Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


PROTEIN
Contents
Chemistry
Food Sources
Determination and Characterization
Requirements
Functional Properties
Interactions and Reactions Involved in Food Processing
Quality
Digestion and Absorption of Protein and Nitrogen Balance
Synthesis and Turnover
Deficiency
Heat Treatment for Food Proteins
Sources of Food-grade Protein
Chemistry
J K P Weder and H-D Belitz
y
,TechnischeUniversita
¨
t
Mu
¨
nchen, Garching, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Like peptides, proteins are formed from amino acids
through amide linkages. Covalently bound hetero
constituents can also be incorporated into proteins.
For example, phosphoproteins such as milk casein or
phosvitin of egg yolk contain phosphoric acid esters
of serine and threonine residues. Glycoproteins, such
as k-casein, various components of egg white and egg
yolk, collagen from connective tissue, and serum pro-
teins of some species of fish contain one or more
monosaccharide or oligosaccharide units bound
O-glycosidically to serine, threonine, or d-hydroxy-
lysine or N-glycosidically to asparagine.
0002 The structure of a protein is dependent on the
amino acid sequence (the primary structure) which
determines the molecular conformation (secondary
and tertiary structures). Proteins sometimes occur
as molecular aggregates which are arranged in an
orderly geometric fashion (quaternary structure).
Amino Acid Sequence
Amino Acid Composition and Subunits
0003 Sequence analysis can only be conducted on a pure
protein. First, the amino acid composition is deter-
mined after acid hydrolysis. The procedure (separ-
ation on a single cation exchange resin column and
color development with ninhydrin reagent) has been
standardized and automated (amino acid analyzers).
More recently, derivatization of amino acids to form
fluorescent derivatives, e.g., with o-phthaldialdehyde
(OPA) and 2-mercaptoethanol, followed by reversed-
phase high-performance liquid chromatography
(RP-HPLC) has been introduced (precolumn deriva-
tization). The use of chiral thiols in this procedure
allows the separation of amino acid enantiomers.
0004Selenocysteine has been detected as a relatively new
protein amino acid in glutathione peroxidase (EC
1.11.1.9) in 1973, and later also in other oxidoreduc-
tases and other proteins (e.g., in selenoprotein P, 10
residues per molecule). The incorporation of seleno-
cysteine occurs during ribosomal protein synthesis
cotranslationally by a complex mechanism in which
a specific tRNA
(ser)sec
is first loaded with serine, then
transformed into selenocysteyl-tRNA
(ser)sec
with sele-
nophosphate as the selenium donor and bound with
its anticodon region to a UGA codon of the mRNA.
Some selenocysteine, however, is also formed by
posttranslational selenium–sulfur exchange, e.g., in
selenoprotein P.
0005It is also necessary to know the molecular weight of
the protein. This is determined by gel column chro-
matography, ultracentrifugation, sodium dodecyl
sulfate–polyacrylamide gel electrophoresis (SDS-
PAGE) or mass spectrometry (matrix-assisted laser
desorption/ionization time-of-flight mass spectrom-
etry, MALDI-TOF-MS, or electrospray ionization
mass spectrometry, ESI-MS). Furthermore, it is neces-
sary to determine whether the protein is a single
molecule or consists of a number of identical or
different polypeptide chains (subunits) associated
through disulfide bonds or noncovalent bonding.
y
Deceased.
PROTEIN/Chemistry 4805

Dissociation into subunits can be accomplished by a
change in pH, by chemical modification of the pro-
tein, such as by succinylation, or with denaturing
agents (urea, guanidine hydrochloride, sodium dode-
cyl sulfate). Disulfide bonds, which are also found in
proteins consisting of only one peptide chain, can be
cleaved by oxidation of cystine to cysteic acid or by
reduction to cysteine with subsequent alkylation of
the thiol group to prevent reoxidation. Separation of
subunits is achieved by chromatographic or electro-
phoretic methods.
Terminal Groups
0006 N-Terminal amino acids can be determined by
treating a protein with 2,4-dinitrofluorobenzene
(DNFB, Sanger’s reagent) or 5-
(dimethylamino)naphthalene-1-sulfonyl chloride
(dansyl chloride). Another possibility is the reaction
with cyanate, followed by elimination of the N-ter-
minal amino acid in the form of hydantoin, and sep-
aration and recovery of the amino acid by cleavage of
hydantoin. The N-terminal amino acid (and the
amino acid sequence close to the N-terminus) is ac-
cessible by hydrolysis with aminopeptidase M (EC
3.4.11.2), in which case it should be remembered
that the hydrolysis rate is dependent on amino acid
side chains and that proline residues are not cleaved.
A special procedure is required when the N-terminal
residue is acylated (N-formyl- or N-acetyl-amino
acids, or pyroglutamic acid).
0007 Determination of C-terminal amino acids is
possible via the hydrazinolysis procedure. The C-ter-
minal amino acid is separated from the amino acid
hydrazides, e.g., by a cation exchange resin, and iden-
tified. The C-terminal amino acids can be removed
enzymatically by carboxypeptidase A (EC 3.4.17.1),
which preferentially cleaves amino acids with aro-
matic and large aliphatic side chains; carboxypepti-
dase B (EC 3.4.17.2), which preferentially cleaves
lysine, arginine, and amino acids with neutral side
chains; carboxypeptidase C (EC 3.4.16.1), which
cleaves with less specificity and cleaves proline; car-
boxypeptidase P (EC 3.4. 16.1), hydrolyzing almost
all amino acids (including proline, aspartic acid,
and glutamic acid; release of serine and glycine is
retarded); or carboxypeptidase Y (EC 3.4. 16.1),
which shows high catalysis rate if the penultimate
and/or terminal residue is an aromatic or aliphatic
amino acid including proline (release of glycine and
aspartic acid is considerably retarded).
