Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

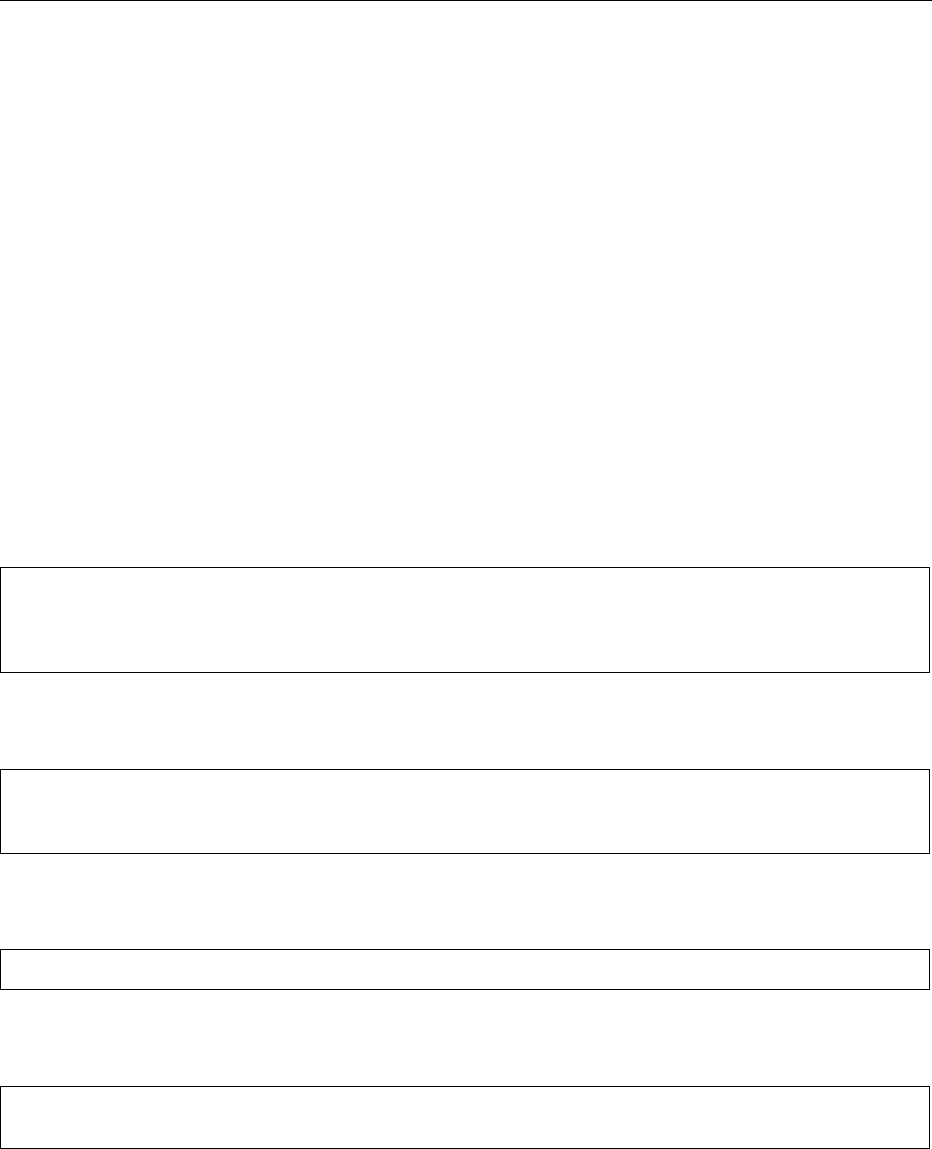
See also: Amino Acids: Metabolism; Freezing: Structural
and Flavor (Flavour) Changes; Irradiation of Foods:
Basic Principles; Lactic Acid Bacteria; Pasteurization:
Principles; pH – Principles and Measurement;
Preservation of Food; Sanitization; Sterilization of
Foods; Water Activity: Effect on Food Stability; Wines:
Production of Table Wines; Production of Sparkling Wines
Further Reading
Deak T (1995) Methods for the detection and identification
of yeasts in foods. Trends in Food Science and Technol-
ogy 6: 287–292.
Deak T and Beuchat LR (1996) Handbook of Food Spoilage
Yeasts. Boca Raton, FL: CRC Press.
Fleet GH (1992) Spoilage yeasts. Critical Reviews in Bio-
technology 12: 1–44.
King AD, Pitt JI, Beuchat LR and Corry JE (eds) (1986)
Methods for the Mycological Examination of Food.
New York: Plenum Press.
Kurtzman CP and Fell JW (eds) (1998) The Yeasts. A Taxo-
nomic Study, 4th edn. Amsterdam: Elsevier.
Pitt JI and Hocking AD (1985) Fungi and Food Spoilage.
Sydney: Academic Press.
Samson RA, Hocking AD, Pitt JI and King AD (eds) (1992)
Modern Methods in Food Mycology. London: Academic
Press.
Skinner FA, Passmore SM and Davenport RR (eds) (1980)
Biology and Activities of Yeasts. London: Academic
Press.
Thomas DS (1993) Yeasts as spoilage organisms in
beverages. In: Rose AH and Harrison JS (eds) The
Yeasts, 2nd edn. vol. 5, pp. 517–561. London: Academic
Press.
Tudor EA and Board RG (1993) Food spoilage yeasts.
In: Rose AH and Harrison JS (eds) The Yeasts, 2nd
edn. vol. 5, pp. 435–516. London: Academic Press.
