Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


FAO (1957) Protein requirements. FAO Nutritional Stud-
ies. 16. Rome: Food and Agriculture Organization.
FAO (1965) Protein requirements. Report of the FAO/
WHO Expert Committee. FAO Nutrition Report Series
37. Rome: Food and Agriculture Organization.
FAO (1973) Energy and protein requirements. Report of the
Joint FAO/WHO Expert Committee. FAO Nutrition
Report Series 52. Rome: Food and Agriculture Organ-
ization.
WHO (1985) Energy and protein requirements. Report of
the Joint FAO/WHO/UNU Expert Consultation. WHO
Technical Report Series 724. Geneva: World Health
Organization.
Functional Properties
J K P Weder and H-D Belitz
y
, Technische Universita
¨
t
Mu
¨
nchen, Garching, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Standardization of food properties, in order to meet
nutritional, physiological and toxicological demands,
and requirements of food-processing operations, is a
perennial endeavor. Food production is similar to a
standard industrial fabrication process: on the one
hand is the food commodity with all its required
properties; on the other hand are the components of
the product, each of which supplies a distinct part of
the required properties. Such considerations have
prompted investigations into the relationship in food
between macroscopic physical and chemical proper-
ties and the structure and reactions at the molecular
level. Reliable understanding of such relationships is
a fundamental prerequisite for the design and oper-
ation of a process, either to optimize the process or to
modify the food components to meet the desired
properties of the product.
Functional Properties
0002 In common with other food constituents, proteins
contribute significantly to the physical properties
of foodstuffs, especially through their ability to
build or stabilize gels, foams, doughs, emulsions and
fibrillar structures. Table 1 shows some typical
examples of functional properties of proteins in rela-
tion to important food systems. Foaming, gelling, and
emulsifying properties will be discussed in more
detail.
Foaming Properties
0003Proteins act as foam-forming and foam-stabilizing
agents in various foodstuffs, e.g., in baked products,
sweets, desserts, and beer. The properties of various
proteins are different: serum albumin foams very
well, whereas ovalbumin does not. Mixtures of pro-
teins, e.g., egg white, can be particularly effective. In
that case, the globulins start the formation of foam;
ovomucin is important for its stabilization, and
ovalbumin and conalbumin are responsible for the
heat-setting properties.
0004Foams are dispersions of gases in liquids. Proteins
stabilize such systems by forming flexible, cohesive
films around the surface of the gas bubbles. During
whipping, the protein is adsorbed at the interface via
hydrophobic areas, followed by partial unfolding
(surface denaturation). The decrease of the surface
tension, caused by protein adsorption, facilitates the
formation of new interfaces and further gas bubbles.
The partially unfolded proteins associate under film
formation.
0005The foamability of a protein molecule depends on
its diffusion rate and the ease with which it is de-
natured. These parameters in turn depend on the
molecular mass, the surface hydrophobicity, and the
stability of the conformation.
0006Foams collapse because large gas bubbles grow at
the expense of smaller ones (‘disproportionation‘).
Therefore, the stability of a foam depends on the
stability of the protein film and its permeability for
gases. The stability of the film in turn depends on the
amount of adsorbed protein and on the ability of the
adsorbed protein to associate. Generally, surface de-
naturation exposes additional amino acid side-chains
that can participate in intermolecular interactions.
The stronger the cross-linking, the more stable is the
film. The pH of the system should be near the iso-
electric points of the involved proteins because the
association is promoted by a small net charge.
0007In summary, the ideal foam-forming and foam-
stabilizing protein is characterized by a low molecular
mass, high surface hydrophobicity, good solubility, a
small net charge at the pH of the food, and easy
denaturability.
0008Foams are destroyed by lipids and organic solvents
such as higher alcohols, which, because of their
hydrophobicity, displace proteins from the surface
of the gas bubbles without being able to form stable
films. Egg yolk, for example, prevents the whipping
of egg white, even at low concentrations. The disturb-
ance of protein association by the lecithins is respon-
sible for that effect.
0009The foaming properties of proteins can be im-
proved by chemical and physical modification. Partial
y
Deceased.
PROTEIN/Functional Properties 4835
enzymatic hydrolysis produces smaller molecules of a
higher diffusion rate, better solubility, and higher
surface hydrophobicity. The disadvantages are the
lower film stability and the loss of heat coagulability.
The introduction of charged or neutral groups or a
partial thermal denaturation (e.g., of whey proteins)
can also improve the desired properties. Recently, the
addition of strongly basic proteins (e.g., clupeines)
has been tested, which obviously increases the protein
association within the films and allows the foaming of
lipid-containing systems.
Emulsifying Properties
0010 Emulsions are disperse systems of two or more im-
miscible liquids. They are stabilized by emulsifiers,
compounds that form interfacial films and prevent
the dispersed phase from coalescing. Proteins are
able to stabilize emulsions (e.g., milk) due to their
amphipathic nature. The emulsifying properties of a
protein depend on the rate at which it diffuses into the
interface, on its adsorbability there, and on the
deformability of its conformation through interfacial
tension (surface denaturation). The diffusion rate
depends on the temperature and the molecular mass
of the protein, which in turn can be influenced by the
pH and the ionic strength. The adsorbability depends
on the exposure of hydrophilic and hydrophobic
groups, and thus on the amino acid composition, as
well as on the pH, ionic strength, and temperature.
The stability of the conformation depends on the
molecular mass, amino acid profile and number of
intramolecular disulfide bonds of the protein.
0011 Thus, a protein with ideal qualities as an emulsifier
for oil-in-water emulsions would have a relatively
low molecular mass, a balanced amino acid compos-
ition in terms of charged, polar and apolar side-
chains, good solubility in water, marked surface
hydrophobicity, and a relatively stable conformation.
Gel Formation
0012Gels are disperse systems of at least two components
in which a solid phase (dispersed phase) forms a
cohesive network in a liquid phase (continuous
phase). Gels are characterized by their lack of fluidity
and their elastic deformability. They are placed
between solutions with repulsive forces between
molecules of the dispersed phase predominating,
and precipitates with strong intermolecular forces
predominating. A differentiation is made between
two types of gels, the ‘polymeric networks‘ and the
‘aggregated dispersions.‘ Intermediate forms are also
known.