Partial Hydrolysis
0008 Longer peptide chains are usually fragmented. The
fragments are then separated and analyzed individu-
ally for amino acid sequences. Selective enzymatic
cleavage of peptide bonds is accomplished with tryp-
sin (EC 3.4.21.4), which only cleaves Lys-X and Arg-
X bonds; chymotrypsin (EC 3.4.21.1), which cleaves
peptide bonds with less specificity (Tyr-X, Phe-X,
Trp-X and Leu-X); and, more recently, with endopro-
teinase Arg-C (EC 3.4.22.8), hydrolyzing only Arg-X
bonds; endoproteinase Asp-N (EC 3.4.24.33), hydro-
lyzing X-Asp and X-CySO
3
H bonds; endoproteinase
Glu-C (EC 3.4.21.19), hydrolyzing Glu-X or Glu-X
and Asp-X bonds depending on pH and buffer;
or endoproteinase Lys-C (EC 3.4.21.50), hydrolyzing
only Lys-X bonds. The enzymatic attack can be
influenced by modification of the protein.
0009The most important chemical method for selective
cleavage uses cyanogen bromide (BrCN) to attack
Met-X linkages. Separation of peptide fragments is
achieved by gel and ion exchange column chromatog-
raphy using a volatile buffer as the eluent (e.g., pyri-
dine, morpholine acetate) which can be removed by
freeze-drying of the fractions collected. Recently the
separation of peptides and proteins by RP-HPLC has
gained great importance, using volatile buffers mixed
with organic, water-soluble solvents (e.g., aceto-
nitrile) as the mobile phase.
0010The fragmentation of the protein is performed by
different enzymatic and/or chemical techniques, at
least by two enzymes of different specificity. The
arrangement of the peptides obtained, in the same
order as they occur in the intact protein, is accom-
plished with the aid of overlapping sequences.
Sequence Analysis
0011The Edman degradation is by far the most important
method in sequence analysis. It involves stepwise
degradation of peptides with phenyl isothiocyanate,
starting at the N-terminus of the polypeptide. The
resultant phenylthiohydantoin is either identified
directly or the amino acid is recovered. The stepwise
reactions are performed in solution or on peptide
bound to a carrier, i.e., to a solid phase. Both ap-
proaches have been automated (sequencer). Carriers
used include resins containing amino groups (e.g.,
aminopolystyrene) or glass beads treated with
aminoalkylsiloxane. The peptides are then attached
to the carrier by carboxyl groups (activation with
carbodiimide or carbonyl diimidazole, as in peptide
synthesis) or by amino groups. Nowadays, Edman
degradation has been miniaturized and is performed
on gas-phase or pulsed-liquid-phase sequencers, in
which the peptide is adsorbed on a polybrene-treated
glass-fiber disk and the reagents are applied either as
vapors in a carrier gas stream or in small liquid doses
separated by carrier gas bubbles.
4806 PROTEIN/Chemistry

0012 Methods for C-terminal sequencing have also
been reported. After activating the polypeptide at
the a -carboxyl group with acetic anhydride, the
activated polypeptide is reacted with thiocyanic
acid (formed from ammonium thiocyanate with an
acid) and the resulting thiohydantoin is cleaved with
a base to give the thiohydantoin of the C-terminal
amino acid and the polypeptide reduced by one
amino acid residue. Another procedure uses diphenyl
phosphoroisothiocyanatidate (DPP-ITC), pyridine
for reaction and cyclization, and sodium trimethylsi-
lanoate for cleavage. A modification of the first
procedure has been automated including S-alkyla-
tion of the thiohydantoin with 2-(bromomethyl)-
naphthalene and cleavage of the C-terminal amino
acid derivative simultaneously with the formation of
the next thiohydantoin by addition of ammonium
thiocyanate plus trifluoroacetic acid. It should be
mentioned that C-terminal sequencing procedures
are by far less sensitive than Edman degradation
and that only a few steps can be interpreted without
doubt.
0013 Methods other than chemical degradations can
provide additional information. These include deter-
mination of terminal residues with amino- and
carboxypeptidases, as already discussed, or mass
spectrometry. Mass spectrometric sequencing can be
done by MALDI-TOF-MS (peptide ladder sequencing
and postsource decay (PSD)-MALDI) or ESI-MS
(atmospheric pressure ionization collision-induced
decay, API-CID; single or multiple fragmentation of
selected ions, MS-MS or MS
n
). Peptide ladder sequen-
cing combines a modified Edman degradation (in-
complete by the addition of 5% phenyl isocyanate)
with the determination of the molecular masses of the
resulting mixture consisting of phenylcarbamoyl de-
rivatives of peptides stepwise reduced by one amino
acid. Because of the small difference between the
molecular mass of aspartic and glutamic acid and
their amides, results can only be interpreted up to
5 kDa. The other two methods include fragmentation
of the peptide chain, and unknown sequences can
only be interpreted up to 2 kDa without doubts. The
main area of mass spectrometric sequencing is the
confirmation of already known sequences. None of
the methods can differentiate between leucine and
isoleucine.
0014 Recently, amino acid sequences of proteins have
increasingly been deduced from the nucleotide
sequences of the corresponding genes. Examples of
food proteins are gliadins and glutenins from wheat.
If the gene responsible for the expression of a protein
is known, the sequence analysis of the nucleic acid
can be performed much easier than that of the protein
itself.
0015Examples for amino acid sequences of food pro-
teins are summarized in Tables 1–3 (legume 11S glo-
bulins, legume 7S globulins, wheat proteins). Further
examples (milk proteins, collagen, monellin, and
thaumatin) are presented in the first edition of this
Encyclopedia.
