Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0005 The final stage of protein digestion is accomplished
by a wide array of aminopeptidases and di- and
tripeptidases manufactured in the absorptive cells of
the intestinal epithelium. Some of these act in the
lumen of the gut, but most of the activity is located
within the brush-border membrane or within the
cytoplasm of the cells. These are mainly exopepti-
dases, which remove single amino acids from the
N-terminal (aminopeptidases) or the C-terminal
(carboxypeptidases).
0006 Both pepsin and the pancreatic proteolytic enzymes
are synthesized in the form of inactive proenzymes
(also termed zymogens) containing one or more ter-
minal peptide regions which prevent the formation of
the three-dimensional structure that confers catalytic
activity. The terminal peptide of pepsinogen (contain-
ing 42 amino acids) is hydrolyzed by hydrochloric
acid in the stomach. Trypsinogen is activated by the
removal of a hexapeptide from the amino-terminal by
enteropeptidase (formerly known as enterokinase),
an enzyme secreted by the small intestine. Trypsin in
turn activates all the pancreatic proenzymes, includ-
ing itself. Hence the presence of trypsin inhibitors in
some legumes plays a crucial role in reducing the
digestibility of these otherwise valuable sources of
protein.
Absorption
0007 Absorption of the products of digestion involves
active transport into the enterocyte, metabolism
within the cell, and diffusion down a concentration
gradient into the portal circulation. Absorption
occurs throughout the length of the small intestine,
but is confined to the cells in the top third of the
villus.
0008 Amino acids and peptides are transported into cells
by a number of active transport systems, most of
which are linked to the simultaneous entry of sodium
ions. Sodium enters the cell down a chemical concen-
tration gradient; this is maintained by the membrane
‘pump‘ which exchanges sodium for potassium
ions and is fueled by the hydrolysis of adenosine
triphosphate (ATP). The specificity of the amino
acid transport systems in the gut is broadly similar
to that of the systems which are found in other tissues,
particularly the brush border of the kidney tubule,
and this is summarized in Table 2. Evidence from
in vitro studies suggests that small peptides may be
transported more rapidly than free amino acids, but
these studies often have not been carried out under
the conditions which occur in vivo after a normal
meal.
0009After transport across the intestinal wall, amino
acids can be degraded, metabolized to other com-
pounds, including other amino acids, incorporated
into proteins, or released unaltered into the circula-
tion. The rate of protein synthesis in the small intes-
tinal mucosa is amongst the highest of any tissue in
the body. This is no doubt related to the high rate of
cell turnover and the requirement for secretion of
digestive enzymes. These cells also utilize energy at a
high rate, and the amino acid glutamine, supplied
both from the lumen of the gut and from the circula-
tion, is a major fuel. Some of the ammonia which is
formed during the utilization of glutamine is released
into the gut, from where it may subsequently be re-
absorbed and transported to other tissues to be used
for the synthesis of nonessential amino acids.
0010Most of the peptides which are absorbed into the
mucosal cells are hydrolyzed to single amino acids
before being released into the portal circulation.
However, some intact peptides have also been ob-
served to enter the portal circulation, and this has
raised some interest because of the possibility of pep-
tides with hormonal activity being absorbed intact.
tbl0002 Table 2 Intestinal amino acid transport systems
System Sodiumdependence Preferred amino acids
A Yes Alanine, serine, glycine, methionine, proline
L No Leucine, isoleucine, valine, methionine, phenylalanine, tyrosine, tryptophan, histidine
ASCP Yes Alanine, serine, cysteine, proline
Ly Yes Lysine, histidine, arginine, ornithine
x
A
Yes Aspartate
x
G
Yes Glutamate
x
C
No Aspartate, glutamate, cystine
y
þ
Yes Lysine, arginine, histidine
b Yes b-alanine, taurine
b
0,þ
No Lysine, leucine
Gly Yes Glycine, sarcosine
N Yes Histidine, glutamine, asparagine
imino Yes Proline
PROTEIN/Digestion and Absorption of Protein and Nitrogen Balance 4855

0011 The circulating concentration of peptides is nor-
mally very low. This may suggest that only small
quantities of peptides are released into the portal
circulation. However, it has also been observed that
when peptides are infused directly into the circulation
they are rapidly metabolized, probably extracellu-
larly, since the plasma concentration of the free
amino acid constituents of the peptides rises almost
immediately. This means that glutamine, an amino
acid which is notoriously unstable during storage in
solution, and sparingly soluble amino acids such as
tyrosine can be supplied in parenteral nutrition mix-
tures in the form of stable and soluble peptides which
are readily available to the tissues.
0012 Very small quantities of whole proteins can also be
absorbed directly from the intestinal lumen, possibly
by diffusion through tight junctions between cells.
There may also be some endocytosis of food proteins
by immunologically active cells within the intestinal
epithelium, particularly in the areas known as Peyer’s
patches, and these proteins may subsequently be
transported by lymphocytes to distant sites. The
absorption of intact proteins from maternal milk
makes an important contribution to the development
of passive immunity by neonates. Where it persists
into later life it is likely to be a major factor in the
development of food allergies.