Spores See Allergens; Food Intolerance: Types; Food Allergies; Milk Allergy; Lactose Intolerance;
Elimination Diets; Microbiology: Classification of Microorganisms; Detection of Foodborne Pathogens and their
Toxins; Spoilage: Chemical and Enzymatic Spoilage; Bacterial Spoilage; Fungi in Food – An Overview; Molds in
Spoilage; Yeasts in Spoilage
Spray Drying See Drying: Theory of Air-drying; Drying Using Natural Radiation; Fluidized-bed Drying;
Spray Drying; Dielectric and Osmotic Drying; Physical and Structural Changes; Chemical Changes; Hygiene;
Equipment Used in Drying Foods
Squashes See Melons, Squashes, and Gourds
Squid See Marine Foods: Production and Uses of Marine Algae; Edible Animals Found in the Sea; Marine
Mammals as Meat Sources
SPOILAGE/Yeasts in Spoilage 5537

STABILIZERS
Contents
Types and Function
Applications
Types and Function
J E Fox, P Ingenpass and S Zachow, GC Hahn and
Company, Lubeck, Germany
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
That familiarity breeds contempt is an oft-quoted
maxim of political life, yet it might also be applied
to many of our foodstuffs, which have acquired the
epithet of common, basic, staple, or simple. The ma-
jority of these foods are, in a physicochemical sense,
far from simple. Almost all are complex multiphase
systems, in which gases, liquids, or solids are dis-
persed in a liquid continuous phase, typically aque-
ous, which itself contains a multitude of solutes.
Examples of such natural foods are milk and eggs,
whilst icecream and mayonnaise are representatives
from manufactured foods.
0002 Disperse systems are inherently instable, and this is
true for many of these complex foods. Thus, full-fat
milk spontaneously creams on standing, and acidified
milk irreversibly curdles. This instability can be
resolved into two principal processes:
1.
0003 Flocculation of fine or colloidally dispersed par-
ticles to aggregates which, if liquid or gaseous,
may coalesce to form larger droplets or bubbles.
2.
0004 Displacement of particles under gravity as a result
of density differences between the phases.
These processes can influence the appearance and
rheology of the food and may detract from its per-
ceived quality and acceptance. The precise effects,
however, will depend on the volume fraction of
the disperse phase, and will naturally be greater
where this is higher. Particularly in such foods, it is
important to control or inhibit flocculation and
gravitationally induced phase separation. (See Floc-
culation.)
0005 The word ‘control’ is included here by virtue of the
fact that, in some foods, it may be advantageous to
promote these processes. Thus, in fat-reduced
mayonnaise, an aggregation of fat droplets may be
required to give the product a structure and plasticity.
Aggregation and structure formation may also be
utilized to prevent phase separation. In fat-rich fresh
cheese products, a casein network prevents the
creaming of the fat, and a similar mechanism is op-
erative in the stabilization of foams by fat crystals.
Although contradictory in terms, a stabilizer may
achieve stability by controlled and partial destabiliza-
tion. (See Casein and Caseinates: Uses in the Food
Industry.)
0006Control of flocculation and separation can be
achieved by the judicious use of polysaccharide
gums, proteins, organic salts (and emulsifiers), alone
or more often in combination. In this role these sub-
stances have become known as stabilizers, a suitable
definition for which would be as follows: food addi-
tives which prevent or control phase separation in
foods consisting of two or more phases.
0007Because stabilizers influence the structure of a
food, and because many of the additives used have,
in their own right, a thickening or gelling function,
the term stabilizer has become loosely used to
describe any additive that influences the rheology of
the products. This is a somewhat dangerous practice
as, in many countries, food-labeling law has led to a
legal definition of the term, and this usually restricts
its use to multiphase systems. It should also be
remembered that the term refers to a functionality;
therefore one and the same additive may not always
be properly described as a stabilizer. Thus, when car-
rageenans are used to prepare a clear jelly – a single-
phase system – it should be declared not as a stabilizer
but as a gelling agent. On the other hand, in chocolate
milk, where the gel serves to hold the cocoa par-
ticles in suspension, the former declaration would be
correct.
Flocculation
0008In order to illustrate how stabilizers influence floccu-
lation, it is necessary to review the forces which are
believed to be acting on dispersed particles. These
were first set out in the theory of Derjaguin Landau
Verwey Overbeck, which recognizes two types of
interparticle interactions:
5538 STABILIZERS/Types and Function
1.0009 Van der Waals forces.
2.
0010 Electrostatic interaction.
Van der Waals forces are strong attractive forces
which are principally attributable to fluctuating po-
larization of the electron distribution in the molecule.
These forces decrease rapidly with distance, being
inversely proportional to the sixth power of the
separating distance.
0011 Dispersed particles may also carry surface charges,
with the resulting formation of an electrical double
layer of counterions in the proximal continuous phase.
When two similarly charged particles approach each
other, these ion layers begin to overlap and interact,
giving rise to repulsive forces. Typically, these forces
are stronger than Van der Waals forces at larger
separations, so resulting in a net repulsive force. As
separating distance decreases these repulsive forces
attain a maximum before Van der Waals attraction
causes flocculation (Figure 1). The height of this bar-
rier will naturally be dependent upon the magnitude
of electric double layer but, in order to limit floccula-
tion, it should be considerably larger than the thermal
energy of the system. The absolute magnitude of these
forces is generally related to the surface area of the
particle. They are therefore important where the ratio
of surface area to weight of the particles is large, and
therefore increase in importance with diminishing
particle size.
0012 From these considerations it follows that a col-
loidal dispersion may be stabilized by increasing the
surface charge and this, in turn, can be achieved by
the adsorption of polyelectrolytes. This approach is
particularly important in the stabilization of protein
dispersions, which tend to flocculation when the pH
of the continuous phase approaches their isoelectric
point. Here sodium carboxymethylcellulose (CMC)
has proved effective down to a pH of 4.2, below
which the polymer itself becomes increasingly insol-
uble. CMC offers the additional advantages that: (1)
its calcium salts are soluble, and there is therefore no
restriction on its use in calcium-rich systems, in par-
ticular milk, and (2) it is in itself a good thickening
agent, and when used in excess, it can retard gravita-
tionally induced phase separation. (See Colloids and
Emulsions.)
0013Below pH 4.2, pectin and, to a lesser degree,
alginate esters can be used. For stabilization, a
high proportion of the carboxylic acid residues
present in these molecules must be blocked, thus
only high-methoxy pectin, with a degree of esterifi-
cation (DE) above 70%, and propyleneglycol alginate
(DE > 60%) are effective. Polymers with a higher
proportion of free acid groups tend to facilitate
flocculation, presumably by bridging the dispersed
particles, and are calcium-sensitive. The esterification
of the acid also promotes the cleavage of the glyco-
sidic bond by b-elimination. These hydrocolloids
are therefore susceptible to thermal degradation,
particularly in neutral systems, and this can be a
handicap for their use in pasteurized and sterilized
products.