0013Examples of polymeric networks are the gels
formed by gelatin or by polysaccharides such as
agarose or carrageenan. The formation of a three-
dimensional network takes place by aggregation of
unordered fibrous molecules via limited ordered
structures, e.g., double helices. Gels of this type are
characterized by a low concentration of polymer
(c. 1%), transparency and fine texture. Gel formation
is started by adjusting to a suitable pH, by adding
suitable ions, or by heating followed by cooling. Since
the aggregation takes place mainly via hydrogen
bonds, which are easily solved when heated, poly-
meric networks are ‘thermo-reversible,‘ i.e., they are
formed when a solution is cooled, and they melt again
when they are heated.
0014Examples of aggregated dispersions are the gels
formed by globular proteins after denaturation by
heat. The thermal unfolding of the protein exposes
amino acid side-chains, which can take part in inter-
molecular interactions. The subsequent association
leads to small spherical aggregates, which combine
into linear strands whose interaction results in the
three-dimensional gel network. Because of the unor-
dered type of aggregation, gel formation requires a
relatively high protein concentration (5–10%). To
tbl0001 Table 1 Typical functional properties caused by proteins in food systems
Functional property Mode of action Food system
Solubility Protein solvation, pH-dependent Beverages
Water absorption and
binding
Hydrogen bonding of water, entrapment of water
(no drip)
Meat, sausages, bread, cakes
Viscosity Thickening, water binding Soups, gravies
Gelation Protein matrix formation and setting Meat, curds, cheese
Cohesion–adhesion Protein acts as adhesive material Meat, sausages, baked goods, pasta products
Elasticity Hydrophobic bonding in gluten, disulfide bridges
in gels (deformable)
Meat, bakery
Emulsification Formation and stabilization of fat emulsions Sausages (e.g., Bologna), soup, cakes
Fat adsorption Binding of free fat Meat, sausages, doughnuts
Flavor binding Adsorption, entrapment, release Simulated meat, bakery, etc.
Foaming Formation of stable films to entrap gas Whipped toppings, chiffon desserts, angel cakes
Modified from Kinsella and Srinivasan (alias Damodaran S) (1981).
4836 PROTEIN/Functional Properties

avoid the formation of coarse and fairly unstructured
gels, in particular in the area of the isoelectric point,
the aggregation rate should be slower than the
unfolding rate. The degree of unfolding necessary to
start aggregation seems to depend on the particular
protein. Since partial denaturation exposes mainly
hydrophobic groups, the aggregation takes place pre-
dominantly via intermolecular hydrophobic bonds,
and ‘thermoplastic‘ (thermo-irreversible) gels are
formed. This type of gel does not liquefy when
heated, but can soften or shrink. Besides hydrophobic
bonds, disulfide bonds formed from exposed thiol
groups can also contribute to cross-linking, as can
electrostatic bonds between proteins with different
isoelectric points in heterogeneous systems (e.g., egg
white).
0015 Addition of salts improves the gel formation. The
moderate increase in ionic strength promotes inter-
action between charged macromolecules or molecule
aggregates through charge shielding without precipi-
tation. An example is the improvement of thermal
gelling of soya globulins in soya milk by calcium
ions (soya curd, tofu).
Modification of Functional Properties
0016 Modification of proteins is still far from being a
common method in food processing, but it is increas-
ingly being recognized as essential, for two main
reasons. First, proteins fulfill many functions in
food, and some of these can be served better by
modified than by native proteins. Second, persistent
nutritional problems the world over necessitate the
utilization of new raw materials. Modifying reactions
can insure that such new raw materials (e.g., proteins
of plant or microbial origin) meet stringent standards
of food safety, palatability, and acceptable biological
value. This review includes several protein modifica-
tion reactions that are being used or are being con-
sidered for use. They involve chemical or enzymatic
reactions or a combination of both. Examples have
been selected to emphasize existing trends. Modifica-
tion of the properties of proteins is possible by
changing the amino acid composition or the size of
the molecule, or by removing or inserting hetero
constituents. Such changes can be accomplished by
chemical and/or enzymatic reactions.
0017From a food processing point of view, the aims of
protein modification are:
.
0018blocking the reactions involved in deterioration of
food (e.g., the Maillard reaction);
.
0019improving some physical properties of proteins
(e.g., texture, foam stability, whippability, solubil-
ity);
.
0020improving the nutritional value (increasing the
extent of digestibility, inactivation of toxic or
other undesirable constituents, introducing essen-
tial ingredients such as some amino acids).
Chemical Modification
0021A selection of chemical reactions of proteins that are
pertinent to, and of current importance in, food pro-
cessing is shown in Table 2.
0022Acylation Treatment with succinic anhydride gener-
ally improves the solubility of a protein. For example,
succinylated wheat gluten is quite soluble at
pH 5. This effect is related to disaggregation of high-
molecular-weight gluten fractions. In the case of
succinylated casein, the modification shifts the isoelec-
tric point of the protein (and thereby the solubility
minimum) to a lower pH. Succinylation of leaf pro-
teins improves the solubility as well as the flavor and
the emulsifying properties. Succinylated yeast protein
not only has an increased solubility in the pH range of
4–6 but is also more heat-stable above pH 5. It has
better emulsifying properties, surpassing many other
proteins, and has an increased whippability.
tbl0002 Table 2 Chemical reactions of proteins significant in food
Reactive group Reaction Product
—NH
2
Acylation —NH—CO—R
—NH
2
Reductive alkylation with HCHO — N(CH
3
)
2
—NH
2
Reductive glycosylation —NH—CH
2
—(CHOH)
n
—CH
2
OH
—CONH
2
Hydrolysis —COOH
—COOH Esterification —COOR
—COOH Amidation (‘glycosylation‘) —CO—NH—CH(CHO)—(CHOH)
n
H
—OH Esterification —O—CO—R
—SH Oxidation —S—S—
—SH Alkylation —S—R
—S—S— Reduction —SH
—CO—NH— Hydrolysis —COOH þH
2
N—
PROTEIN/Functional Properties 4837

0023 Introduction of aminoacyl groups into protein
can be achieved by reactions involving amino acid
carboxy anhydrides, amino acids and carbodiimides,
or by t-butyloxycarbonyl (BOC)-amino acid N-
hydroxysuccinimide esters with subsequent removal
of the amino-protecting group (BOC). Feeding tests
with casein with attached methionine, as produced by
this method, have demonstrated a satisfactory avail-
ability of methionine. Such covalent attachment of
essential amino acids to a protein may avoid the
problems associated with food supplementation with
free amino acids: losses during processing, develop-
ment of undesired aroma due to methional, etc. With
b-casein as an example, it was shown that the associ-
ation of a protein is significantly affected by its acyla-
tion with fatty acids of various chain lengths.