0013 It is now known that some small molecules can be
absorbed from the colon as well as the small intestine,
so that the products of bacterial metabolism can be an
important source of nutrients. In the case of nitrogen-
ous compounds the main process appears to be secre-
tion of urea into the colon, bacterial hydrolysis of this
urea to ammonia, and subsequent absorption of the
ammonia into the body. The ammonia may then be
used for the synthesis of amino acids by transamina-
tion. This process is referred to as urea salvage, since
it results in rather less urea being excreted in the urine
than has been produced by the liver. This may make
an important contribution to the maintenance of ni-
trogen balance when protein intake is low. There is
also some evidence that essential amino acids synthe-
sized by colonic bacteria may also be absorbed into
the body, but the quantitative significance of this
observation is not clear.
Amino Acid Pools
0014 Amino acids are transported in the plasma and are
taken up into tissues by mechanisms similar to the
systems referred to in Table 2. Amino acids are also
present in blood cells, but the interaction of these
amino acids with other tissues may be quite different
from that of plasma amino acids. For example, the
plasma amino acid concentration increases as blood
traverses the gastrointestinal tract after a meal,
whereas the amino acid content of blood cells
decreases.
0015The intracellular free amino acid content varies
between different tissues, and is affected by hormonal
and nutritional influences. However, the intracellular
concentration is greater than the plasma concentra-
tion for most amino acids in most tissues. The distri-
bution ratio (intracellular concentration divided by
plasma concentration) is close to unity for many of
the essential amino acids, particularly in muscle, but
is severalfold higher for some of the nonessential
acids, with a value as high as 70 having been reported
for aspartic acid in liver. The interpretation of changes
in amino acid concentrations in particular situations
is complex. Changes in concentration may result
from changes in outflow to or inflow from other
tissues, or pools of other metabolites in the same or
different tissues, including utilization for protein
synthesis and supply from protein degradation.
0016Only about 2% of the amino acids in the body are
present as free amino acids, the rest being present
as protein. Again there is considerable variation
between different amino acids and different tissues
in the ratio of the free to protein-bound amino
acids, with much lower values generally being found
for essential than nonessential amino acids. Given the
currently accepted values for rates of protein synthe-
sis, it can be shown that in some tissues the entire free
pool of some essential amino acids is incorporated
into protein within seconds, emphasizing the rapidity
with which free amino acids must be resupplied.
Nitrogen Balance
0017The state of protein metabolism in the body as a
whole may be assessed by measuring nitrogen bal-
ance. This is because almost all the nitrogen in the
body is in the form of protein, and the nitrogen con-
tent of a wide range of proteins is relatively constant,
at around 16%. Thus, if the body is in positive nitro-
gen balance, it must be laying down new protein, and
this is normally associated with growth in children or
recovery from illness. Conversely, if the body is in
negative nitrogen balance, it must be losing tissue
protein, either because the diet is inadequate or be-
cause of a pathological response to injury or illness.
Nitrogen balance in an individual will fluctuate from
day to day by a few grams either side of zero, but over
a period of weeks a nongrowing adult will normally
be in zero nitrogen balance.
0018Every protein in the body has a specific functional
role. These roles include the catalytic function of
enzymes, the contractile function of muscle proteins,
the structural role of connective tissue proteins, roles
4856 PROTEIN/Digestion and Absorption of Protein and Nitrogen Balance

in transport, immunological recognition, hormone
receptors, and many others. There is no inert store
of protein to be drawn on in times of need, as exists
for fat (adipose tissue triacylglycerol) and carbohy-
drate (glycogen); any sustained period of negative
nitrogen balance is likely to have functional conse-
quences. Nevertheless, different tissues do lose pro-
teins at different rates when the body goes into
negative nitrogen balance, with muscle protein (in-
cluding the smooth muscle of the gut) being lost in the
early stages while the liver and the heart are relatively
protected.
Measurement of Nitrogen Balance
0019 Nitrogen balance is defined as the difference between
intake and output, and may be formally represented
by the following equation:
Balance ¼ I ðU þ F þ MÞ
where I is nitrogen intake, U is urinary nitrogen
excretion, F is fecal nitrogen excretion, and M is the
sum of all the other routes by which nitrogen is lost
from the body.
0020 Nitrogen intake is mainly in the form of protein
and can conveniently be measured by the Kjeldahl
method, which measures virtually all nitrogen except
that bonded to oxygen (e.g., nitrates and nitrites). The
same method is thus also appropriate for measure-
ment of the various components of nitrogen excretion.
Accurate determination of nitrogen balance requires
direct analysis of duplicate portions of diet, although
a reasonable approximation can be made using a
weighed-food record and tables of food composition.
An alternative approach is to feed the subjects a
constant amount of a diet which has been specially
formulated using known quantities of ingredients.
0021 The urine is the major route for excretion of nitro-
gen, so that urinary nitrogen is the major determinant
of nitrogen balance and reliable collection of 24-h
urine specimens is required for measuring nitrogen
balance. Most of the nitrogen in urine is in the form
of urea, which is the end product of amino acid
oxidation. Other nitrogenous compounds in the urine
include ammonia, creatinine, and uric acid, as well as
small quantities of peptides, amino acids, and other
small molecules. The amount of ammonia depends on
the body’s acid–base status and the amount of uric
acid varies with nucleic acid intake. The amount of
creatinine is fairly constant from day to day within a
healthy individual, and shows a good correlation
with muscle mass. When protein intake is low, the
proportion of urea in the urine will decrease, so that
urinary urea content is not a reliable indicator of total
urinary nitrogen.
0022Fecal nitrogen is mainly composed of bacterial cells
from the large intestine, together with mucosal cells
which have been sloughed off from the intestinal
wall, and some remnants of undigested food proteins.