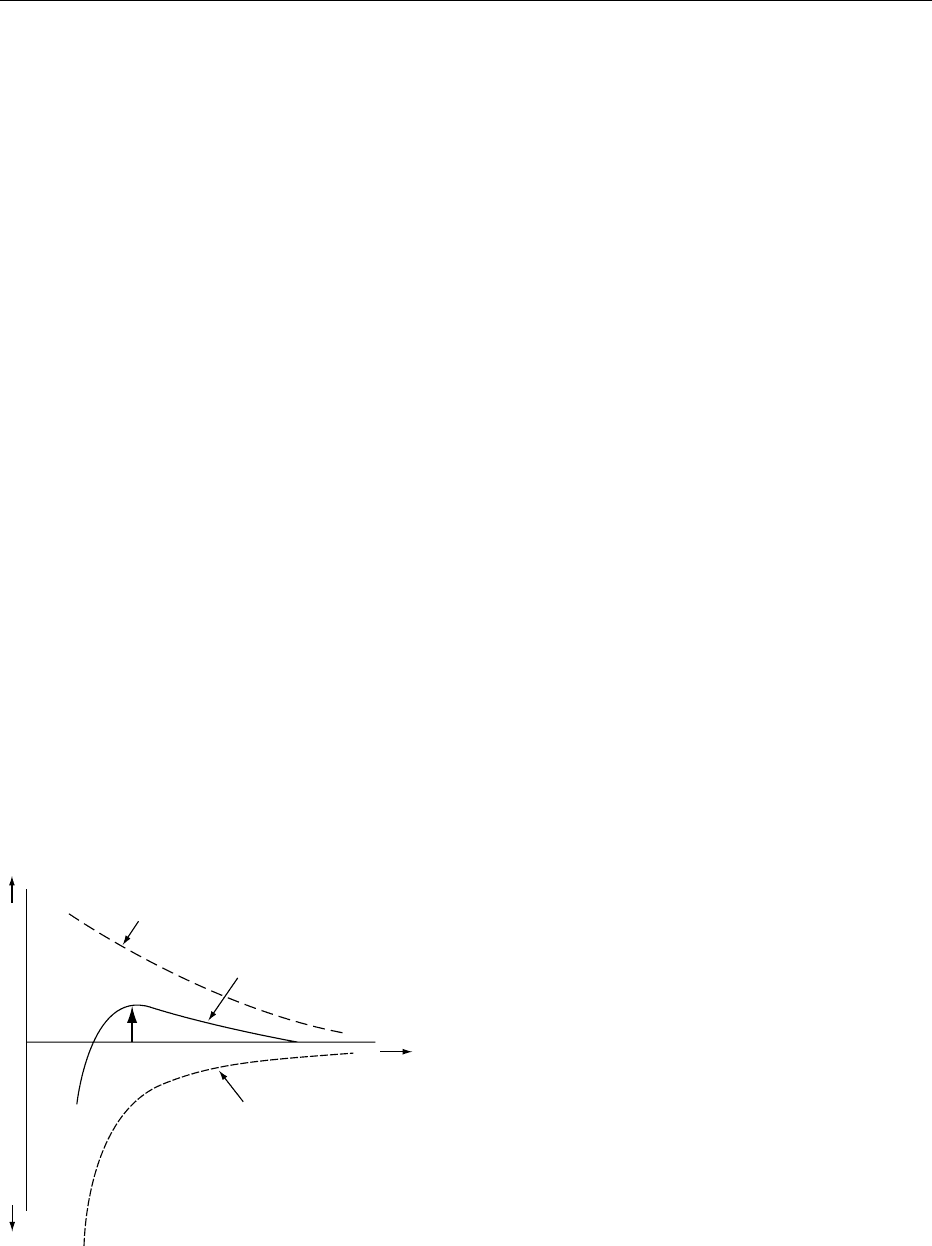
0014With these pectins and alginate derivates, a further
mechanism of stabilization may be operative. The
steric effects of the long molecules adsorbed on to
the surface of the particles may prevent their mutual
approach. In order to function in this way, the stabil-
izing polymer must have an appreciable portion of its
length as a fully solvated chain in the proximal solv-
ent (Figure 2). This requirement can rarely be met by
homopolymers and usually requires block polymer
species, such as high-methoxy pectin, containing
both lyophilic and lyophobic segments. This stabiliz-
ing mechanism will be affected by the solvent quality
with respect to the lyophilic portion; reducing the
quality will decrease stability. The stabilization also
requires that the surface be fully coated with polymer,
which should therefore be used in excess. If added
sparingly, lyophobic portions of the same molecule
may cause bridging between particles and thereby
induce flocculation.
Gravitationally Induced Phase Separation
0015Neglecting interparticle forces, the movement of par-
ticulate phase under gravity is governed ideally by
Stokes law. This law relates the terminal velocity u
of a spherical body to the viscosity of continuous
Electrostatic double-layer
repulsion
Resultant force with
energy barrier to flocculation
Interparticle
separation
Van der Waals
attraction
Attractive force Repulsive force
0
fig0001 Figure 1 Schematic representation of particle interaction. Re-
produced from Stabilizers/Types and Function, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
STABILIZERS/Types and Function 5539
phase, Z, the radius of the particle, r, and the force
acting on the particle, f:
¼ð6f Þ=r
f ¼ð
p
s
Þ
4
3
r
3
The density of the particle is r
p
, and that of the
continuous phase is r
s
.
0016 It follows from these equations that the speed of
creaming or sedimentation increases with particle size
and decreases with the viscosity of the continuous
phase. This is, of course, the rationality behind the
use of homogenization to delay creaming in milk, or
the use of thickening agents to delay sedimentation.
0017 In order to have appreciable influence on stability,
the viscosity must be drastically increased and this
can best be achieved by the use of polysaccharide
gums. These substances are extremely efficient
thickening agents, being effective at concentrations
typically below 1.0%. (See Gums: Properties of Indi-
vidual Gums.)