Conformation
0016Information about conformation is available through
X-ray crystallographic analysis of protein crystals
and through the determination of the H–H distances
by proton nuclear magnetic resonance (NMR) spec-
troscopy in solution. In principle, the conformation of
the protein in crystalline form can be assumed to be
similar to that of the protein in solution. In 1960 the
structure of myoglobin (17.8 kDa) was elucidated
with a resolution of 0.2 nm. Individual atoms are
well revealed at 0.11 nm. Such a resolution has only
been achieved with few proteins. Reliable localization
of the C
a
atoms of the peptide chain requires a reso-
lution of less than 0.3 nm. Most protein conform-
ations have been studied with a resolution between
0.2 and 0.3 nm and, more recently, between 0.15 and
0.2 nm.
Extended Peptide Chains
0017X-ray structural analysis and other physical measure-
ments of a fully extended peptide chain reveal the
lengths and angles of bonds. The peptide bonds have
partial (40%) double-bond character with p-electrons
shared between the C
0
–OandC
0
–N bonds. The res-
onance energy is about 83.6 kJ mol
1
. Normally the
bond has a trans configuration, i.e., the oxygen of the
carbonyl group and the hydrogen of the NH group
are in the trans position; a cis configuration only
occurs in exceptional cases (e.g., in small cyclic
peptides or in proteins before proline residues). Be-
cause of the partial double-bond character, six atoms
of the peptide bonds, C
i
a
,C
i
0
,O
i
,N
iþ1
,H
iþ1
and C
a
iþ1
,
lie in one plane. For a trans peptide bond, o
i
is 180
.
The position of two neighboring planes is determined
by the numerical value of the angles c
i
(rotational
bond between a carbonyl carbon and an a-carbon)
and f
i
(rotational bond between an amide nitrogen
and an a-carbon). For an extended peptide chain c
i
¼
180
and f
i
¼ 180
. The position of side chains can
also be described by a series of angles w
i
1n
.
Secondary Structure (Regular Structural Elements)
0018The primary structure gives the sequence of amino
acids in a protein chain whereas the secondary and
tertiary structure reveal the arrangement of the chain
in space. The peptide chains are not in an extended or
PROTEIN/Chemistry 4807

unfolded form (c
i
, f
i
6¼ 180
). It can be shown with
models that c
i
and f
i
, at a permissible minimum
distance between atoms can assume only particular
angles. It has been shown for many proteins empiric-
ally that they have values of c
i
, f
i
pairs within the
permissible range. When a multitude of equal c
i
, f
i
pairs occurs consecutively in a peptide chain, the
chain acquires regular repeating structural elements
(secondary structure). Examples of some important
secondary structures are shown in Figures 1–3.
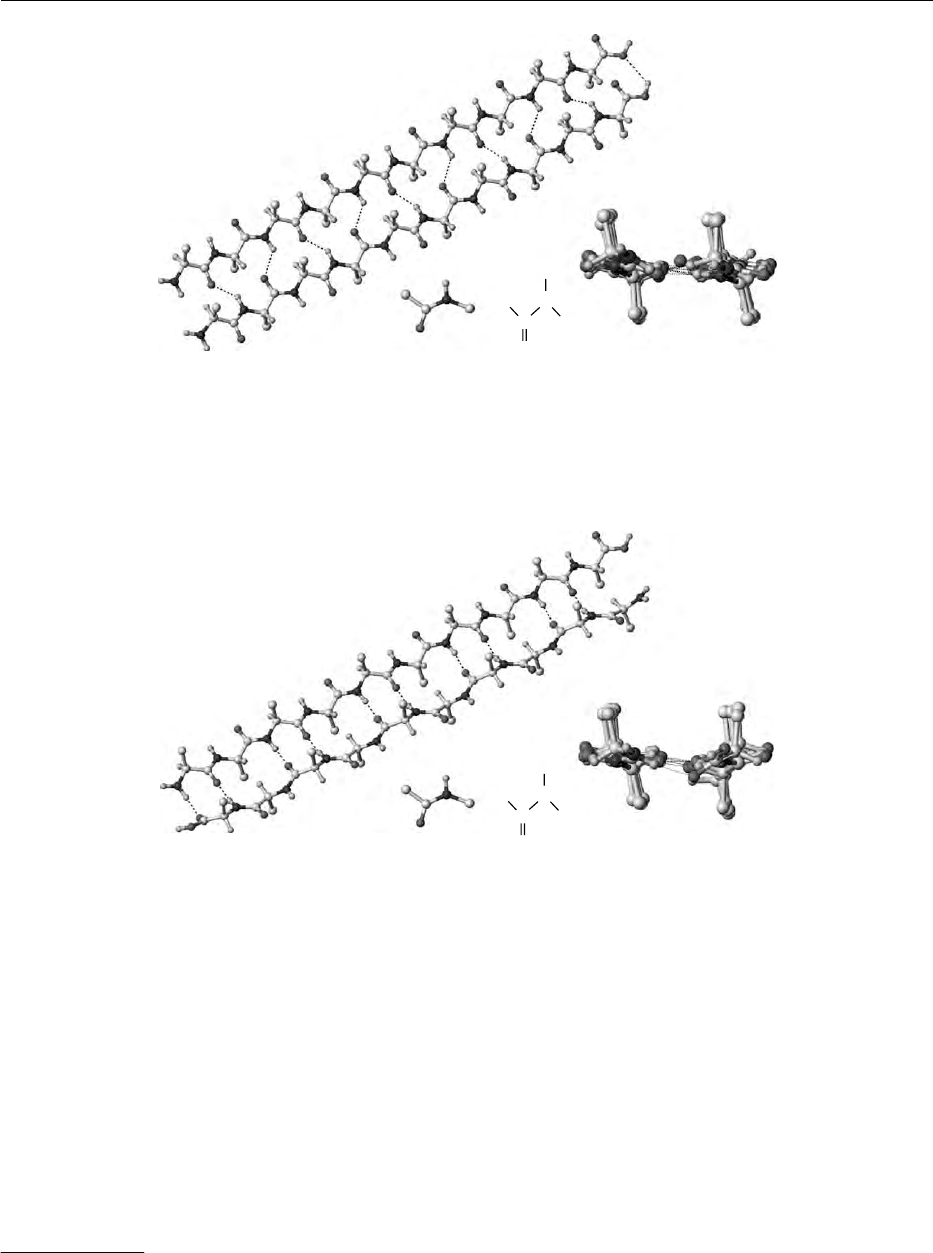
0019 b-Sheet Three regular structural elements (pleated-
sheet structures) have values in the range of f ¼
120
and c ¼þ120
. The peptide chain is always
lightly folded on the C
a
atom, thus the R side chains
extend perpendicularly to the extension axis of the
chain, i.e., the side chains change their projections
alternately from þ z to z. Such a pleated structure
is stabilized when more chains interact along the x
axis by hydrogen bonding, thus providing the cross-
linking required for stability. When adjacent chains
run in the same direction, the peptide chains are
parallel. This provides a stabilized, planar, parallel
sheet structure (Figure 1). When the chains run in
opposite directions, a planar, antiparallel sheet struc-
ture is stabilized (Figure 2). The lower free-energy,
twisted sheet structures, in which the main axes of the
neighboring chains are arranged at an angle of 25
,
are more common than planar sheet structures.