On a normal mixed diet fecal nitrogen amounts to a
fairly constant 8% of nitrogen intake, although on
very-high-fiber diets and particularly those contain-
ing large quantities of legumes this proportion may
increase. In the measurement of nitrogen balance it is
conventional to use a nonabsorbable marker to mark
the beginning and end of the fecal collection, or to use
a continuous fecal marker.
0023The other routes by which nitrogen is lost from the
body include the shedding of hair, nails and dead skin
cells, sweat, saliva, semen, blood lost during menstru-
ation or removed for clinical testing, and the ammo-
nia exhaled in the breath. Dermal losses vary with the
rate of sweating and with protein intake. Many of
these losses are small and hard to measure, so that a
single figure of 0.5 g of nitrogen per day may be taken
to approximate the sum of these miscellaneous routes.
0024The most important use of nitrogen balance is in
evaluating the adequacy of dietary intake. It is the
major criterion which is used to assess quantitative
protein requirements. Such studies have demon-
strated that energy intake is also a major determinant
of nitrogen balance. Measurement of nitrogen bal-
ance is also the basis of many biological methods for
measuring protein quality.
Amino Acid Imbalance and Antagonism
0025It is normally the case that once the minimum require-
ments for total nitrogen and for each essential amino
acid have been met, any surplus protein intake has no
deleterious effect on nitrogen balance. However,
there are reports of the addition of a very large quan-
tity of a single essential amino acid causing a reduc-
tion in the biological value of an otherwise adequate
protein. This has been called amino acid imbalance
and appears to occur only when protein intake is
marginally adequate. It can thus be overcome by
increasing the intake of either total protein or of the
limiting amino acid.
0026The related phenomenon of amino acid antagon-
ism refers to a situation in which the addition of one
amino acid affects the efficiency of utilization of an-
other related amino acid. For example, the addition
of one of the branched-chain amino acids (leucine,
isoleucine, or valine) will impair the utilization of
dietary protein, and this can be overcome by adding
the other two branched-chain amino acids. It is
tempting to speculate that this is because of competi-
tion for membrane transport, or because of increased
activity of the common pathway by which these
PROTEIN/Digestion and Absorption of Protein and Nitrogen Balance 4857

amino acids are oxidized, but no such mechanism has
yet been confirmed.
See also: Amino Acids: Properties and Occurrence;
Determination; Metabolism; Enzymes: Functions and
Characteristics
Further Reading
Alpers DH (1986) Uptake and fate of absorbed amino acids
and peptides in the mammalian intestine. Federation
Proceedings 45: 2261–2267.
Bender DA (1985) Amino Acid Metabolism, 2nd edn.
Chichester: John Wiley.
Bender DA and Bender AE (1997) Nutrition: A Reference
Handbook. Oxford: Oxford University Press.
Calloway DH, Odell ACF and Margen S (1971) Sweat and
miscellaneous nitrogen losses in human balance studies.
Journal of Nutrition 101: 775–786.
Gardner MLG (1984) Intestinal assimilation of intact
peptides and proteins from the diet – a neglected field?
Biological Reviews 59: 289–331.
Jackson AA (1995) Salvage of urea-nitrogen and protein
requirements. Proceedings of the Nutrition Society 54:
535–547.
Waterlow JC, Garlick PJ and Millward DJ (1978) Protein
Turnover in Mammalian Tissues and the Whole Body.
Amsterdam: North Holland.
Webb KE (1986) Amino acid and peptide absorption from
the gastrointestinal tract. Federation Proceedings 45:
2268–2271.
Yudilevitch DL and Boyd CAR (eds) Amino Acid Transport
in Animal Cells. Manchester: Manchester University
Press.
Synthesis and Turnover
P W Emery, King’s College London, London, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 It is self-evident that growth must involve the synthe-
sis of new protein. Equally, there are situations, usu-
ally associated with illness or undernutrition, when
the organism loses protein, indicating that a mechan-
ism must also exist for degrading tissue proteins.
However, not until the 1940s was there direct evi-
dence that the processes of protein synthesis and deg-
radation continue to occur in healthy nongrowing
adult animals which are neither gaining nor losing
weight. The amino acid tyrosine, labeled with the
stable isotope
15
N, was fed to an adult rat, and after
10 days only half the isotope had been excreted in the
urine while most of the rest had been incorporated
into tissue proteins. Protein turnover includes both
the synthesis of protein from amino acids and the
breakdown of protein to amino acids. Much experi-
mental work over the last 40 years has been devoted
to measuring the rates at which these two processes
occur and identifying the factors which modulate
them. It is now clear that both processes occur con-
tinuously and simultaneously, and it is estimated that
an adult man synthesizes and degrades about 300 g of
protein each day, which is three or four times as much
as is consumed in the diet.
Mechanism of Protein Synthesis
0002The major biochemical pathways by which protein is
synthesized in cells are now well established. Infor-
mation specifying the primary structure is carried in
the sequence of base pairs in the DNA molecules
in the nucleus. This information is transcribed by
the formation of short-lived molecules of messenger
ribonucleic acid (mRNA) with base sequences com-
plementary to those of the deoxyribonucleic acid
(DNA). The mRNA moves out of the nucleus into
the cytosol, where it associates with ribosomes to
allow translation of the message as a sequence of
amino acids. Every three bases on the mRNA specify
a single amino acid which then binds to the ribosome
in association with a specific molecule of transfer
RNA (tRNA). A peptide bond is formed to join the
new amino acid to the growing polypeptide chain,
and the deacylated tRNA is released from the ribo-
some.