0024 Alkylation Modification of proteins by reductive
methylation of amino groups with formaldehyde
and sodium borohydride (NaBH
4
) retards Maillard
reactions. The resultant methyl derivative, depending
on the degree of methylation, is less accessible to
proteolysis. Hence, its value from a nutritional and
physiological point of view is still an open question.
0025 Sugars such as glucose, fructose, maltose, or lactose
can be bound to the amino groups of proteins (casein,
legumin, b-lactoglobulin) by reductive glycosylation
with sodium cyanoborohydride. Whereas neutral
sugars favor foaming capacity and foam stability,
charged carbohydrates improve emulsifying proper-
ties. Amino sugars can also be bound to the carboxyl
group of proteins through an amide bond by the
carbodiimide method.
0026 Reactions involving cysteine and cystine Disulfide
bonds exert a strong influence on the properties of
proteins. Wheat gluten can be modified by reduction
of its disulfide bonds to sulfhydryl groups and subse-
quent reoxidation of these groups under various con-
ditions. Reoxidation of a diluted suspension in the
presence of urea results in a weak, soluble, adhesive
product (gluten A), whereas reoxidation of a concen-
trated suspension in the presence of a higher concen-
tration of urea yields an insoluble, stiff, cohesive
product (gluten C). Additional viscosity data have
shown that the disulfide bridges in gluten A are
mostly intramolecular, whereas those in gluten C are
predominantly intermolecular.
0027 Disulfide cleavage in soya protein by 0.1 M sodium
sulfite produces an adhesive with an acceptable vis-
cosity and improved adhesive strength and hydropho-
bicity compared with unmodified soya protein when
spray-dried, but a very low viscosity when freeze-
dried.
Enzymatic Modification
0028Of the great number of possible enzymatic reactions
with protein as a substrate, only a small number have
been found so far to be suitable for use in food
processing.
0029Dephosphorylation With b-casein as an example, it
was shown that the solubility of a phosphoprotein in
the presence of calcium ions is greatly improved by
enzymatic dephosphorylation.
0030Plastein reaction The enzyme-catalyzed formation
of larger polypeptides (about 3 kDa) from peptide
fragments of a hydrolysate, i.e., the formation of
peptide bonds, is called the ‘plastein reaction.‘
Amino acid esters that are added to the hydrolysate
are incorporated into the resulting products, which
are called ‘plasteins.‘ Incorporation of amino acid
esters into the polypeptide is affected by the nature
of the amino acid side-chain and by the length of the
alkyl chain of the ester. Amino acids with hydro-
phobic side-chains and long-chain alkyl esters are
preferred. Thus, an amino acid with a short side-
chain should be applied as an ester of a long-chain
alcohol. The plastein reaction can help to improve the
biological value of a protein. An example is the plas-
tein enrichment of zein with tryptophan, threonine,
and lysine.
0031Enrichment of a protein with selected amino acids
can be achieved with the corresponding amino acid
esters or, equally well, by using suitable partial hydro-
lysates of another protein. For example, the enrich-
ment of soya protein with sulfur-containing amino
acids is possible through ‘adulteration‘ with the
partial hydrolysate of wool keratin. The protein effi-
ciency ratio (PER) value of such a plastein is signifi-
cantly improved. In this way, the production of
plasteins with an amino acid profile very close to
that recommended by the Food and Agriculture
Organization and the World Health Organization
(FAO/WHO) can be achieved from very diverse
proteins, e.g., from leaf, bacterial and algal proteins.
0032The plastein reaction also makes it possible to im-
prove the solubility of a protein, e.g., by increasing
the content of glutamic acid. Soya protein (24% glu-
tamic acid) yields a plastein with 25% glutamic acid
and a Glu-plastein with 42% glutamic acid. Soya
protein has a pronounced solubility minimum in the
pH range of 3–6. The minimum is much less pro-
nounced in the case of the plastein, and the glutamic
acid-enriched Glu-plastein has a satisfactory solubil-
ity over the whole pH range and is also resistant
to thermal coagulation. Proteins with an increased
content of glutamic acid show an interesting sensory
4838 PROTEIN/Functional Properties

effect: partial hydrolysates of such plasteins do not
taste bitter, but exhibit a pronounced ‘meat broth‘
flavor.
0033 Elimination of the bitter taste from a protein
hydrolysate is also possible without incorporation of
hydrophilic amino acids. Bitter-tasting peptides, such
as leucyl-phenylalanine, which are released by partial
hydrolysis of proteins, react preferentially in the
subsequent plastein reaction and are incorporated
into higher-molecular-weight peptides with a neutral
taste.
0034 The versatility of the plastein reaction is also
demonstrated by examples wherein undesired amino
acids are removed from a protein. A phenylalanine-
free diet, which can be prepared by mixing amino
acids, is recommended for certain metabolic defects.
However, the use of higher-molecular-weight phenyl-
alanine-free peptides is more advantageous from a
sensory and osmotic point of view. Such peptides
can be prepared from proteins by the plastein reac-
tion. First, the protein is partially hydrolysed with
pepsin (pepsin A, EC 3.4.23.1). Then, treatment
with pronase (mycolysin, EC 3.4.24.31) under suit-
able conditions preferentially releases amino acids
with long hydrophobic side-chains such as phenyl-
alanine. The remaining peptides are separated by gel
chromatography and subjected to the plastein reac-
tion in the presence of added tyrosine and tryptophan
ethyl esters. This yields a plastein that is practically
phenylalanine-free and has a predetermined ratio of
other amino acids, including tyrosine. The plastein
reaction can also be carried out as a one-step process,
thus putting these reactions to economic, industrial-
scale use.