0020The b-structures can also be regarded as special
helical structures with a continuation of two residues
per turn. With proline, the formation of a b-structure
is not possible.
0021Helical structures There are three regular structural
elements in the range f ¼60
and c ¼60
,in
which the peptide chains are coiled like a threaded
screw. These structures are stabilized by intrachain
hydrogen bridges which extend almost parallel to the
helix axis, cross-linking the CO and NH groups,
specifically the CO group of amino acid residue i
with the NH group of residue iþ3(3
10
-helix), iþ4
(a-helix) or iþ5(p-helix). The a-helix, and for poly-
peptides from l-amino acids exclusively the right-
handed a-helix, is the most common (Figure 3). The
3
10
-helix is only observed at the ends of a-helices,
whereas the existence of a p-helix is hypothetical. A
helix is characterized by the angles f and c, or by the
tbl0001 Table 1 Amino acid sequences of 11S globulins from legumes
Glycinin A
2
B
1a
from soya bean, a polypeptide
505050 5050
1 L REQAQQNE CQI QKL N AL KPDNRI ESEGGF I ET WNPN NKPFQC AGVAL SR
51 CTLNRNALRRPSYT NGPQEI YI QQGNGI F GMI FP GCP STYQEP QESQQRG
101 RSQRPQDRHQKVHRFREGDLI AV PTGVAWWMYNNEDT PVVAVS I I DTNSL
151 ENQL DQMPR RF YL A GN QE QEF L K Y QQQQQ GGSQS QKG KQQE E E NE GSN I L
201 SGFAPEF LKEAFGV NMQI VRNLQGENEEE DSGAI VTVKGGLRVTAPAMRK
251 PQQEEDDDDEEEQP QCVETDKGCQRQSKRS RN
Glycinin A
2
B
1a
from soya bean, a polypeptide
505050 5050
1 GI DETI CTMRL RQN I GQNSSPDI YNPQAGS I T T ATSL DF PALWL L KLS AQ
51 YGSL RKN AMFVPHY TL NANSI I Y AL NGRA L V QVV NCN GERVF D GELQE GG
101 VL I VPQNFAVAAKS QS DNF EYVS FKTNDR P S I GNL AGANSL L N ALPEE VI
151 QHT F NLKSQQARQV KNNNPFSF L VPPQES QR RAV A
Legumin J from pea, b polypeptide
505050 5050
1 L ATSSEF DR L NQCQL DSI NAL EP DHRVVS E A GL T ETWNPNHPE L KCAGVS
51 LI RRTI DPNGLHLP SF SPSPQLI FI I QGKGVLGL SFPGCPETYEEPRSSQ
101 SRQESRQQQGDSHQKV RRFRKGDI I AI PS GI PYWT YN HGDEPL VAI SL L D
151 TSNI ANQLDST PRV FY L GGNPET EFPET QE E QQGRHR QKHSYP VGRRS GH
201 HQQEEESEQQNEGNSVLSGFSSEFLAQTF NT EEDTAKRLRSPRDERSQI V
251 RVEGGLRI I KGRTE EE KEQSHSHSHREEKE E EEE EEE DEEEKQRSEERKN
Legumin J from pea, b polypeptide
505050 5050
1 GLEETI C SAKI RENI ADAARADL YNPRAGR I STA NSL T L PVLR YLRLS AE
51 YVRLYRNGI YAPHWNI NANSL L Y VI RGEGRV RI RSCE LPT NTMF DNKL RK
101 GHLVVVPQNFVVAE QAGEEEGLEYVVFKT NDRAAVSHVQQVFRATPSEVL
151 ANAFGL R QR QVTEL KL SGNRGPMVHPRSQS QSH
Amino acids are given in the International Union of Pure and Applied Chemistry one-letter notation. The two polypeptides of glycinin and legumin
are connected by a disulfide bond Cys(a84)-Cys(b7) and Cys(a89)-Cys(b7), respectively. The two remaining cysteine residues of the a subunits (10/43 and
13/46, respectively) probably form an intrachain disulfide bridge.
Data from Wright DJ (1987) The seed globulins. In: Hudson BJF (ed.) Developments in Food Proteins, vol. 5, pp. 81–157. London: Elsevier Applied Science.
4808 PROTEIN/Chemistry

parameters derived from these angles: n, the number
of amino acid residues per turn; d, the rise along
the main axis per amino acid residue; and r, the radius
of the helix. Thus, the equation for the pitch, p,is
p ¼nd.
0022 Reverse turns An important conformational feature
of globular proteins is the reverse turns, b-turns, or
b-bends. They occur at ‘hairpin‘ corners, where the
peptide chain changes direction abruptly. Such
corners involve four amino acids residues, among
them frequently proline. Glycine is favored in the
third position of b-bends on the basis of energy con-
siderations. Different types of b-turns are known, for
which different amino acids are allowed.