0003Even when the completed polypeptide has been
released from the ribosome it may undergo consider-
able posttranslational modification. Some amino acid
residues may be hydroxylated or methylated, while
others may form covalent links with other parts of
the molecule as the protein adopts a stable three-
dimensional structure. Nonprotein molecules, par-
ticularly carbohydrates, may be added. Finally, parts
of the protein may be removed altogether, possibly
after being transported to a different compartment of
the cell, to allow activation of an inactive precursor or
to facilitate secretion of export proteins.
0004Regulation of the rate of protein synthesis within a
given cell occurs at two levels. There is specific con-
trol of the types of protein synthesized, which occurs
mainly by controlling the transcription of particular
genes. There is also general control of the total
amount of protein synthesized, which occurs mainly
at the level of translation. This includes regulation of
the number of ribosomes present in the cell, and
hence its capacity for protein synthesis, as well as
regulation of the efficiency of translation, which
4858 PROTEIN/Synthesis and Turnover

appears to be achieved largely by changes in the rate
of initiation of peptide synthesis, although elongation
and termination may also be modulated under some
circumstances.
Protein Degradation
0005 The mechanisms by which proteins are broken down
are much less well understood. A large number
of proteolytic enzymes have been identified, with
differing substrate specificities and conditions for op-
timal function. This may reflect the complexity and
variety of the substrates on which they operate, and
may also indicate a degree of cooperative activity,
since once a protein is committed to degradation it
appears to be broken down to its constituent amino
acids very rapidly.
0006 Many of the proteolytic enzymes appear to operate
within a specific organelle, the lysosome. This pro-
vides the acidic environment in which enzymes such
as the cathepsins are optimally active. Lysosomes can
engulf and degrade large structures such as whole
mitochondria, but individual soluble proteins can
also enter the lysosome from the cytosol.
0007 Lysosomes are believed to be responsible for the
majority of protein degradation, particularly
in tissues such as the liver where the overall rate of
protein turnover is high. However, there are also
a number of proteinases which operate in the
cytosol, and are active at neutral or even alkaline
pH. These include the following: the calpains,
which are activated by calcium ions and may be
particularly active in muscle; a large multicatalytic
proteinase sometimes called a proteosome; and a
series of enzymes which commit proteins to degrad-
ation by binding them to a 76-amino-acid polypep-
tide called ubiquitin.
0008 There also exist extracellular proteinases which de-
grade specific extracellular proteins such as collagen.
0009 The control of protein degradation may be exer-
cised at both a general and a specific level, as with
protein synthesis. Some of the factors that affect the
rate of protein degradation are intrinsic to the protein
substrate itself, for example, increasing size and
acidic nature favor more rapid degradation. At least
some protein degradation appears to require energy.
There also appears to be a requirement for continuing
protein synthesis, although this may simply reflect the
fact that the enzymes which degrade proteins are, of
course, proteins themselves. However, this associ-
ation may at least partly explain the observation
that rates of protein synthesis and degradation often
change in the same direction in response to external
influences.
Measurement of Protein Synthesis and
Breakdown
0010Having identified the pathways by which proteins are
synthesized and degraded it is desirable to measure
the rates at which these processes occur. Such meas-
urements can be made in vivo, in humans or experi-
mental animals, or in vitro, in perfused or incubated
tissues or organs, cell cultures, or cell-free systems.
Unfortunately there are quantitative differences be-
tween the results obtained from different systems,
which limits the usefulness of in vitro measurements.
For example, rates of protein synthesis measured in
incubated muscle preparations tend to be lower, and
rates of protein breakdown are always considerably
higher than those observed in vivo. This occurs des-
pite careful attention being paid to factors which
are known to affect the viability of the model such
as tissue oxygenation, muscle integrity, stretch and
electrical stimulation, as well as nutrient supply
and hormonal environment. Hence such preparations
are always in net negative protein balance, whereas
in vivo muscle switches between negative balance, in
the postabsorptive state, and positive balance after a
meal.
0011The ultimate aim is to be able to measure the
turnover rates of individual proteins. This can be
done in vitro, and it may soon be possible to make
such measurements in vivo as well. However, for
technical reasons the methods that are currently
available for use in vivo are only able to give reliable
data for turnover rates in whole tissues or even the
whole body. Nevertheless, such data do provide a
useful insight into the mechanisms by which nutri-
tionally significant quantities of protein are gained or
lost, and such measurements are being made increas-
ingly frequently in both clinical and agricultural
research.