0035 Associations involving cross-linking Cross-linking
between protein molecules is achieved with peroxid-
ase (EC 1.11.1.7). The cross-linking occurs between
tyrosine residues when a protein is incubated with
a mixture of peroxidase and hydrogen peroxide
(H
2
O
2
). Incubation of a protein with a mixture of
peroxidase, H
2
O
2
, and catechol also results in cross-
linking. In this case, oxidative deamination of lysine
residues takes place, followed by aldol and aldimine
condensations, i.e., reactions analogous to those cata-
lyzed by lysyl oxidase in connective tissue.
Texturized Proteins
0036 The world protein production for nutrition is cur-
rently about 22% from animal sources and 78%
from plant sources. The plant proteins are primarily
from cereals (53%) and oilseed meal (18%). Some
nonconventional sources of protein (single cell pro-
teins, leaves) have also acquired some importance.
Proteins are responsible for the distinct physical struc-
ture of a number of foods, e.g., the fibrous structure
of muscle tissue (meat, fish), the porous structure of
bread, and the gel structure of some dairy and soya
products.
0037Many plant proteins have a globular structure and,
although available in large amounts, are used to only
a limited extent in food processing. In an attempt to
broaden the use of such proteins, a number of pro-
cesses were developed in the mid-1950s that confer a
fiber-like structure to globular proteins. Suitable pro-
cesses yield products with cooking strength and a
meat-like structure. They are marketed as meat
extenders and meat analogs and can be used
whenever a lumpy structure is desired.
0038Starting material The following protein sources are
suitable for the production of texturized products:
soya; casein; wheat gluten; oilseed meals such as
from cottonseed, groundnut, sesame, sunflower, saf-
flower or rapeseed; zein (corn protein); yeast; whey;
blood plasma; or packing plant offal such as lungs or
stomach tissue. The required protein content of the
starting material varies and depends on the process
used for texturization. The starting material is often a
mixture such as soya protein with lactalbumin, or
protein plus acidic polysaccharide (alginate, carra-
geenan, or pectin).
0039The suitability of proteins for texturization varies,
but the molecular weight should be in the range of
10–50 kDa. Proteins of less than 10 kDa are weak
fiber builders, whereas those higher than 50 kDa are
disadvantageous due to their high viscosity and their
tendency to gel in the alkaline pH range. The propor-
tion of amino acid residues with polar side-chains
should be high in order to enhance intermolecular
chain binding. Bulky side-chains hinder such inter-
actions, so that the proportions of amino acids with
these structures should be low.
0040Texturization The globular proteins are unfolded
during texturization by solving the intramolecular
bonds. The resultant extended protein chains are sta-
bilized through interactions with neighboring chains.
In practice, texturization is achieved in one of two
ways:
.
0041The starting protein is dissolved, and the resultant
viscous solution is pressed through a spinning
nozzle into a coagulating bath (‘spin process‘).
.
0042The starting protein is moistened slightly, and then,
at high temperature and pressure, the molten pro-
tein is extruded with shear force through the orifice
of a die (‘extrusion process‘).
PROTEIN/Functional Properties 4839

In the spin process, the starting material (protein
content > 90%, e.g., soya protein isolate) is sus-
pended in water and dissolved by the addition of
alkali. The 20% solution is then aged at pH 11
through constant stirring. The viscosity increases
during this step as the protein unfolds. The solution
is then pressed through the orifices of a nozzle
(5000–15 000 orifices, each with a diameter of
0.01–0.08 mm) into a coagulating bath of pH 2–3.
The bath contains an acid (citric, acetic, phosphoric,
lactic, or hydrochloric) and, usually, 10% sodium
chloride. Spinning solutions of protein–acidic poly-
saccharide mixtures also contain earth alkali salts.
The resulting protein filaments are extended further
(to about two to four times the original length) in a
‘winding up‘ step, and are bundled into thicker fibers
with diameters of 10–20 mm. The molecular inter-
actions are enhanced during stretching of the fibers,
thus increasing the mechanical strength of the fiber
bundles.
0043 The adherent coagulation solvent is then removed
by pressing the fibers between rollers, followed by
passing a neutralizing bath (sodium bicarbonate plus
sodium chloride) of pH 5.5–6 and, occasionally, also
a hardening bath (concentrated sodium chloride).
The fiber bundles may be combined into thicker
cords with diameters of 7–10 cm. Additional treat-
ment involves passage of the bundles through a bath
containing a binder (a protein that coagulates when
heated, such as egg protein) and other additives
such as modified starch or other polysaccharides,
aroma compounds or lipids. This treatment produces
bundles with improved thermal stability and aroma.
A typical bath for fibers that are processed to produce
a meat analog might consist of 51% water, 15%
ovalbumin, 10% wheat gluten, 8% soya flour, 7%
onion powder, 2% protein hydrolysate, 1% sodium
chloride, 0.15% monosodium glutamate, and 0.5%
pigments. Finally, the soaked fiber bundles are heated
and chopped.
0044 In the extrusion process, the moisture content
of the starting material (protein content about 50%,
e.g., soya concentrate) is adjusted to 30–40%,
and additives (sodium chloride, buffers, aroma com-
pounds, pigments) are incorporated. Aroma com-
pounds are added in fat as a carrier, when necessary,
after the extrusion step to compensate for aroma
losses. The protein mixture is fed into the extruder
(a thermostatically controlled cylinder or conical
body that contains a polished, rotating screw with
a gradually decreasing pitch) that is heated to
120–180
C and develops a pressure of 3–4 10
6
Pa.
Under these conditions, the mixture is transformed
into a plastic, viscous state in which solids are dis-
persed in the molten protein. Hydration of the protein
takes place after partial unfolding of the globular
molecules and stretching and rearrangement of the
protein strands along the direction of mass transfer.