0023 Super secondary structures Analysis of known
structures has demonstrated that regular elements
can exist in combined forms. Examples are the
coiled-coil a-helix, chain segments with antiparallel
b-structures (b-meander structure) and combinations
of a-helix and b-structure (e.g., baba).
Tertiary and Quaternary Structure
0024Proteins can be divided into two large groups on the
basis of conformation: (1) fibrillar (fibrous) or sclero-
proteins, and (2) folded or globular proteins.
0025Fibrous proteins The entire peptide chain is packed
or arranged within a single regular structure for a
variety of fibrous proteins. Examples are wool
keratin (a-helix), silk fibroin (b-sheet), and collagen
(a triple helix). Stabilization of these structures is
achieved by intermolecular binding (electrostatic
interaction and disulfide linkages, but primarily
hydrogen bonds and hydrophobic interactions).
tbl0002 Table 2 Amino acid sequences of 7S globulins from legumes
b-Conglycinin from soya bean, a subunit
50 5 0 5 05 050
1 VEE EEECEEGQI P RPRP QHPERE RQQHGE KEEDEGEQP RPF PFPRPRQPH
51 QEEEHEQKEEHEWHRKE EKHGGKGSEEEQDEREHPRP HQPHQKEEEKHEW
101 QHK QEKHQGKESE EEEE DQDEDE EQDKES QESEGSES QREPRRHKNKNPF
151 HF NSKRFQTL F KN QYGHVRVL QR F NKRSQQLQNLRDY R I L EFNSKPNTLL
201 L PH HADADYL I VI L NGT AI L T L V NNDDRDSYNL QSGD A LRVPAGTTFYVV
251 NPDNDENL RMI AGTTFY VVNPDN DENL RMI T LAI PVNK PERF ESFFL SST
301 QAQQSYL QGF SKN I LEA SYDT KF EEI NKV LF GREEGQQQGEERLQESVI V
351 EI S KKQI REL SKHAKSS SRKTI S SEDKPF NL GSRDPI Y SNKL GKL F EI TQ
401 RNP QLRDL DV FLS VVDMNEGAL F L PHF NS KAI VVLVI N EGEANI ELVGI K
451 EQQQRQQQEE QPL EVRKYRAELSEQDI FVI PAGYPVMVNATSDLNFFAFG
501 I NA ENNQRNF L AGSKDNVI SQI P SQVQEL AF PRSAKDI ENL I KSQSESYF
551 VDAQPQQKEE GNK GRKGPL SSI L RAF Y
Vicilin from pea
50 5 0 5 05 050
1 RSDPQNPFI F KSNKFQTLFENENGHI RLLQKFDQRSKI FENLQNYRLLEY
51 KSK PHTI F LP QHT DADY I LVVL S GKAI LT VL KPDDRNS FNERGDTI KL PA
101 GTI AYLVNRDDNEELRVLDLAI PVNRPGQLQSFLLSGNQNQQNYLSGFSK
151 NI L EASF NT D YEE I EKV L L EEHE KET QHRRSL KDKRQQSQEENVI VKL SR
201 GQI EEL SKNAKST SKKS VSSESE PFNL RS RGPI YSNE P GKFF EI T PEKNP
251 QLQDLDI F VNSVE I KEGSL L L PH YNSRAI VI VTVNEGK GDFEL VGQRNEN
301 QQE QRKEDDE EEE QGEE EI NKQV QNYKAKL SSGDVF V I PAGHPVAL KASS
351 NL DLLGF GI NAEN NQRNFL AGDE DNVI SQVQRPVKEL AFPGSAQEVDRI L
401 ENQKQSHF AD AQP QQRE RGSRET RDRL SS V
Phaseolin from garden bean, b subunit
50 5 0 5 05 050
1 ATS LREEEES QDN PF YF NSDNSWNTLF KN QYGHI RVL QRFDQQSKRL QNL
51 EDY RLVEF RS KPE TLLL PQQADA ELLL VV RSGSAI L V L VKPDDRREYFFL
101 TQGDNPI FSDNQKI PAGTI FYLVNPDPKEDLRI I QLAMPVNNPQI HEFFL
151 SSTEAQQSYL QEFSKHI LEASFNSKFEEI NRVLFEEEGQQEEGQQEGVI V
201 NI D SEQI EEL SKH AKSS SRKSHS KQDNTI GNEFGNL T E RTDNSL NVL I SS
251 I EMKEGALFV PHYYSKAI VI LVVNEGEAHVELVGPKGNKETLEFESYRAE
301 L SKDDVFVI P AAY PVAI KATSNV NFTGF GI NANNNNRNLLAGKTDNVI SS
351 I GR AL DGKDV L GL T F S G S GE E V MKL I NKQSGS Y F VDGH HHQQE QQKGSHQ
401 Q E Q Q K G R K G A F V Y
Data from Wright DJ (1988) The seed globulins – part II. In: Hudson BJF (ed.) Developments in Food Proteins, vol. 6, pp. 119–178. London: Elsevier Applied
Science, and references therein.