Measurement of Whole-Body Protein
Turnover
0012The measurement of protein turnover necessitates a
model which is simple enough to analyze fully but can
be reasonably interpreted in terms of what happens in
a living animal or person. The model used most com-
monly is the two-pool model, shown in Figure 1. All
the protein in the body is represented by one pool,
while the other pool represents the total amount of
one particular free amino acid (both intracellular and
extracellular). The flux of that amino acid (Q)is
defined as the sum of all the processes by which the
amino acid leaves the free pool:
Q ¼ O þ S þ M
PROTEIN/Synthesis and Turnover 4859
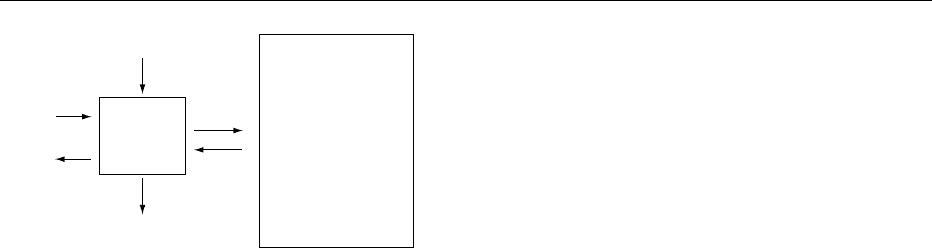
In this equation O is the rate at which the amino acid
is oxidized, S is the rate at which it is incorporated
into protein, and M is the rate at which it is metabol-
ized to other compounds. Measurements are nor-
mally made in a steady state, when the amino acid
pool is neither expanding nor contracting, so that flux
is also equal to the sum of all the processes by which
the amino acid enters the pool:
Q ¼ I þ B þ N
where I is the intake of the amino acid from dietary
protein, B is the rate at which it is released by protein
breakdown, and N is the rate at which it is synthe-
sized. Judicious choice of amino acid allows these
equations to be simplified: for essential amino acids,
N ¼0, and several of the amino acids have no meta-
bolic fates other than oxidation, so that M ¼0. Diet-
ary intake can be controlled during the period of
measurement, and is often set to zero. Thus, if the
rates of flux and oxidation can be measured, the rates
of incorporation of amino acid into protein (i.e., pro-
tein synthesis) and release from protein (i.e., protein
degradation) can be calculated. Knowledge of the
proportion of this amino acid in tissue proteins then
allows the overall rates of protein synthesis and
degradation to be calculated.
0013 There are two commonly used methods for meas-
uring amino acid flux. One involves the administra-
tion of a single dose (d) of an amino acid (often
glycine) labeled with
15
N and measurement of the
cumulative urinary excretion of label in an end
product of amino acid metabolism, usually either
urea or ammonia, over a period of up to 60 h. The
flux is calculated from the following equation:
Q ¼ dðE=etÞ
In this equation e is the cumulative excretion of the
labeled end product and E is the total excretion rate
of that end product. Oxidation rate can be estimated
from the rate of excretion of urea nitrogen expressed
as a proportion of the nitrogen flux. This method is
noninvasive and therefore particularly suitable for
certain clinical situations. However, from a theoret-
ical point of view it has major disadvantages in that
the long period necessary to obtain a representative
collection of labeled end product allows some isotope
to reenter the free amino acid pool after incorpor-
ation into rapidly turning over proteins, leading to
an underestimate of flux. In addition, the fact that
very different values for flux are obtained using
different end products even in the same experiment
suggests that the end products may not actually be
derived from the same pool as the precursors for
protein synthesis. This therefore casts doubt on the
validity of the method, particularly when comparing
different clinical and metabolic conditions.
0014The other method for measuring protein turnover
is to give a constant infusion of labeled amino acid (at
a rate i) and to measure the isotopic enrichment of the
amino acid in the plasma when this reaches a steady-
state value (L
max
), at which time isotope is entering
and leaving the plasma pool at the same rate. The flux
is then calculated from
Q ¼ i=L
max
L
max
is expressed as the ratio of plasma enrichment to
infusate enrichment. The most commonly used amino
acid is leucine labeled with
13
C in the carboxyl group
so that the oxidation rate can be obtained by measur-
ing the rate at which
13
C-labeled carbon dioxide
(
13
CO
2
) is excreted in the breath. By giving priming
doses of labeled amino acid and bicarbonate, steady-
state labeling of both plasma leucine and breath
CO
2
can be reached very quickly and the whole pro-
cedure can be completed in 4 h, although the need to
take repeated blood samples is considered a practical
disadvantage. The major theoretical limitation is that
the enrichment of free amino acids in the plasma may
not be the same as that at the site of protein synthesis,
which is likely to be intracellular and will thus vary
between different tissues anyway. The enrichment of
the intracellular amino acid pool will be lower than
that of the plasma pool, so that this method tends to
underestimate the true flux rate. When
13
C-leucine is
being used as the tracer the enrichment of its transmi-
nation product, a-ketoisocaproic acid, in plasma is
often taken as an estimate of the enrichment of intra-
cellular free leucine.
Measurement of Protein Synthesis in
Individual Tissues
0015The rate of protein synthesis in an individual tissue
can be measured directly by administering a labeled
amino acid and measuring the amount of the
Free
amino
acids
I
N
S
M
O
B
Protein
fig0001 Figure 1 Two-pool model for investigating whole-body protein
turnover. I, intake of amino acid from dietary protein; S, protein
synthesis; B, protein degradation; O, amino acid oxidation; N,
synthesis of amino acid; M, metabolism to other compounds.
4860 PROTEIN/Synthesis and Turnover

labeled amino acid that has been incorporated into
the proteins within the tissue after a given time. The
enrichment of the precursor pool for protein synthesis
during the incorporation time must also be measured
in order to calculate the rate of protein synthesis. This
can be problematical since the enrichment of the free
amino acid pool following administration of a tracer
dose of labeled amino acid follows rather complex
kinetics, and erroneous conclusions have been drawn
from experiments in which the enrichment of the
precursor pool was not measured.