0018 The effect on viscosity is believed to be the result
of nonspecific entanglement of the randomly coiled
polysaccharide chains. It follows from this model that
the more extended the polysaccharide molecules are
in solution, the more entanglement would be likely
and the higher the viscosity obtained. The extension,
and thereby the viscosity, is governed by size and
topology of the molecule, its flexibility, and its inter-
action with the solvent. Given the same molecular
weight, a linear molecule will be more extended and
more prone to entanglement than a highly branched
species. For linear molecules the extension should be
directly related to the molecular weight. (See Carbo-
hydrates: Classification and Properties.)
0019It is therefore not surprising to find that all com-
mercially important gum thickeners are linear poly-
mers, and that they are available in a wide range of
viscosities which reflect their degree of polymeriza-
tion.
0020The fact that these substances show a much larger
effect on viscosity as compared with equally large
synthetic polymers or proteins has been ascribed to
the bulky monomer sugar which restricts chain flexi-
bility. None of these polymers are rigid rods, however,
so that the quality of the solvent will influence the
degree of extension. In thermodynamically poor
solvents, and in the presence of salts which shield
electrostatic repulsive forces in polyelectrolytes, the
molecules tend to assume more compact conform-
ations and the efficiency of thickening will be
reduced. It also follows from the model of molecular
entanglement that the concentration must be such
that physical contact is possible. This condition is
usually satisfied when the concentration of hydrocol-
loid gums, c, is such that:
c:½4
In this equation, [Z] is the intrinsic viscosity of the
polysaccharide; c.[Z] is known as the overlap factor.
The intrinsic viscosity is a measure of the extension of
the molecule in solution and can be related to its
radius of gyration.
0021The flow properties of hydrocolloid-thickened so-
lutions are markedly pseudoplastic (Figure 3). Under
the influence of shear, the dynamic viscosity falls.
This is thought to be the result of orientation of the
extended molecules under the shear gradient, with a
concomitant reduction in entanglement. This prop-
erty can be used to great advantage in stabilizers. The
shear stresses operative in gravitational sedimenta-
tion or creaming are in the order of 10
0
10
1
mPa.
At these extremely low shear stresses, hydrocolloids
exhibit extremely high dynamic viscosities. A 1%
xanthan solution shows a viscosity of 10
5
mPa s
1
under a shear stress of 10 mPa. At shear rates of
between 10 and 50 s
1
, believed to be present in the
mouth during mastication, the viscosity of the same
Hydrated lyophilic
segments
Adsorbed lyophobic segments
(a)
(b)
fig0002 Figure 2 (a) Steric stabilization and (b) flocculation. Repro-
duced from Stabilizers/Types and Function, Encyclopaedia of
Food Science, Food Technology and Nutrition, Macrae R, Robinson
RK and Sadler MJ (eds), 1993, Academic Press.
5540 STABILIZERS/Types and Function
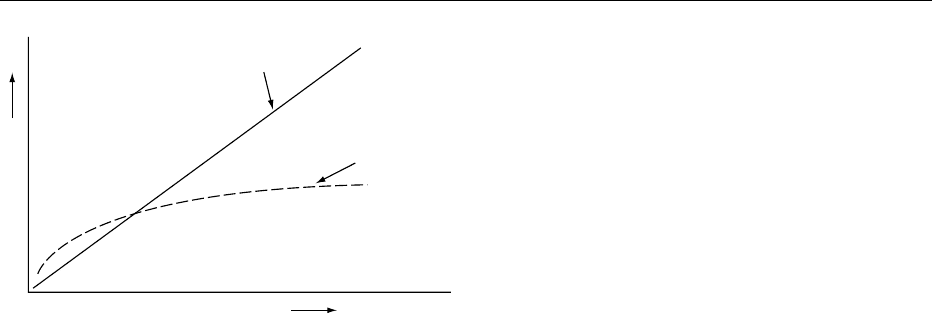
xanthan solution falls to 10
2
10
3
mPa s
1
. Thus,
foods thickened in this manner exhibit a light,
pleasant mouth feel.
0022 The same stabilization effect cannot be obtained
with a thickener showing Newtonic flow characteris-
tics in which viscosity is independent of shear. If the
high viscosities could be obtained at low shear then
the product would be unacceptably thick on eating.
This can be illustrated by honey, a Newtonic fluid
thickened by mono- and disaccharides, which has a
viscosity of only 10
4
mPa s
1
, independent of shear
rate. Organoleptically, such Newtonically thickened
solutions are perceived as long and slimy.
0023 This approach to stabilization, and the use of non-
absorbed polysaccharides in food in general, is
limited by thermodynamic incompatibility with
other colloidally dispersed polymers. This prevents
the polysaccharide and its incompatible neighbor
from occupying the same space, the so-called ex-
cluded volume, and can drive the excluded colloid
to flocculate or lead to phase separation. Such ther-
modynamic incompatibility is known to exist be-
tween proteins and polysaccharides and the effect is
therefore important in foods containing colloidally
dispersed proteins such as milk or gelatin solutions.
Thus, in milk, the addition of a low concentration of
hydrocolloid with a large hydrodynamic volume (i.e.,
extended conformations) will cause curdling, while
gelatin solution, in the presence of larger concentra-
tions, will exhibit phase separation. Indeed this effect
may be used to advantage in the gelatin encapsulation
of fats or, possibly, in the stabilization of foams.
(See Carbohydrates: Interactions with Other Food
Components; Protein: Interactions and Reactions
Involved in Food Processing.)
0024A further explanation for the flocculation of
colloidal particles has been proposed; this relies on
the driving force of the osmotic gradient, caused
by the exclusion of bulky polymer molecules from
the narrow gap between two approaching par-
ticles. This phenomenon, christened depletion floccu-
lation, has yet to be definitively observed in food
systems.
0025The seed gums, guar and locust bean gum (LBG),
which are both galactomannans, together with the
microbiological slime, xanthan, are the principal hy-
drocolloids used commercially for thickening foods.
Of these, xanthan shows the most pronounced and
desirable pseudoplastic flow and, in its purified form,
is colorless and tasteless. Although in many respects
an ideal stabilizer, xanthan is considerably more ex-
pensive than the seed gums and, presumably for this
reason, its use has been somewhat restricted. Guar
suffers from the problem of taste and, although it can
be reduced by steam treatment, this erodes the price
advantage. For LBG the critical quality characteristic
is the presence of dark specks, which originate
from the seed coat and which can prohibit its use in
white products. (See Gums: Nutritional Role of
Guar Gum.)