PROTEIN/Chemistry 4809

tbl0003 Table 3 Amino acid sequences of gluten proteins from wheat
HMW subunit of glutenin (x-type), 1Bx7 (Cheyenne)
5050505050
1EGE AS GQL QC E H EL EA C QQV V DQQ L RD VS P G CR P I T V SPGT R QYE Q QP V V
51PSKAGSFYPSETTPSQQLQQMI FWGI PALLRRYYPSVTSSQQGSYYPGQA
101SP Q QS GQ G Q Q P G QE QQP GQGQ QHQ Q P GQR QQ GY Y P T S PQQ P GQGQQL GQG
151QP G Y Y P T S Q Q PG QKQQA GQGQQS G Q GQQGYY P T S P Q Q SGQGQQP GQ GQP G
201YY P T S P QQ S GQWQQP G Q G QQP GQG Q QS GQ GQ QGQQP G QGQRP GQGQ QG Y Y
251P I S P QQP G Q G QQ S GQGQP GY Y P T S L RQ P GQWQQ P GQG QQP GQ GQQG QQ P G
301QG Q QS GQ G QQ GY Y P T S L Q QP G QGQ Q L GQG QP GY Y P T S QQS E Q GQQP GQ GK
351QP GQGQQGYY PT SPQQSGQGQQLGQGQPGYY PT SP QQSGQGQQSGQGQQG
401YY P T S P QQS GQG QQP GQGQSGYF P T S R QQSG QG QQ P GQGQQS GQGQ QG Q Q
451P G Q GQQA Y Y P T S S QQS R QRQQ AGQ WQR PGQG QP GY Y P T S P QQP GQE QQ S G
501QAQQS GQWQL VYYPTSPQQPGQLQQPAQGQQPAQGQQSAQEQQPGQAQQS
551GQWQWQL V Y Y PT SPQQP GQLQQPAQGQQGYYPT SP QQSGQGQQGYYPT SP
601QQ S GQ GQ Q GY Y P T S PQQ S GQGQQP G QGQQP R QGQQGY YP I S P QQS G QGQQ
651PGQGQQGYYPTS PQQS GQGQQPGHEQQPGQWLQPGQGQQGYYPTSS QQSG
701QGH QS GQGQQGY YPTS L WQPGQGQQGY AS PY HV SAE Y QAA RL KVAKAQQL
751AA QLP AMC R L EGSDAL S T RQ
HMW subunit of glutenin (y-type), 1Dy10 (Cheyenne)
5050505050
1EGE AS RQL QCER EL QE S S L EACRQV VD QQL A GR L P WS TGL QMRCCQQL RD
51VSAKCRSVAVSQVARQYEQTVVPPKGGSFYPGETTPLQQLQQGI FWGTSS
101QT V QGYY P GV T S P RQGS Y YP G QAS P QQ PGQG QQ P GK WQEP GQGQQWYY P T
151SL QQP GQ G Q QI G KGQQ GY Y P T SL QQ P GQGQQ GY YP T S L QH T GQRQQ P V Q G
201QQ P E Q GQ Q P GQWQQGY Y P T SP QQL GQGQQ P R QWQQ S G QGQQGHYP T SL QQ
251P G Q GQQGH Y L AS QQQP G Q GQQGHY P AS QQ QP GQGQQ G HYP AS QQQP GQGQ
301QGH YP AS Q Q E P G QGQQ G Q I P A SQQQ PGQGQQ GH YP A S L QQPGQGQQ GH Y P
351T S L QQ L GQ GQ QT GQP GQ K QQP GQGQ QT GQGQQP E Q E Q QP GQGQQGY YP T S
401L QQ P G QG Q Q Q GQ GQQG Y Y P T S L QQ P GQ GQ QG HY P A S L QQP GQ GQPG QR QQ
451PGQGQHP E QGKQPGQGQQGYYPTS P QQPGQGQQLGQGQQGYYPTSP QQP G
501QGQQP GQGQQGHCPTS PQQSGQAQQPGQGQQI GQVQQPGQGQQGYYPT SV
551QQ P GQ GQQS G QGQQSGQGHQP GQG Q QS GQ E Q QG Y D S P Y HV SA E QQA AS P M
601VAKAQQPATQLPTVCRMEGGDALSASQ
LMW subunit of glutenin, clone LMWG-1D1 (Chinese spring)
5050505050
1RCI PGLERPWQQQPLPPQQTFPQQPLFSQQQQQQLFPQQPSFSQQQPPFW
51QQ QP P F S Q Q Q PI L PQQP P F SQ QQQ L V L P Q QP P F S Q QQ QP V L P PQQS P F P Q
101QQ Q QH QQ L V Q QQ I P VV Q P SI L QQL N PC KV F L QQ QC S P VAMPQ RL AR SQML
151QQ S S C HV MQQ QC CQQL P Q I PQ QS R Y E A I R AI I Y S I I L QEQQQ V QGS I Q S Q
201QQQPQQL GQCVS QPQQQS QQQLGQQPQQQQL AQGT F L QPHQI AQLEVMT S
251I AL RI LPT MCSVNVPL YRTTT SVP F GV GT GV GAY
a-Gliadin clone A 1235 (Cheyenne)
5050505050
1VRV PVPQL QP QNPSQQQP QEQVPL MQQQQQF PGQQE QFPPQQPYPHQQPF
51P S Q QP Y P Q P Q PF P P QL P Y P QT QP F P P Q QP YP QP QP QY P QP QQ PI S Q QQA Q
101QQ Q QQQQ T L Q QI L QQQ L I P CR DVV L QQHN I A HA SS Q V L QQSS YQQL QQL C
151CQQLF QI P E QSR CQAI H NVVHAI I L HHHQQQQQQP S S QVS YQQPQE QY P S
201GQV SF QSSQQNPQAQGSVQPQQLP QFQEI RNLALQT L PAMCNVYI P PY CS
251TTI APFGI F GTN
g-Gliadin clone gene A (Yamhill)
5050505050
1NMQ V D P S G Q V QWP QQQP V L L P QQP F SQQP QQ T F P QP Q QT F P H QP QQ QF P Q
51PQQPQQQF L QPQQPFPQQPQQPYP QQP QQPF PQTQQP QQL FP QSQQPQQP
101YP Q QP QQP F P QT QQPQ Q Q F PQSQQ P QP F P QP QQ P QQ S F PQ QQ P SF I QP S L
151QQ Q L N P C K N L L L QQCR P V S L V S S L WS MI WP Q SA CQV MRQQ CC QQL A QI P Q
201QL QCA AI H S V V H SI S MQ E QQQQQQQ QQQQQQ QQGMR I L L P L Y QQQQ VGQG
251T L V QGQGI I QPQQPAQL E AI RSL V L QT L P TMCN VY V P PECSI I KAP F A S I
301VT GI GGQ
The high-molecular-weight (HMW) subunit (x-type) contains one intramolecular disulfide bond (Cys (10)-Cys (17)), whereas the remaining two cysteines
(Cys (32) and Cys (758)) form intermolecular disulfide bonds probably with other HMW subunits. In HMW subunits (y-type), the two adjacent cysteines
(Cys(43) and Cys(44)) form two intermolecular disulfide bonds with two adjacent cysteines of another y-type HMW subunit, Cys(513) forms an
intermolecular disulfide bond with low-molecular-weight (LMW) subunits, whereas the linkage of the remaining four cysteines is still unknown (probably
one intermolecular disulfide bond, Cys(10)-Cys(22)). The LMW subunit contains three intramolecular disulfide bonds (Cys(127)-Cys(162), Cys(135)-
Cys(155) and Cys(163)-Cys(260)), whereas the remaining two cysteines (Cys(2) and Cys(210)) form intermolecular disulfide bonds with other LMW
4810 PROTEIN/Chemistry
0026 Globular proteins Regular structural elements are
mixed with randomly extended chain segments
(random-coiled structures) in globular proteins.