The process is affected by the rotation rate and shape
of the screw, and by the heat transfer and viscosity of
the extruded material and its residence time in the
extruder. As the molten material exits from the extru-
der through a die of 4 mm diameter, the water vapor-
izes, leaving behind vacuoles in the ramified protein
strands.
0045The extrusion process is more economical than the
spin process. However, it yields fiber-like particles
rather than well-defined fibers. A great number and
variety of extruders are now in operation. As with
other food processes, there is a trend toward develop-
ing and utilizing high-temperature/short-time (HTST)
extrusion cooking.
See also: Aerated Foods; Casein and Caseinates: Uses
in the Food Industry; Colloids and Emulsions;
Emulsifiers: Organic Emulsifiers; Enzymes: Uses in
Food Processing; Functional Foods; Protein: Chemistry;
Quality; Sources of Food-grade Protein; Rheological
Properties of Food Materials; Single-cell Protein:
Yeasts and Bacteria; Soy (Soya) Beans: Processing for
the Food Industry; Soy (Soya) Cheeses; Stabilizers:
Types and Function; Wheat: Grain Structure of Wheat and
Wheat-based Products
Further Reading
Damodaran S and Paraf A (eds) (1997) Food Proteins and
their Applications. New York: Marcel Dekker.
Froning GW (1988) Nutritional and functional properties
of egg proteins. In: Hudson BJF (ed.) Developments
in Food Proteins, vol. 6, pp. 1–34. London: Elsevier
Applied Science.
Hettiarachchy NS and Ziegler GR (eds) (1994) Protein
Functionality in Food Systems. New York: Marcel
Dekker.
Kinsella JE (1978) Texturized proteins: fabrication,
flavoring, and nutrition. Critical Reviews in Food
Science and Nutrition 10: 147–207.
Kinsella JE and Shetty KJ (1978) Yeast proteins: recovery,
nutritional and functional properties. Advances in
Experimental Medicine and Biology 105: 797–825.
Kinsella JE and Srinivasan D (1981) Nutritional, chemical
and physical criteria affecting the use and acceptability
of proteins in foods. In: Solms J and Hall RL (eds)
Criteria of Food Acceptance, pp. 296–332. Zurich:
Forster Verlag.
Mitchell JR (1986) Foaming and emulsifying properties of
proteins. In: Hudson BJF (ed.) Developments in Food
Proteins, vol. 4, pp. 291–338. London: Elsevier Applied
Science.
Morrissey PA, Mulvihill DM and O’Neil EM (1987) Func-
tional properties of muscle proteins. In: Hudson BJF
4840 PROTEIN/Functional Properties

(ed.) Developments in Food Proteins, vol. 5, pp. 195–
256. London: Elsevier Applied Science.
Morrissey PA, Mulvihill DM and O’Riordan D (1991)
Functional properties of blood proteins. In: Hudson
BJF (ed.) Developments in Food Proteins, vol. 7, pp.
125–166. London: Elsevier Applied Science.
Phillips LG, Whitehead DM and Kinsella J (1994)
Structure–Function Properties of Food Proteins. San
Diego, CA: Academic Press.
Pomeranz Y (1991) Functional Properties of Food Com-
ponents, 2nd edn. San Diego, CA: Academic Press.
Poole S and Fry JC (1987) High-performance protein
foaming and gelation systems. In: Hudson BJF (ed.)
Developments in Food Proteins, vol. 5, pp. 257–298.
London: Elsevier Applied Science.
Puigserver AJ, Sen LC, Clifford AJ, Feeney RE and
Whitaker JR (1978) A method for improving the nutri-
tional value of food proteins: covalent attachment of
amino acids. Advances in Experimental Medicine and
Biology 105: 587–612.
Watanabe M and Arai S (1988) The plastein reaction and its
applications. In: Hudson BJF (ed.) Developments in
Food Proteins, vol. 6, pp. 179–217. London: Elsevier
Applied Science.
Whitaker JR, Shahidi F, Munguia AL, Yada RY and Fuller G
(eds) (1998) Functional Properties of Proteins and
Lipids, ACS Symposium Series 708. Washington, DC:
American Chemical Society.
Interactions and Reactions
Involved in Food Processing
J K P Weder and H-D Belitz
y
, Technische Universita
¨
t
Mu
¨
nchen, Garching, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The nature and extent of the chemical changes
induced in proteins by food processing depend on a
number of parameters, for example, food compos-
ition and processing conditions, such as temperature,
pH, or the presence of oxygen. The consequences of
such reactions may be desirable or undesirable. For
example, the biological value of proteins may be
decreased by destruction of essential amino acids,
conversion of essential amino acids into derivatives
that are not metabolized, or reduced digestibility of
protein as a result of intra- or interchain cross-
linking. The formation of toxic degradation products
is also possible. The nutritional/physiological and
toxicological assessment of changes induced by pro-
cessing of foods is a subject of some controversy and
opposing opinions.
Reactions with Carbohydrates
0002Many foodstuffs contain reducing sugars and amino
compounds such as proteins, peptides, amino acids,
and amines. Reactions between these components are
usually classed under the term ‘Maillard reaction‘ or
‘nonenzymatic browning.‘ They occur especially at a
higher temperature, low water activity and during
longer storage. Reactive sugars are glucose, fructose,
maltose, lactose, and, to a smaller extent, reducing
pentoses. On the side of the amino components, pri-
mary amines are more important than secondary
amines because their concentration in foods is usually
higher. Exceptions are, for example, malt and corn
products, which have a high proline content. In the
case of proteins, the e-amino groups of their lysine
residues react predominantly, but guanidino groups
of arginine residues can also react. These reactions
result in:
.
0003Brown pigments, known as ‘melanoidins,‘ which
contain nitrogen in variable amounts, and have
varying molecular masses and solubilities in water.
Little is known about their structure. Browning is
desired in baking and roasting, but not in foods
which are only slightly colored or have a distinct
color (condensed milk, faintly colored dried soups,
tomato soup).
.