0026These hydrocolloid thickeners can be used indi-
vidually, but it is well known that galactomannans
interact with xanthan and other hydrocolloids. This
so-called synergism results, in the case of guar and
xanthan, in the mixture having a higher viscosity than
predicted on the basis of the individual concentra-
tions. In the case of LBG and xanthan, the mixture
gels. These effects are believed to be the result of
ordered associations forming cross-links between the
chains. The naked mannan segments of the galacto-
mannans are implicated in the formation of these
junction zones with xanthan, although their exact
form is at present unknown.
0027Generally speaking, viscosity increase should only
be used to stabilize suspensions in which the particle
size is small. With macroparticular suspension, such
as herbs in oil-free dressings or chocolate particles in
milk, greater resistance to movement is usually re-
quired and here the elastic properties of the continu-
ous phase should be augmented, i.e., the gel character
of the continuous phase increased.
0028The origin of gelling in polysaccharide solution is
believed to lie in formation of intermolecular cross-
links between the individual chains. With the onset of
cross-linking, the solution becomes progressively
more viscous until a distinct gel begins to form.
With increasing cross-linking the gel loses its elasti-
city, becomes more brittle, and exhibits syneresis.
Eventually, the hydrocolloids may precipitate out
of solution. The cross-links, or junction zones, are
Newtonian flow
Shear stress, τ
Pseudoplastic flow
Shear strain, d g
fig0003 Figure 3 Schematic representation of pseudoplastic and New-
tonian flow. Z ¼ t/dg, where Z is dynamic viscosity. Reproduced
from Stabilizers/Type and Function, Encyclopaedia of Food Sci-
ence, Food Technology and Nutrition, Macrae R, Robinson RK and
Sadler MJ (eds), 1993, Academic Press.
STABILIZERS/Types and Function 5541

complex, involving at least two polysaccharide
strands, and are believed to be crystalline in nature.
The driving force in their formation is hydrogen
bonding between hydroxyl groups and/or ionic
bonding where polyelectrolytes are involved.
0029 For the stabilization of liquid suspensions gener-
ally, a weak and, preferably, thixotropic gel should
be used, and the breaking strength should be chosen
so that the weakly gelled aqueous phase flows when
subject to the high shear stresses on pouring. In
principle, any gelling polysaccharide can be employed
but, in practice, carrageenans enjoy the widest
commercial use. At dosage levels of 0.5%, Jota carra-
geenans give a weak thixotropic gel suitable for
water-based dressings. It is a requirement of their
use, however, that the dressing is filled at a tempera-
ture of around 20
C. This may be bacteriologically
undesirable and, in such cases, a weakly gelling
xanthan galactomannan mixture may be used,
hot-filled. Where milk is the suspending medium, k-
carrageenans at dosage levels of 0.02% provide a
satisfactory gel.
Ostwald Ripening
0030 One further mechanism of destabilization is known.
0031 In polydisperse systems it can be observed that
larger particles grow at the expense of smaller ones.
This disproportionation phenomenon, which was
first described by Ostwald in crystallization pro-
cesses, is only important in the context of food
systems in the destabilization of foams. Gas trapped
in a foam bubble is subject to a pressure, the Laplace
pressure, due to the surface tension of the surround-
ing, typically aqueous, interface. The magnitude of
this internal pressure is inversely proportional to the
diameter of the bubble, i.e., the smaller the bubble,
the higher the pressure. If the gas is soluble in the
continuous phase then an equilibrium will be estab-
lished between the pressure of the gas in the bubble
and the concentration of dissolved gas in the continu-
ous phase immediately surrounding the interface.
This dissolved gas may diffuse away from the inter-
face or the continuous phase itself may be transported
in bulk through the foam. If this becomes adjacent to
a larger bubble with a lower Laplace pressure then
gas will be released from solution to reestablish the
equilibrium.
0032 The speed of Ostwald ripening in foams may be
reduced by decreasing the surface tension of the aque-
ous interface or decreasing the solubility of the gas in
the aqueous continuous phase. In practice, however,
longer-term stability can be conferred on the foam by
gelling the continuous phase so that bubble dispro-
portionation cannot take place.
See also: Carbohydrates: Classification and Properties;
Interactions with Other Food Components; Casein and
Caseinates: Uses in the Food Industry; Colloids and
Emulsions; Flocculation; Gums: Properties of
Individual Gums; Nutritional Role of Guar Gum; Protein:
Interactions and Reactions Involved in Food Processing
Further Reading
Dickinson E and Stainsby G (1988) Advances in Food
Emulsions and Foams. Essex: Elsevier Science Pub-
lishers.
Larsson K and Friberg SE (eds) (1990) Food Emulsions.
New York: Marcel Dekker.
Mitchell JR and Ledward DA (1986) Functional Properties
of Food Macromolecules. Essex: Elsevier Science Pub-
lishers.
Philips GO, Williams PA and Wedlock DJ (eds) (1989)
Gums and Stabilisers for the Food Industry, vol. 5.
Oxford: IRL.
Tanford C (1961) Physical Chemistry of Macromolecules.
Chichester: John Wiley.
Applications
J E Fox, P Ingenpass and S Zachow, GC Hahn and
Company, Lubeck, Germany
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Neutral Milk Products
0001The inhabitants of Normandy and Ireland’s west
coast were historically the first to use hydrocolloids
as food additives. With the help of calcium-sensitive,
gelling polysaccharides which were extracted from
red seaweeds, and which later became known as
carrageenans, a gelled milk pudding or blancmange
was prepared.
0002Carrageenans are still used in food in this capacity.
k-Carrageenan dosages between 0.2% and 0.3% pro-
duce a brittle-gelled pudding when hot-filled above
the gel melting point of about 70
C, but should the
product be subject to shear whilst gelling and cold-
filled, then a smooth, creamy, custard-type product
results. The consistency and mouth feel of both the
hot- and cold-filled products can be modified by the
incorporation of other polysaccharides. Starch, for
example, will improve the body and locust bean
gum (LBG) the elasticity. (See Gums: Properties of
Individual Gums.)
5542 STABILIZERS/Applications

0003 The classification of carrageenans as stabilizers is,
in these products, a somewhat moot point. There are,
however, two well-established applications of carra-
geenans in which their stabilizing functionality is
undisputed.