The proportion of regular structural elements is
highly variable: 20–30% in casein, 45% in lyso-
zyme, and 75% in myoglobin. Five structural sub-
groups are known in this group of proteins: (1)
a-helices occur only; (2) b-structures occur only;
(3) a-helical and b-structural portions occur in sep-
arate segments on the peptide chain; (4) a-helices
and b-structures alternate along the peptide chain;
and (5) a-helices and b-structures do not exist. The
process of peptide chain folding is not yet fully
understood. It occurs spontaneously, probably
arising from one center or from several centers of
high stability in larger proteins. Folding of the pep-
tide chain packs it densely, by formation of a large
number of intramolecular noncovalent bonds. Data
about the nature of the bonds involved are provided
in Table 4.
subunits(headtoheadorheadtotail),y-typeHMWsubunitsorglutenin-bound g-gliadins. In a-gliadins,threeintramoleculardisulfidebondsoccur
(Cys(120)-Cys(150),Cys(151)-Cys(241)andCys(163)-Cys(249)). In g-gliadins,fourintramoleculardisulfidebondsoccur(Cys(157)-Cys(191),Cys(165)-
Cys(184),Cys(192)-Cys(282)andCys(204)-Cys(290)).
DatafromM
u
¨
llerSandWieserH(1997)Thelocationofdisulphidebondsinmonomeric a-typegliadins.JournalofCerealScience26:169–176,ShewryPR
andTathamAS(1997)Disulphidebondsinwheatglutenproteins.JournalofCerealScience25:207–223andreferencestherein.
(a)
(b)
R1
=
H
R2
O
C
N
fig00 01 Figure 1 Parallel b-sheet( f ¼119
, c ¼þ113
,n¼2.0,d¼0.32nm,r¼0.11nm;seetext).(a)Perspectiveviewoftwodecapeptide
chainsconsistingof10alanineresidueseach. TheN-terminusofbothchainsisontheleft;hydrogenatomsofthemethylgroupshave
beenomittedforclearness;hydrogenbridgesarepresentedasbrokenlines. (b)Viewinthedirectionofthechainaxis.
(a)
(b)
R1
=
H
R2
O
C
N
fig00 02 Figure 2 Antiparallel b-sheet( f ¼139
, c ¼þ135
,n¼2.0,d¼0.34nm,r¼0.09nm).(a),(b)Cf. Figure 1,buttheN-terminusofthe
upper chain is on the left and that of the lower chain is on the right.
PROTEIN/Chemistry 4811
0027 The hydrogen bonds formed between main chains,
main and side chains, and side-side chains are of
particular importance for folding. The portion of
polar groups involved in hydrogen bond build-up in
proteins of > 8.9 kDa appears to be fairly constant at
about 50%. The hydrophobic interaction of the non-
polar regions of the peptide chains also plays an
important role in protein folding. These interactions
are responsible for the fact that nonpolar groups are
folded to a great extent towards the interior of the
protein globule. The surface areas accessible to water
molecules have been calculated for both unfolded and
native folded forms for a number of monomeric pro-
teins with known conformations. The proportion of
the accessible surface of a stretched state which tends
to be fixed in the interior of the globule as a result of
folding is a simple linear function of the molecular
weight.
0028Proteins with disulfide bonds fold at a significantly
slower rate than those without disulfide bonds.