0004Volatile compounds, which often contribute to the
aroma. Aroma formation through the Maillard re-
action is desired in cooking, baking, roasting, and
frying. However, the generation of off-flavors in
food during storage, especially in the dehydrated
state, or during pasteurization, sterilization, or
roasting is not desired.
.
0005Bitter substances, which are partially desired
(coffee), but can also cause an off-flavor, e.g., in
grilled meat or fish.
.
0006Reductones, which have highly reductive proper-
ties and contribute to the stabilization of foods
against oxidative deterioration.
.
0007Losses of essential amino acids (e.g., lysine,
methionine).
.
0008Mutagenic compounds.
.
0009Cross-linking of proteins.
The Maillard reaction of the e-amino group of lysine
prevails in the presence of reducing sugars, for
example, lactose or glucose, which yield the protein-
bound Amadori compounds, e-N-deoxylactulosyl-1-
lysine or e-N-deoxyfructosyl-1-lysine, respectively.
Lysine is not biologically available in these forms.
y
Deceased.
PROTEIN/Interactions and Reactions Involved in Food Processing 4841

Acid hydrolysis of such primary reaction products
yields lysine as well as the degradation products fur-
osine and pyridosine in a constant ratio. A nonredu-
cing sugar (e.g., sucrose) can also cause a loss of lysine
when conditions for sugar hydrolysis are favorable,
leading to the formation of the reactive sugars glucose
and fructose.
0010 Very recently, two intensely red-colored compounds
were isolated from brown–orange melanoidins with
mass > 10 000 Da obtained by thermal treatment of
an aqueous solution of casein and furan-2-carbox-
aldehyde (furfural), a well-known product of the
Maillard reaction. These compounds were identified
as the so-far unknown chromophoric amino acids
(S)-2-amino-6-{4-[ (E)-1-formyl-2-(2-furyl)ethenyl]-
5-(2-furyl)-2-[ (E)-(2-furyl)methylidene]-2,3-dihy-
dro-3-oxo-1H-pyrrol-1-yl}hexanoic acid and its
2-[ (Z)-(2-furyl)methylidene] isomer.
0011 In the late 1970s, it was shown that charred surface
portions of grilled fish and meat, as well as the smoke
condensates, isolated during grilling, exhibit highly
mutagenic effects in microbial tests (Ames test with
Salmonella typhimurium mutant strain TA 98). Model
experiments showed that pyrolysates of amino acids
and proteins are responsible for those effects. Table 1
lists several mutagenic compounds (pyridoindoles,
pyridoimidazoles, pyridocarbazoles, and tetraaza-
fluoranthenes) that were isolated from such pyro-
lysates.
0012 Mutagenic compounds can also be formed at lower
temperatures from amino acids and proteins. The
compounds listed in Table 2 were isolated from
meat extract, deep-fried meat, grilled fish, and heated
model mixtures of creatinine, an amino acid
(glycine, alanine, or threonine), and glucose. The
compounds, mainly imidazoquinolines and imidazo-
quinoxalines, are formed from creatinine, subsequent
products of the Maillard reaction (pyridines, pyra-
zines), and amino acids. Their toxicity is based on
the heteroaromatic amino function.
0013 Pentosidine (N
5
-[N-(5-amino-5-carboxy-1-pentyl)-
pyrido[4,5-b]imidazol-2-yl]-2,5-diaminopentanoic
acid), a cross-linked amino acid in which one arginine
and one lysine residue are linked together by a pen-
tose, was recently detected in foods. The low levels
found in commercial thermally processed food
samples (up to 4 mg kg
1
protein in milk products,
up to 40 mg kg
1
protein in roasted coffee beans
and bakery products) indicate that pentosidine
does not contribute significantly to food protein
cross-linking.
0014CROSSPY (1,4-bis-(5-amino-5-carboxy-1-pentyl)-
pyrazinium radical cation), a cross-linked amino acid
in which two lysine residues are linked together
through reaction with two molecules of glycolalde-
hyde, a Maillard reaction product, was more recently
isolated from heated aqueous solutions of bovine
serum albumin and glycolaldehyde and detected also
in toasted wheat bread crust and dark brown roasted
cacao as well as coffee beans by ESR (electron spin
resonance) spectroscopy.
Reactions with Lipid Oxidation Products
Products Formed from Hydroperoxides
0015Fatty acid hydroperoxides formed thermally or enzy-
matically in food are usually degraded further. This
degradation can also be of nonenzymatic nature. In
nonspecific reactions involving heavy metal ions,
heme(in) compounds or proteins, hydroperoxides
are transformed into oxo, epoxy, mono-, di-and tri-
hydroxy carboxylic acids. Unlike hydroperoxides,
i.e., the primary products of autoxidation, some of
these derivatives have a bitter taste. Such compounds
are detected in legumes and cereals. They may play a
role in other foods rich in unsaturated fatty acids and
proteins, such as fish and fish products.
Lipid–Protein Complexes
0016Studies related to the interaction of hydroperoxides
with proteins have shown that, in the absence of
oxygen, linoleic acid 13-hydroperoxide reacts with
N-acetylcysteine, yielding an adduct that consists of
tbl0001 Table 1 Mutagenic compounds from pyrolysates of amino acids and proteins
Mutagenic compound Short form Pyrolyzedcompound
3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole Trp-P-1 Tryptophan
3-Amino-1-methyl-5H-pyrido[4,3-b]indole Trp-P-2 Tryptophan
2-Amino-6-methyldipyrido[1,2-a:3
0
,2
0
-d]imidazole Glu-P-1 Glutamic acid
2-Aminodipyrido[1,2-a:3
0
,2
0
-d]imidazole Glu-P-2 Glutamic acid
3,4-Cyclopentenopyrido[3,2-a]carbazole Lys-P-1 Lysine
4-Amino-6-methyl-1H-2,5,10,10b-tetraazafluoranthene Orn-P-1 Ornithine
2-Amino-5-phenylpyridine Phe-P-1 Phenylalanine
2-Amino-9H-pyrido[2,3-b]indole AaC Soya globulin
2-Amino-3-methyl-9H-pyrido[2,3-b]indole MeAaC Soya globulin
Modified from Chen C, Pearson AM and Gray JI (1990) Meat mutagens. Advances in Food and Nutrition Research 34: 387–449.