0016 One way of overcoming this problem is again to
give a constant infusion of the labeled amino acid, so
that the free amino acid enrichment remains constant
for a period of several hours. Since the practical ap-
proach is similar this can conveniently be combined
with measuring whole-body protein turnover. The
major limitation of this technique is again that the
enrichment of free amino acids reaches different
steady-state values in the plasma and within the
cells. It is not clear which value best reflects the
precursor pool for protein synthesis, since there is
some evidence that extracellular amino acids may be
incorporated directly into proteins. Furthermore, in
some tissues which contain proteins that turn over
very rapidly, there is the possibility of some reentry
of label from protein after several hours of infusion.
0017 A method which largely overcomes these problems
involves givinga verylarge single dose oflabeled amino
acid. This effectively floods all the free amino acid
pools, so that they reach virtually the same level of
isotopic enrichment, and this value remains constant,
or declines slowly and linearly for an adequate period,
after which the tissue sample is taken. This period is
usually 10 min in rats or mice and 90 min in humans.
0018 The practical drawback to both these methods for
measuring protein synthesis in tissues is the need to
take a sample of the tissue. This can be achieved in
animals which can be killed at the end of the experi-
ment, but studies in humans are restricted to those
tissues which can safely be sampled by percutaneous
biopsy (such as muscle) or those which can be sampled
during scheduled surgical operations.
0019 An alternative approach which obviates the need
for tissue sampling involves sampling arterial and
venous blood simultaneously during a constant infu-
sion of a labeled amino acid. The decrease in amino
acid enrichment between arterial and venous blood
samples reflects the rate at which free amino acid is
being produced in the tissue, and at steady state, for
an amino acid that is not synthesized in that tissue,
this is assumed to equate with the rate of release by
protein degradation. This value can then be sub-
tracted from the net difference in amino acid concen-
tration across the tissue to calculate the rate of amino
acid incorporation into tissue protein, so long as the
amino acid has no other metabolic fate within that
tissue. This method has been used to measure protein
synthesis and degradation in muscle using deuterated
or tritiated phenylalanine as the tracer. However
there are practical limitations, including the difficulty
of measuring small differences in isotopic enrichment,
measuring blood flow accurately, and reliably sam-
pling venous blood from a single tissue.
Measurement of Protein Degradation
0020The method outlined above produces values for the
rate of protein degradation as well as protein synthe-
sis. This is important since there are no other methods
for measuring protein degradation in single tissues
directly. Other methods based on isotopic labeling
are limited by the problem of reutilization of amino
acids after release from protein. Thus many workers
have concluded that the most reliable estimate of
protein breakdown is obtained from the difference
between protein synthesis and the net rate of change
of the protein mass of the tissue. This involves
sequentially killing closely matched groups of animals
over a period of days, and thus cannot give any infor-
mation about short-term fluctuations in protein
degradation.
0021One approach to estimating protein degradation
specifically in skeletal muscle involves 3-methylhisti-
dine. This amino acid is formed by post translational
modification of certain histidine residues in the myo-
fibrillar proteins actin and myosin, and once it is
released from degraded protein it cannot be reutilized
for protein synthesis. 3-Methylhistidine is not metab-
olized, except for N-acetylation in certain species,
including the rat; assessment of its production rate,
from measurement of urinary excretion, should there-
fore indicate the rate of myofibrillar protein break-
down. Unfortunately, it is now known that many
other tissues, including smooth muscle and skin, con-
tain significant quantities of actin, and can make
appreciable contributions to 3-methylhistidine excre-
tion, and this has led to erroneous conclusions about
muscle protein degradation in certain circumstances
such as fasting. Some useful data have been derived
from measurements of arteriovenous differences in
3-methylhistidine across muscles, but results based
solely on measurement of urinary excretion of
3-methylhistidine should be interpreted with extreme
caution.
Rates of Protein Synthesis
0022Whole-body protein turnover has been measured in a
number of species. Values (per kg of body weight per
PROTEIN/Synthesis and Turnover 4861

day) reported for mature animals range from 40 g in
the mouse to 3 g in the cow and 4–5 g in humans
(Table 1). Both synthesis and degradation are consid-
erably increased in young growing animals, with
values of 12 g kg
1
day
1
being reported for prema-
ture infants, falling to 7 g kg
1
day
1
in 1-year-old
children.
0023 Whole-body protein turnover represents the sum of
the contributions from many different tissues. The
contribution of each tissue depends on the fractional
rate of protein synthesis in the tissue and on the
proportion of the body’s protein mass present in
that tissue. Table 2 shows representative data for a
number of tissues in the rat; isolated values are avail-
able for several other species, including humans but
these do not yet form a comprehensive picture.
0024 It should be noted that protein turnover in different
tissues is controlled independently. Thus a simple
measurement of whole-body protein turnover may
fail to detect changes in opposite directions in two
different tissues, such as muscle and liver.
Regulation of Protein Synthesis and
Degradation
0025 The rates of both protein synthesis and degradation
change in response to nutritional and other environ-
mental influences. For example, muscle protein syn-
thesis increases rapidly after a meal, as the tissue goes
temporarily into positive balance, then subsequently
declines as amino acids are mobilized during the post-
absorptive phase. There is a similar cycle of positive
and negative balance with feeding and fasting at the
level of the whole body, although the changes in
protein synthesis and degradation are more equivo-
cal. Whole-body protein degradation is suppressed by
feeding, but the rate of whole-body protein synthesis
does not always increase. The magnitude of the
changes in protein balance is affected by the habitual
diet, so that people who are habituated to a high-
protein diet lose more protein during the postabsorp-
tive period. This has important implications for the
estimation of protein requirements, which will appear
to be higher in people who are accustomed to a high
protein intake.