Ultra-heat-treated (UHT) Whipping Cream
0004 One aspect of the instability of milk, and particularly
cream, arises from the flocculation and creaming of
the milk fat. In short-life products, this can be ad-
equately controlled by homogenizing the product to
reduce the oil droplet size. However, this cannot be
done with long-life UHT whipping cream because it
would impair the whipping properties. The problem
can be solved by the incorporation of stabilizers
which form weak gels. (See Cream: Types of Cream.)
0005 Carrageenans have proved efficient here when
added at levels between 0.01% and 0.02%. The sta-
bilizer should be dissolved at 70
C in the skimmed
milk used to standardize the fat content, before UHT
treatment. In these systems, the casein micelles appear
to play an active role in the gel structure. The carra-
geenan becomes attached to the micelle surface by a
process other than that requiring the intermediacy of
calcium ions, and probably involving the cationically
charged section of the casein molecules. It is therefore
not surprising to find that the strength of the gel is
dependent on casein quality or, more precisely, on the
thermal history of the cream. Where the heat treat-
ment was prolonged or repeated, absorption or bind-
ing is poor and a higher dosage of carrageenan will be
required to obtain the required stability. Carragee-
nans are also amongst the most thermal-instable
hydrocolloids. Prolonged storage at elevated tem-
peratures is therefore to be avoided.
Cocoa Drinks
0006 A similar problem arises with chocolate milk. The
cocoa particles tend to sediment to the bottom of
the bottle, giving a deep brown, compact layer
below a somewhat pale (milk) fluid. This can be
corrected in the same way as the creaming in UHT
cream by the formation of a weak gel using about
0.02% of carrageenans. Other hydrocolloids may
also be included to improve the mouth feel of the
drink. The dosage of carrageenan is critical. Too
much carrageenan results in the formation of a gel
which is too strong and shows syneresis on standing,
leaving an almost clear serum at the top of the bottle.
Too low a dosage results in a gel which is insuffi-
ciently strong to support the cocoa particles which
sediment in the bottle. The quality of the cocoa can
also affect the stability. Cocoa which has been treated
with alkali to obtain a good color is difficult to
stabilize. (See Cocoa: Production, Products, and Use.)
0007To obtain stabilization, the UHT or sterilized drink
must also be bottled at a temperature below the gel-
ling point of the system, and ideally 24
C should not
be exceeded.
Foamed Milk Products
0008Two groups of foamed milk products may be recog-
nized: those containing fat in which the fat plays a
functional role in stabilization, and those based on
skimmed milk.
0009In fat-free systems, the milk proteins themselves are
sufficient to lower surface tension and form a stabil-
izing layer around the air cells. This can be reinforced
by the addition of other hydrolyzed proteins, and
thickening agents can be added to reduce the speed
of drainage of the aqueous phase from the lamellae
between the air cells, which would otherwise lead to
collapse of the foam. Thickening agents will not pre-
vent Ostwald ripening, so that to insure complete
stability the aqueous continuous phase must be gelled
as in a mousse-type product.
0010An entirely different situation exists in fat-contain-
ing products. Here the fat displaces the protein from
the air surface and prevents its stabilization of the
foam. In such products, the foam is stabilized by an
entirely different mechanism. An emulsifier, typically
an acetic acid or lactic acid ester of a mono- or
diglyceride, which is capable of displacing the protein
from the oil-water interface, is included in the stabil-
izing system. On whipping or aeration, the emulsi-
fiers promote coalescense of the fat globules, and the
fat crystals, which must be present in the fat phase at
the aeration temperature, form a supporting network
around the air cell. Such a mechanism is operative in
artificial cream, icecream, aerosol cream, cream top-
pings, milkshakes, and other products. (See Emulsi-
fiers: Uses in Processed Foods.)
0011In addition to emulsifiers, a variety of gums are
added to these stabilizer systems to modify the rhe-
ology of the aqueous phase. This is particularly
important where the fat content is low or the crystal-
lization poor. In cream toppings for desserts and
cakes, 1.0–1.5% gelatin is added directly before aer-
ation to stabilize the foam. In addition, carrageenans,
guar, and starches may be added to influence the
eating quality of the topping. In aerosol cream in
spray cans, the cream must remain fluid in the can,
and this system therefore cannot be used. Here the
protein content of the cream is enhanced and a low
dosage of gelling polysaccharide, carrageenan, or
pectin added.
0012Surface-active polysaccharides, such as propylene
glycol alginate or methylcellulose, may be used to
enhance foaming and foam stability. Where the
foam must be stable at elevated ambient temperature,
STABILIZERS/Applications 5543

the gelatin may be totally or partially replaced by
xanthan or gelling mixtures of xanthan and LBG.
Icecream
0013 Hydrocolloids and gelatin are also widely used in
icecream, and are customarily described and declared
as stabilizers, although their funtionality here has
little in common with stabilization in its strict sense.
Depending upon the type of icecream to be produced
and its fat content, typically between 0.5% and 0.8%
of a stabilizer mixture is used. Of this, about 50–60%
is a monoglyceride emulsifier to destabilize the fat
globules; the remaining 0.2–0.4 % is hydrocolloid,
of which about 80% is a thickening gum such as
LBG, guar, or sodium carboxy-methylcellulose
(CMC), and 20% a gelling agent, such as alginate or
carrageenan (0.01–0.03%). The gums serve to im-
prove mouth feel and limit ice crystal growth on
freezing and storage. The gelling agents are added to
prevent whey separation on thawing and give a slow
meltdown. (See Ice Cream: Methods of Manufacture;
Properties and Analysis.)
Sour Milk Products
Yogurt
0014 On approaching the isoelectric point, the casein mi-
celles in milk flocculate to give a delicate gel structure
typified by natural yogurt. With the natural solid
content of milk, the gel formed is extremely weak
and fragile and unsuitable for commercial exploit-
ation. The mouth feel is also poor and watery. The
addition of 2–5% milk solids as milk powder to the
base milk before incubation can remedy this situ-
ation. However, the problem remains that on aging
or exposure to high ambient temperature the casein
gel shows syneresis or wheying-off. To inhibit this,
the milk powder may be totally or partially replaced
by modified starches, gelatin, isolated milk protein,
or combinations thereof. Although from an economic
point of view the use of chemically modified starches
is very attractive, too high a dosage gives the
product a heavy, pasty and, for yogurt, very unchar-
acteristic mouth feel. Gelatin typically endows the
product with a smooth, light texture. (See Starch:
Modified Starches; Yogurt: The Product and its
Manufacture.)