4842 PROTEIN/Interactions and Reactions Involved in Food Processing
several isomers. However, in the presence of oxygen,
covalently bound amino acid–fatty acid adduct for-
mation is significantly suppressed; instead, oxidized
fatty acids are formed.
0017 The difference in reaction products is explained by
different reaction pathways. The thiyl radical, de-
rived from cysteine by abstraction of a hydrogen
atom, is added to the epoxyallyl radical only in the
absence of oxygen. In the presence of oxygen, oxida-
tion of cysteine and fatty acids to their oxidized forms
occurs with a higher reaction rate than in the previous
reaction. As a consequence, a large portion of the
oxidized lipid from a protein-containing food stored
in air does not have lipid–protein covalent bonds and,
hence, is readily extracted with a lipid solvent such as
chloroform/methanol (2:1, v/v).
Protein Changes
0018 Some properties of proteins change when they react
with hydroperoxides or their degradation products.
This is reflected by changes in food texture, decreases
in protein solubility (formation of cross-linked
proteins), changes in color (browning), and changes
in nutritive value (loss of essential amino acids).
0019The radicals generated from hydroperoxides
can abstract hydrogen atoms from proteins, preferen-
tially from the amino acids tryptophan, histidine,
lysine, arginine, cysteine and tyrosine, wherein
the nitrogen-, sulfur- or phenolic hydroxyl-containing
group reacts. Protein radicals combine with each
other, resulting in the formation of a protein net-
work.
0020Malonaldehyde is generated under certain condi-
tions during lipid peroxidation. As a bifunctional
reagent, malonaldehyde can cross-link proteins
through a Schiff base reaction with the e-amino
groups of two lysine residues. The Schiff base product
is a conjugated fluorochrome that has distinct spec-
tral properties (l
max
excitation *350 nm; l
max
emis-
sion *450 nm). Hence, it can be used to detect lipid
peroxidation and the reactions derived from it with
the proteins present.
tbl0002 Table 2 Mutagenic compounds from various heated foods and model systems
MutageniccompoundShortformFood/modelsystem
a
2-Amino-3-methylimidazo[4,5-f]quinoline IQ 1, 2, 3
2-Amino-3,4-dimethylimidazo[4,5-f]quinoline MeIQ 3
2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline MelQx 2, 3
2-Amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline 4,8-DiMelQx 2, 3, 5, 6
2-Amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline 7,8-DiMelQx 4
2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine PhIP 2
a
1, meat extract; 2, deep-fried meat; 3, grilled fish; 4, model mixture of creatinine, glycine and glucose; 5, as 4, but with alanine; 6, as 4, but with threonine.
Modified from Chen C, Pearson AM and Gray JI (1990) Meat mutagens. Advances in Food and Nutrition Research 34: 387–449.
tbl0003 Table 3 Amino acid losses occurring in proteins exposed to peroxidized lipids
ReactionsystemReactionconditionsAminoacidslost
a
(% loss)
Protein Lipid Time Temperature (
C)
CaseinLinoleic ac idethylester24h55
b
Lys(10),Thr(10),Val(10),Ala(9),Tyr(8),Phe(8),Ser(8),
Arg(8),Asp(8)
c,d
CaseinLinoleic ac idethylester4days60
e
Lys(50),Met(47),Ile(30),Phe(30),Arg(29),Asp(29),Gly(29),
His(28),Thr(27),Ala(27),Tyr(27)
c,d
OvalbuminLinoleic ac idethylester24h55
b
Met(17),Ser(10),Lys(9),Ala(8),Leu(8)
c,d
OvalbuminLinoleic ac idethylester4days60
e
Lys(50),Met(42),Leu(22),His(21),Val(21)
c,d
RibonucleaseLinoleicacid40min37
b
Met(99),Tyr(62),His(54),Lys(51),Cys(40)
c
TrypsinLinoleic ac id40min37
b
Met(83),His(12)
c
LysozymeLinoleic ac id8days37
b
Trp(56),His(42),Lys(17),Met(14),Arg(9)
a
Only the most labile amino acids are listed.
b
Aqueous system.
c
Trp not analyzed.
d
Cys not analyzed.
e
80% relative humidity.
Data from Gardner HW (1979) Lipid hydroperoxide reactivity with proteins and amino acids: a review. Journal of Agricultural and Food Chemistry 27: 220–
229 and references therein.
PROTEIN/Interactions and Reactions Involved in Food Processing 4843
0021 Reactions resulting in the formation of a protein
network like that outlined above have practical
implications; for example, they are responsible for
the decrease in solubility of fish protein during frozen
storage. Also, the monocarbonyl compounds derived
from autoxidation of unsaturated fatty acids readily
condense with free amino groups of the proteins, thus
forming Schiff bases that can provide brown poly-
mers by repeated aldol condensations. The brown
polymers are often nitrogen-free since the amino
compound can be readily eliminated by hydrolysis.
When hydrolysis occurs in the early stages of aldol
condensations (after the first or second condensation)
and the released aldehyde, which has a powerful odor,
does not re-enter the reaction, the condensation pro-
cess results not only in discoloration (browning) but
also in a change in aroma.
Decomposition of Amino Acids
0022Studies of model systems have revealed that protein
cleavage and degradation of side-chains, rather than
the formation of protein networks, are the preferred
reactions when the water content of protein–lipid
mixtures decreases. Examples for the extent of
losses in amino acids in a protein in the presence
of an oxidized lipid are presented in Table 3. The
strong dependence of this loss on the nature of the
protein and on reaction conditions is obvious. Deg-
radation products obtained in model systems of
pure amino acids and oxidizing lipids are listed in
Table 4.
Reactions Under Alkaline Conditions
0023Losses of available lysine, cystine, serine, threonine,
arginine, and some other amino acids occur at
high pH values. Hydrolysates of alkali-treated pro-
teins often contain some unusual compounds, such
as ornithine, b-aminoalanine, lysinoalanine, ornithi-
noalanine, lanthionine, methyllanthionine and
d-allo-isoleucine, as well as other d-amino acids.