0026It is possible that the concentrations of free amino
acids within the cell have an influence on the rate of
protein synthesis, but this view is hard to reconcile with
the observation that the Michaelis constants (K
m
)of
the amino acyl tRNA ligases are generally well below
the intracellular amino acid concentrations, so that the
tRNAs are normally fully charged. Moreover, intracel-
lular amino acid concentrations have been observed
to increase in starvation, when the rate of protein
synthesis is known to go down. Thus it is likely that
other factors such as hormones play a more important
role in regulating protein synthesis.
0027However, it is still possible that the concentration
of one or another amino acid may affect protein
turnover. Tryptophan is the amino acid which is gen-
erally present in the lowest concentrations in intracel-
lular free amino acid pools, at least in mammalian
tissues, and there is evidence from in vitro studies
using rat liver cells that changes in tryptophan supply
can affect the rate of protein synthesis by affecting the
aggregation of ribosomes into polysomes (the form in
which they are active). However, the circumstances in
which such large changes in tryptophan concentra-
tion would occur in vivo are not clear.
0028In vitro studies have also suggested that the
branched-chain amino acids, or leucine alone, may
regulate protein synthesis in skeletal muscle, and that
the deaminated metabolite of leucine, a-ketoisoca-
proic acid, may regulate protein degradation. How-
ever, injection of a large quantity of leucine has been
tbl0001 Table 1 Representative values for whole-body protein turnover
(i.e., synthesis or degradation) rates in adult animals
Species Protein turnover (g kg
1
body weight day
1
)
Mouse 40
Rat 20
Rabbit 10
Sheep 6
Human 4–5
Cow 3
tbl0002 Table 2 Contributions of tissues to whole-body protein synthesis (average values for 100 g rats)
Tissue Protein content (% of wholebody) Fractional synthetic rate (% per day) Absolute synthesis (% of wholebody)
Whole body 100 34 100
Muscle 45 17 23
Skin 10 64 19
Liver 4 90 12
Gut 4 100 11
Bone 3 90 8
Remainder 34 26 27
4862 PROTEIN/Synthesis and Turnover

shown to have no effect on muscle protein synthesis
in vivo.
0029 Recent evidence has indicated that the rate of
protein synthesis in muscle correlates with the intra-
cellular concentration of glutamine, particularly in
circumstances where both are reduced, including
injury and infection. However, increasing muscle glu-
tamine concentration has not been shown to alter the
balance between protein synthesis and degradation.
0030 Of the many hormones that are known to affect
protein turnover, insulin has perhaps received the
most attention. Studies in rats show that insulin in-
creases protein synthesis, and this may be the major
mechanism by which food intake affects protein me-
tabolism. In contrast, insulin’s main action in humans
appears to be to decrease protein degradation. Glu-
cocorticoids depress muscle protein synthesis, and
thyroid hormones increase protein breakdown. Tes-
tosterone and its synthetic derivatives appear to exert
their anabolic effects either by increasing protein
synthesis or by decreasing protein degradation,
depending on concentration.
0031 The rate of protein synthesis in muscle is affected
by physical activity. Exercise causes muscles to hyper-
trophy by increasing the rate of protein synthesis.
During normal growth an increase in the length of
long bones tends to stretch the muscles which are
attached to them, and this stimulates muscle protein
synthesis, resulting in the growth of the muscle.
Factors that Depress Protein Synthesis
0032 One of the major reasons for studying protein turn-
over in relation to nutrition is to investigate the mech-
anisms by which growth rate is reduced or by which
lean body mass is lost in situations of undernutrition
or disease. It is perhaps not surprising that retard-
ation or even cessation of growth is associated with
a reduction in the rate of protein synthesis. There is
now considerable evidence that the net loss of lean
body mass in adults suffering from chronic diseases
such as cancer as well as primary muscle-wasting
diseases is also caused by a decrease in protein
synthesis. Protein breakdown appears not to be in-
creased, and may even be decreased in many tissues as
a secondary response to decreased protein synthesis.
This has major implications for therapy, which
should be directed towards augmenting protein syn-
thesis rather than suppressing protein breakdown.
0033 Several factors appear to be involved in the depres-
sion of protein synthesis. Patients with chronic dis-
eases often have inadequate intakes of protein and
energy, and this is likely to depress protein turnover.
Lack of physical activity, particularly in bedridden
patients, is another important factor. Changes in
conventional hormones are likely to be involved, as
are cytokines such as the interleukins and tumor ne-
crosis factor. These are peptides produced by the cells
of the immune system in response to infection and
injury which, apart from their immediate local action,
also appear to have systemic effects, including sup-
pressing muscle protein synthesis. This presumably
evolved as an adaptive response to acute injury or
infection, when it would be more important to insure
the supply of substrates for such processes as gluco-
neogenesis and acute-phase protein synthesis than to
synthesize muscle proteins. However, this now
appears as a maladaptive response to chronic disease,
since prolonged or severe muscle wasting is associ-
ated with considerable morbidity and mortality. At
the cellular level there is some evidence that these
cytokines, and indeed some conventional hormones,
may act by modulating the production of eicosanoids.
In this context prostaglandin F
2a
appears to increase
the rate of protein synthesis while prostaglandin E
2
promotes protein breakdown.