0015 Polysaccharide hydrocolloids are not generally
employed because of their flocculation of milk pro-
teins at the isoelectric point, owing to the excluded
volume effects when added at effective concentra-
tions. The exception is the use of low-methoxy pectin,
at low dosages of about 0.1%, to promote floccula-
tion and thereby strengthen the gel.
0016Set yogurts, fermented in the tub, are inefficient to
produce on an industrial scale. Much more suitable to
the restraints of an industrial process are stirred
yogurts, in which the fermentation can take place in
larger vessels under carefully controlled conditions.
Unfortunately, however, once the yogurt gel is broken
by stirring, pumping, and filling, it will only partially
reform in the tub. This situation can be remedied
by the use of stabilizers which, if they are to be
added to the milk before culture, are limited to
those used in set yogurt, although they differ qualita-
tively from these.
0017With yogurt as a pumpable liquid, it becomes pos-
sible to pasteurize or even sterilize it in a plate heat
exchanger. This removes one of the major hurdles to
wide-scale distribution, i.e., the limited shelf-life. At a
pH not far below the isoelectric point of the proteins,
the stability of the dispersed protein is delicate and
thermal energy can easily lead to flocculation. This
flocculation is manifested as an unpleasant, sandy
texture in thermized yogurt. This phenomenon can
be delayed by the addition of high-methoxy pectin,
typically at levels between 0.5% and 0.7%, before
pasteurization. Pectin is believed to function by
forming a protective layer on the surface of the casein
micelles. A finer dispersion with a larger surface will
therefore require a higher dosage. The dosage is also
related to the thermal stress which the product must
survive. Indeed, under ideal technology and recipe, a
commercially satisfactory thermized product may be
manufactured with almost no stabilizer. However, if
the product is kept at elevated temperature over
hours, no amount of protective colloid can prevent
unacceptable sandiness. At extremely high dosage,
pectin also causes flocculation owing to excluded
volume effects. As an alternative to pectin, propylene
glycol alginate can be, but seldom is, used commer-
cially. CMC may be used above pH 4.2. (See Pectin:
Food Use.)
0018Pasteurization also presents the opportunity to add
new sterile ingredients to the sour product before
thermization. Thus low concentrations of gums, typ-
ically guar and LBG at 0.1% dosage levels, can be
added to improve the mouth feel of the product. (See
Pasteurization: Principles.)
Quarg and Fresh Cheese Products
0019The problems associated with the production of fresh
cheese products have their roots in the same phenom-
ena as those that destabilize yogurt. (See Cheeses:
Quarg and Fromage Frais.)
0020Collapse and contraction of the casein gel lead to
syneresis or wheying-off, and heating of the casein
enhances flocculation and causes sandiness in the
product. Both these effects can be eliminated or
5544 STABILIZERS/Applications

reduced by the use of stabilizers; the type and dosage
depend greatly on the recipe, on the technology, and
the type of product to be manufactured. A typical
thermized quarg stabilizer contains 0.3% gelatin,
0.3% starch, and small amounts of gum, including
CMC, guar, and LBG. Gelatin gives the quarg a
pleasant, smooth, and light mouth feel and appears
to function, at least partially, as a protective colloid
for underefficient thermization processes – such as
those occurring in a scraped-surface heat exchanger
– and in the presence of moderate amounts of fat, no
sandiness occurs.
0021 When the product is subject to high thermal stress,
such as prevails in a batch process with a long cooling
time or when the product is fat-free, other protective
colloids, such as CMC, may have to be included.
Starches are usually included to give the product
body. Gums confer viscosity on the product. This is
of special importance in hot-whipped products,
where the viscosity of the hot quarg would be insuffi-
cient to sustain a foam. In whipped products a higher
dosage of gelatin is also used to stabilize the foam
when cold.
Sour Milk Drinks
0022 Compared to yogurt or quarg, sour milk drinks con-
tain appreciably less protein, and the problem is
not so much one of stabilizing a casein gel network
as maintaining the individual casein flocculates in
suspension. To this end, almost all sour milk drinks
are homogenized under high pressure to break down
the casein flocculates and give a stabilizable par-
ticle density. The resulting high surface area dictates
that a higher than normal concentration of protective
colloids must be employed. Typically, this is high-
methoxy pectin for drinks below pH 4.2, and CMC
for a pH above 4.2. Below pH 4.2, CMC becomes
increasingly insoluble, and chain association leads
to flocculation enhancement. Above this pH, how-
ever, CMC has the added advantage of increasing
viscosity and thereby slowing sedimentation. With
pectin, other gums may be added to increase vis-
cosity.
Emulsified Foods
0023 Two types of emulsified foods can be recognized:
those such as mayonnaise and dressings, in which
the oil is dispersed in a continuous aqueous phase
(o/w), and those typified by such products as butter
and margarine, which are water-in-oil (w/o) emul-
sions. Both these systems are inherently unstable
owing to their high surface energy content and density
differences between the phases.
Oil-in-water Emulsion
0024Traditional mayonnaise is an o/w emulsion contain-
ing 80% oil, in which egg yolk serves as an emulsifier.
The short plastic structure of this product is attribut-
able to three factors:
1.
0025The fine oil droplet size, 1–10 mm, results in a
relatively high Laplace pressure which resists
droplet deformation.
2.
0026The high volume of oil is somewhat in excess of
the optimal packing condition, thus forcing the
droplets to be in intimate contact.
3.
0027Attractive forces between proteins on the aqueous
face of the droplet surface cause adhesion and
restrict movement. (See Dressings and Mayon-
naise: The Products and Their Manufacture;
Chemistry of the Products.)
