is also observed for other sugars, which produce
higher glycemic responses, including glucose and
polycose. Therefore, a rapid increase in blood glucose
following ingestion of rapidly digestible carbohy-
drates does not negatively impact food intake regula-
tion. In general, 50 g of sugar within 20–60 min of a
meal is sufficient to reduce meal intake.
0029Mechanisms controlling the regulation of food
intake remain poorly understood. It seems clear,
however, that the cause of obesity is an excess of
energy intake over expenditure. It is far too simple
to attribute its origin to the composition of the food
supply or of a particular food. Many foods and many
dietary habits probably contribute to this imbalance,
but for most the causative factor of greatest impact is
reduced energy expenditure in the form of physical
activity.
See also: Diabetes Mellitus: Etiology; Glucose:
Maintenance of Blood Glucose Level; Obesity:
Epidemiology; Etiology and Diagnosis; Sugar:
Sugarcane; Refining of Sugarbeet and Sugarcane;
Sweeteners: Intensive
5644 SUCROSE/Dietary Importance
Further Reading
American Diabetes Association Position Statement (2002)
Evidence-based nutrition principles and recommenda-
tions for the treatment and prevention of diabetes and
related complications. Diabetes Care 25: 202–212.
Anderson GH (1989) Sugar consumption. Are dietary
guidelines needed? Journal of the Canadian Dietetic
Association 50: 229–232.
Anderson GH (1998) Sugar consumption and health. Food
Science Biotechnology 7(4): 229–235.
Duffy VD and Anderson GH (1998) Position of the
American Dietetic Association: use of nutritive and
nonnutritive sweeteners. Journal of American Dietetic
Association 98: 580–587.
FAO/WHO (1997) Carbohydrates in Human Nutrition.
FAO/WHO Expert Consultation on Carbohydrates in
Human Nutrition. Rome, Italy: FAO/WHO.
Glinsmann WH, Irausquin H and Park YK (1986) Evalu-
ation of health aspects of sugars contained in carbo-
hydrate sweeteners: report of the Sugars Task Force.
Journal of Nutrition 116(11S): S1–216.
Vettorazzi G and Macdonald I (1988) Sucrose: Nutritional
and Safety Aspects. London: Springer-Verlag.
WHO (1997) Obesity: Preventing and Managing the
Global Epidemic. Report of a WHO Consultation on
Obesity. Geneva, Switzerland: WHO.
Woodend DM and Anderson GH (2001) Effect of sucrose
and safflower oil preloads on short term appetite and
food intake in young men. Appetite 37: 185–195.
USDA/Economic Research Service (1998) US Food Con-
sumption Sources of Data and Trends. Washington,
DC: USDA/Economic Research Service.
US Department of Agriculture, US Department of Health
and Human Services (2000) Nutrition and Your Health:
Dietary Guidelines for Americans. Home and Garden
Bulletin No. 232. Washington, DC: Government
Printing Office.
SUGAR
Contents
Sugarcane
Sugarbeet
Palms and Maples
Refining of Sugarbeet and Sugarcane
Sugarcane
M A Godshall, Sugar Processing Research Institute,
New Orleans, LA, USA
B L Legendre, US Dept of Agriculture, Houma, LA,
USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 During the decade of the 1980s, word sugar produc-
tion held steady at around 100 10
6
t, with cane
sugar accounting for 60–64% of the total, and beet
sugar for the remainder. The decade of the 1990s was
characterized by a steadily increasing output of sugar,
so that total world sugar production was forecast to
reach 121.5 10
6
t in 1999–2000. Cane sugar now
accounts for about 70% of total world sugar produc-
tion. Although most tropical and subtropical coun-
tries produce cane sugar, 10 countries account for
74% of global cane sugar production, as shown in
Table 1.
Taxonomy and Origin
0002Sugarcane is a large perennial tropical grass belonging
to the tribe Andropogoneae of the family Gramineae
and the genus Saccharum. The Andropogoneae
tbl0001Table 1 Raw cane sugar production for the 10 leading
producingcountries(1997–98crop)
a
Country Raw sugar (10
6
t) Harvest season
Brazil 14.557 June–May
India
b
12.523Oct–Sep
China 6.705 Sep–Jan
Australia 5.350 June–Dec
Mexico 4.997 Nov–Sep
Thailand 3.913 Nov–May
Pakistan 3.400 Oct–July
Cuba 2.951 Nov–June
USA 2.692 Oct–Mar
South Africa 2.334 May–Feb
a
Total world cane raw sugar production in 1997–98 was 30.212 10
6
t.
b
Includes khandsari, a cottage-industry-produced, semiwhite centrifugal
sugar.
SUGAR/Sugarcane 5645

are characteristically tropical or subtropical with a
high concentration of genera in two geographical
areas: India and Indonesia. The genera Saccharum,
Erianthus (sect. Ripidium), Sclerostachya, and Nar-
enga, most cited in the origin of sugarcane, constitute
an interbreeding group that, along with three species
of Saccharum (S. officinarum L., S. barberi Jeswiet,
and S. sinense Roxb) were used for commercial sugar
production. Saccharum officinarum is a progenitor
of all modern sugarcane cultivars. However, the
presence of the interbreeding Saccharum complex of
the three sugar species as well as its wild relatives,
S. spontaneum L. and S. robustum Brandes and Jes-
wiet ex Grassl, has provided a genetic pool of unpar-
alleled diversity, allowing for the development of
thousands of cultivars that are adapted to the areas
where sugarcane is grown. Today, most cultivars of
sugarcane are interspecific hybrids of two or more of
the five Saccharum species.
Description
0003 The sugarcane plant is a large, jointed grass 2.5–4m
in height, with robust stems up to 5 cm in diameter
and a tendency to tiller profusely in clumps called
stools (Figure 1). The stem is made up of 10–40
internodes with leaves borne at the nodes. The inflor-
escence (Figure 2) is a large, terminal panicle, feathery
in appearance, white to purplish in color.
History
0004The evolution of sugarcane is postulated to have had
three foci – Polynesia, China, and India – and must
have started many thousands of years ago. Sugarcane
is a readily transported food plant and capable of
germination several weeks after cutting from the
parent stool. The ‘noble’ cultivars (clones of S. offici-
narum) were undoubtedly grown in native gardens
of Polynesia as a food plant since earliest times.
They were also carried throughout the Pacific Island
archipelago by Polynesian navigators more than 1000
years ago.
0005There are well-documented records of sugarcane in
both China and India several centuries before the
Christian era. Sugarcane was carried from India to
Persia in the sixth century, after which the Arabs took
it to the Mediterranean littoral. The Egyptians were
the first to refine sugar.
0006Sugarcane reached Spain and Portugal in the
eighth century and was subsequently brought to the
Canary Islands, the Azores, and East Africa. The first
fig0001 Figure 1 Example of sugarcane growth habit. Courtesy of Mary An Godshall and Alfred D French.
5646 SUGAR/Sugarcane