See also: Amino Acids: Properties and Occurrence;
Exercise: Muscle; Nucleic Acids: Properties and
Determination; Physiology; Protein: Functional
Properties
Further Reading
Emery PW (1999) The protein synthetic response to feed-
ing: a fundamental question revisited. Nutrition 15:
241–242.
Garrow JS and Halliday D (eds) (1984) Substrate and
Energy Metabolism in Man. London: John Libbey.
Millward DJ (1990) The hormonal control of protein turn-
over. Clinical Nutrition 9: 115–126.
Pacy PJ, Price GM, Halliday D et al. (1994) Nitrogen
homeostasis in man: the diurnal responses of protein
synthesis and degradation and amino acid oxidation to
diets with increasing protein intake. Clinical Science 86:
103–118.
Rennie MJ, Edwards RHT, Emery PW et al. (1983) Hypoth-
esis: depressed protein synthesis is the dominant
characteristic of muscle wasting and cachexia. Clinical
Physiology 3: 387–398.
Rennie MJ, Hundal HS, Babij P et al. (1986) Characteristics
of a glutamine transporter in skeletal muscle may have
important consequences for nitrogen loss in injury,
infection and chronic disease. Lancet ii: 1008–1012.
Waterlow JC (1984) Protein turnover with special reference
to man. Quarterly Journal of Experimental Physiology
69: 409–438.
Waterlow JC and Stephen JML (eds) (1981) Nitrogen
Metabolism in Man. Essex: Elsevier Science.
Waterlow JC, Garlick PJ and Millward DJ (1978) Protein
Turnover in Mammalian Tissues and in the Whole Body.
Amsterdam: Elsevier Science.
PROTEIN/Synthesis and Turnover 4863

Deficiency
C Leitzmann, Justus-Liebig University, Giessen,
Germany
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001 Protein deficiency indicates a lack of body protein or
a relative deficiency of one or several essential amino
acids. Thus, protein deficiency is synonymous with a
negative nitrogen balance. The deficiency can result
from a protein-deficient diet or other events, such as
diseases, and it must be distinguished from the multi-
factorial syndrome of kwashiorkor. However, the
clinical features and physiological effects are gener-
ally similar to those of kwashiorkor.
0043 In this article the causes and groups at risk of
protein deficiency are described. Its clinical features,
physiological effects and treatment are explained.
The adaptation to low-protein intake and protein
reserves of the body are discussed.
Causes and Groups at Risk
Causes
0002 Although the main cause of protein deficiency is a
protein-deficient diet, seen in children in developing
countries, it can also occur in patients suffering from
various diseases. In this sense, protein deficiency (as
well as other nutritional deficiencies) is a secondary
consequence of the particular disease. (See Malnutri-
tion: The Problem of Malnutrition.)
0003 Secondary protein deficiencies can be ascribed to
six causes:
1.
0004 Irregular food habits, e.g., in the case of chronic
alcoholics. The diet of alcoholics can be severely
deficient in protein.
2.
0005 Inability to digest and absorb the protein that is
consumed; this occurs in patients with chronic
gastrointestinal disorders, such as celiac disease
or enteritis. (See Celiac (Coeliac) Disease.)
3.
0006 A disturbed protein metabolism, which may exist
in patients with cirrhosis of the liver but also in
patients with hormonal disorders or in some cases
of diabetes.
4.
0007 A continuous loss of protein; this predominates in
patients with diseases such as chronic renal
disease, bleeding or exudative gastroenteropathy.
High losses of albumin into the urine are indica-
tors of the nephrotic syndrome. (See Renal
Function and Disorders: Kidney: Structure and
Function.)
5.
0008An increased protein turnover, which is character-
istic in cases of infection, fever, or gastroenteritis.
6.
0009Enhanced catabolism of protein, with increased
nitrogen losses, seen in patients with severe injur-
ies, especially burns, or in postoperative stress.
(See Burns Patients – Nutritional Management.)
0010Protein losses after operations depend on the kind
of surgery (Table 1). If patients are unable to eat
normally, insufficient food intake worsens the protein
deficiency.
Groups at Risk
0011The groups at risk can be classified according to the
causes of protein deficiency (see above) or better
according to socioeconomic parameters. According
to socioeconomic parameters, by far the largest
group is the poor population in developing countries,
primarily children in the second year of life. This
group is at risk from primary protein deficiency be-
cause of a protein-deficient diet, caused by economic,
ecological, and political factors. In developed soci-
eties, groups at risk are those who adhere to extreme
diets or suffer from anorexia nervosa or bulimia
nervosa. (See Anorexia Nervosa; Bulimia Nervosa.)
Clinical Features
Fatty Liver
0012Protein deficiency contributes to fatty infiltration of
the liver and results in hepatomegaly. This can be seen
in healthy subjects receiving a low-protein diet as well
as in protein-deficient animals fed adequate quan-
tities of other nutrients. Because of a decreased syn-
thesis of b-lipoproteins needed for the transport of
triglycerides, the fat accumulates in small droplets
within the cells, first accumulating in the periphery
of the lobules and then spreading to the center of the
tbl0001Table 1 Postoperative protein losses
Operation Loss (grams per day)
Stomach removal 110–112
Strumectomy 72
Cholecystectomy 71
Herniotomy 11
Amputation of the breast 9–18
Fractures of long bones 86–312
Skull injuries 67
Hip joint dislocation 17
From Welsch A (1986) Krankenerna€hrung, 5th edn, p. 410. Stuttgart: Thieme
Verlag.
4864 PROTEIN/Deficiency