With no room for gravitational separation and an
adequate protein layer around the droplets, mayon-
naise represents a perfectly stable system. The
modern consumer, aware of the dangers of a high-
lipid diet, has shied away from such products and
demanded surrogate products showing identical
rheological behavior, but with 50%, 30%, and even
15% oil contents. Although milk powder or case-
inate, which promotes droplet adhesion, is used as
the emulsifier, the volume of oil is too low either to
form a continuous agglomerated network and to
endow the product with stability against creaming,
or to give the desired rheological properties. This
problem may be solved by including a starch paste
(10–12% solids) to replace the oil in a 1:1 ratio. This,
especially in very-low-oil emulsions, gives the prod-
uct a cheap and pasty mouth feel. A better and more
acceptable salad or low-energy mayonnaise can
be stabilized by replacing some of the starches by
hydrocolloids. For example, for the cold preparation
of a 30% emulsion, a blend of either LBG or guar
gum and xanthan at a total dosage of 0.4–0.6% can
be used in combination with starch. Guar gum or
CMC can be used in addition where the fat content
is very low in order to achieve a mayonnaise-like
consistency. The stabilizers can be used with emulsi-
fiers such as milk protein or egg yolk.
0028For the stabilization of hot-prepared dressings and
mayonnaises, guar gum and xanthan in the ratio of
3:1 at an addition of about 0.50% can be employed.
The viscosity can be adjusted by varying the combin-
ation and quality of raw material and stabilizer(s).
0029Mayonnaises are widely used for the preparation
of salads. There are three special applications of
hydrocolloid stabilized products. When marinated
fish or fresh vegetables are included they tend to
lose appreciable amounts of cell fluids which can
STABILIZERS/Applications 5545

form unsightly pools in the mayonnaise. The incorp-
oration of gums can help bind this water. For
example, guar gum is used in combination with
xanthan, LBG, and propylene glycol alginate. For
dressings with high fat or cream contents, milk pro-
teins, especially whey protein and caseinates, are
widely used.
0030 Fresh fruit and vegetables often contain amylases
which attack and hydrolyze the starch in the mayon-
naise with the concomitant loss of viscosity and sta-
bility. For such applications, special mayonnaises
have been developed which are wholly stabilized by
enzyme-resistant hydrocolloids. Typical are blends
of guar gum and xanthan in the ratio of 3:1 at an
addition rate of 0.50%. The water-binding ability
and ‘cling’ of the dressing will be increased by adding
CMC to this blend. Where the oil content of the
mayonnaise has been reduced in the formulation of
a calorie-reduced or ‘light’ product, the consequent
reduction in opacity can be compensated by the
addition of milk proteins.
0031 In some salads, where the proportion of mayon-
naise is low, it must be prevented from running-off the
other components. This feature, known as cling, is
important in such products as coleslaw and may be
achieved by improving the pseudoplastic flow char-
acteristics of the emulsion by the use of stabilizers.
Good cling to salad ingredients, also to hot salads,
will be obtained by using balanced combination of
water-binding and gellifying hydrocolloids.
Water-in-Oil Emulsions
0032 Those arguments which led to the introduction of
low-oil mayonnaises have also promoted the develop-
ment of fat-reduced spreads. In butter or traditional
margarine, the distribution of the finely dispersed
aqueous phase is stabilized by the crystal matrix in
the fat. With half-fat products and spreads containing
more than 60% aqueous phase, the crystal matrix is
depleted and insufficient to insure stability. Hydro-
colloid stabilizers are, of course, unable to influence
the rheology of the fat phase. The usual approach is
therefore to prevent coalescence of the droplets by
increasing their viscosity or gelling. The viscosity
can be raised by the addition of proteins or gums.
The use of 2% caseinate in combination with other
milk proteins at a total dosage of about 4% is neces-
sary for the stability of the emulsion, possibly in
combination with fatty acid emulsifiers. The quality
of the product with regard to melting and spreadabil-
ity can be improved when adding hydrocolloids such
as carrageenan and xanthan. For gelling, gelating is
customarily used by virtue of its low melting tempera-
ture. (See Margarine: Types and Properties; Methods
of Manufacture.)
Deep-Frozen Foods
0033The crystallization of both oil and water phases in
deep-frozen products presents a special stabilization
problem. In addition to damaging and weakening cell
structures, crystal growth also physically concen-
trates the dispersed phase and thereby promotes ag-
glomeration. Icecream is typical of the foods in which
this can occur. In an analogous manner, ice crystal
growth within polysaccharide gels forces the chains
together; these may then form macroscopic, solid-
phase regions between the crystals which will not
rehydrate on thawing. The effect is most pronounced
in native starch gels where, after freezing, the gel
resembles a sponge from which the water can be
removed by simply squeezing. This situation can
be remedied by the addition of low levels of a hydro-
colloidal gum to the paste. Typically, 0.15% xanthan
and CMC is used. Alternatively, chemically deriva-
tized starches which show little or no retrogration,
such as hydroxypropyl derivatives, can be used. The
addition of emulsifiers and special starch-degradation
products counteracts crystallization. (See Freezing:
Principles.)
0034This behavior is not restricted to starch. Where the
chemical nature of the gelling polysaccharide is con-
ducive to ordered association, considerable syneresis
results from a freeze–thaw cycle. Such gels are, by
virtue of their propensity to cross-linking, naturally
strong and brittle. As with starch, syneresis can be
limited by the addition of small amounts of nongel-
ling gums. With k-carrageenan – a typical brittle,
freeze–thaw-unstable gel – small additions of LBG
may be used, but this will also influence gel rheology.
As would be expected, weak, elastic, and preferably
thixotropic gels may survive freeze–thaw cycles un-
damaged. Commercially, i-carrageenans are widely
used in this role.
0035Crystallization of the fat in an o/w emulsion usu-
ally results in a penetration of the protective layer
around the oil droplet with subsequent coalescence
on thawing. Provided that the oil content remains
low, preferably < 20%, emulsions which do not
break can be prepared by using propylene glycol al-
ginate as the emulsifier in combination with xanthan
as the stabilizer at levels of 0.5–1.0%.
Miscellaneous Applications
0036More than 10 years ago, the first oil-free dressing
appeared on the market in which the herbs were
suspended in a clear aqueous phase. In these products
the herbs are held in a suspension by a weak, prefer-
ably thixotropic gel, usually based on carrageenans.
This technique may be applied to a wide range of
5546 STABILIZERS/Applications