The formation of these compounds is based on
the following reactions: 1,2-eliminations in the
case of hydroxy amino acids and thio amino acids
tbl0004 Table 4 Products formed from amino acids exposed to peroxidizing lipids
Reactionsystem Compoundsformed fromaminoacids
Amino acid Lipid
Histidine Methyl linoleate 3-(Imidazol-4-yl)lactic acid, 3-(imidazol-4-yl)acetic acid; histamine, valine, aspartic acid,
ethylamine
Cysteine Ethyl arachidonate Cystine, hydrogen sulfide, alanine
Linoleic acid Cystine, cysteic acid, cystine disulfoxide
Methionine Methyl linoleate Methionine sulfoxide
Lysine Methyl linoleate Diaminopentane, aspartic acid, glycine, alanine, a-aminoadipic acid, pipecolinic acid,
1,10-diamino-1,10-dicarboxydecane
Modified from Gardner HW (1979) Lipid hydroperoxide reactivity with proteins and amino acids: a review. Journal of Agricultural and Food Chemistry
27: 220–229.
tbl0005 Table 5 Formation of unusual amino acids by alkali treatment
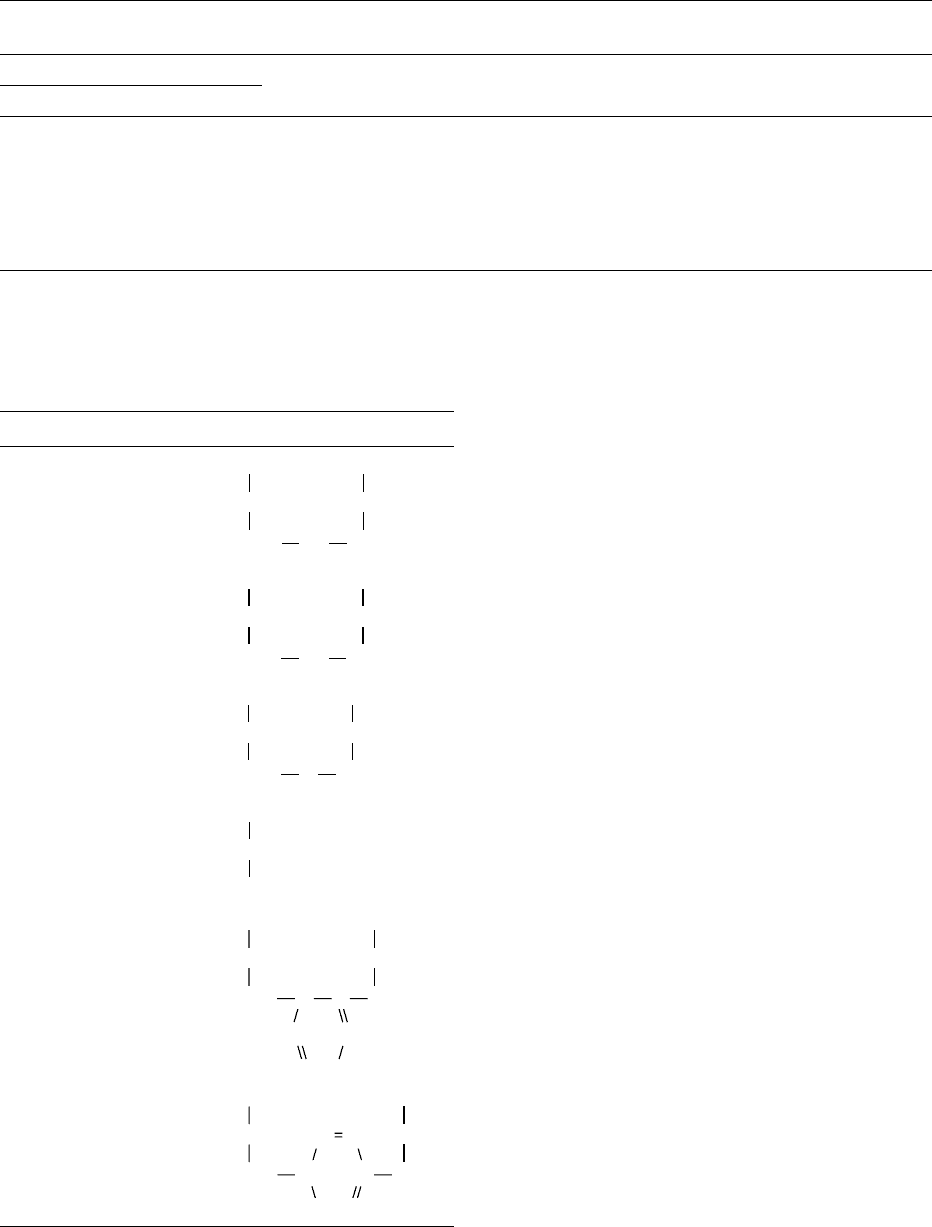
of proteins
Name Formula
3-N
6
-Lysinoalanine (R
—
—
H)
3-N
6
-Lysino-3-methylalanine
(R
—
—
CH
3
)
COOH
CHNH
2
COOH
CHNH
2
(CH
2
)
4
CHR NH
3-N
5
-Ornithinoalanine (R
—
—
H)
3-N
5
-Ornithino-3-
methylalanine (R
—
—
CH
3
)
COOH
CHNH
2
COOH
CHNH
2
(CH
2
)
3
CHR NH
Lanthionine (R
—
—
H)
3-Methyllanthionine (R
—
—
CH
3
)
COOH
CHNH
2
COOH
CHNH
2
CH
2
CHR S
3-Aminoalanine (R
—
—
H)
2,3-Diaminobutyric acid
(R
—
—
CH
3
)
COOH
CHNH
2
CHRNH
2
3-N
p
-Histidinoalanine
COOH
CHNH
2
CH
2
N
N
HC CH
C
COOH
CHNH
2
CH
2
3-N
t
-Histidinoalanine
COOH
CHNH
2
CH
2
N
N
CH
HC
C
COOH
CHNH
2
CH
2
4844 PROTEIN/Interactions and Reactions Involved in Food Processing