Folding is not limited by the reaction rate of disulfide
formation. Therefore the folding process of disulfide-
containing proteins seems to proceed in a different
way. The reverse process, protein unfolding, is very
much slowed down by the presence of disulfide
bridges which generally impart great stability to
globular proteins. This stability is particularly effect-
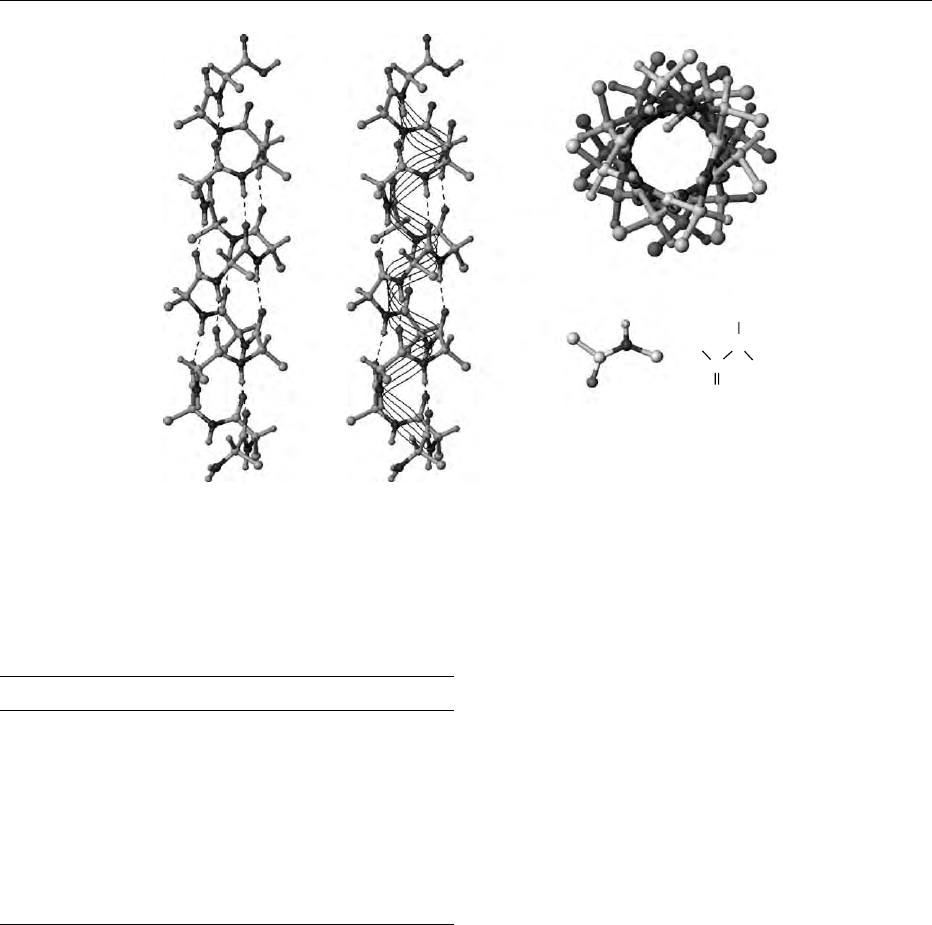
ive against denaturation. An example is the Bowman–
Birk inhibitor from soya beans, which inhibits the
activity of trypsin and chymotrypsin. Its tertiary
structure is stabilized by seven disulfide bridges. The
reactive sites of inhibition are Lys(16)-Ser(17) and
Leu(43)-Ser(44), i.e., both sites are located in rela-
tively small rings, each of which consists of nine
amino acid residues held in ring form by a disulfide
bridge. The thermal stability of this inhibitor is high
(Figure 4).
0029Quaternary structures In addition to the free energy
gain by folding of a single peptide chain, association
of more than one peptide chain (subunit) can provide
(a) (b)
(c)
R1
=
H
R2
O
C
N
fig0003 Figure 3 Right-handed a-helix (f ¼57
, c ¼47
, n ¼ 3.3, d ¼ 0.15 nm, r ¼ 0.23 nm). (a) Perspective view of a helical peptide chain
consistingof15alanineresidues;theN-terminusisatthebottom;inotherrespectsitisthesameas Figure 1. (b)Samerepresentation
as (a) with a lined band elucidating the right-handed helix running from bottom to top. (c) View in the direction of the helix axis.
tbl0004 Table 4 Bond-types in proteins
Type Examples Bond strength (kJmol
1
)
Covalent bonds -S-S- 230
Electrostatic bonds -COO
...H
3
N
þ
-21
a
>C¼O...O¼C<þ1
a,b
Hydrogen bonds -O-H . . . O< 17
>N-H . . . O¼C< 13
Hydrophobic bonds -Ala . . . Ala- 3
-Val . . . Val- 8
-Leu . . . Leu- 9
-Phe . . . Phe- 13
-Trp . . . Trp- 19
a
For a dielectric constant of the surrounding medium e ¼ 4;
b
weak
repulsion due to the negative partial charge of the oxygens in a polarized
C–O double bond.
Data from Belitz H-D and Grosch W (1999) Food Chemistry, 2nd edn. Berlin:
Springer–Verlag.
4812 PROTEIN/Chemistry
further gains in free energy. For example, hemoglobin
(four associated peptide chains) DG
¼46 kJ mol
1
and the trypsin–trypsin inhibitor complex (associ-
ation of two peptide chains) DG
¼75.2 kJ mol
1
.
In principle, such associations correspond to the
folding of a larger peptide chain around several
structural domains without covalently binding the
subunits.
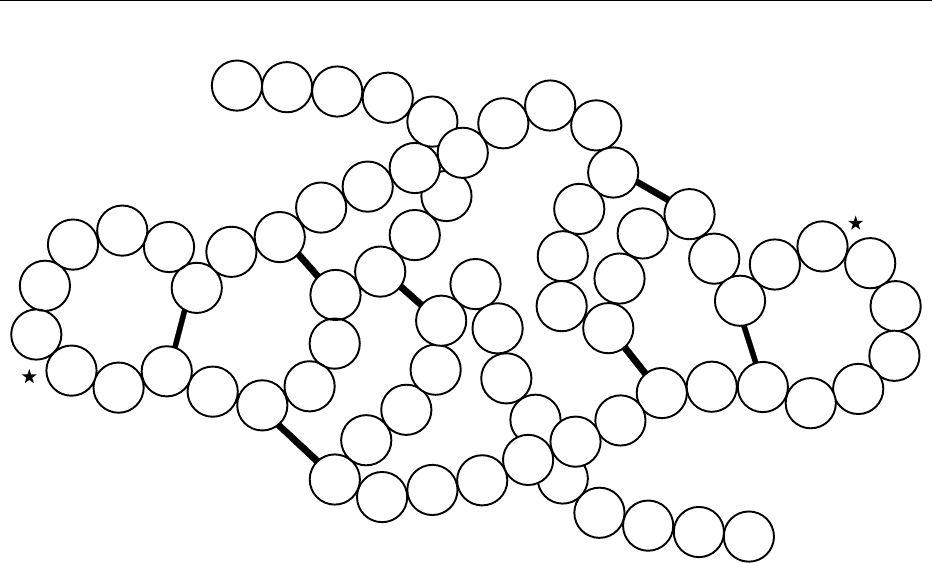
0030 Examples for protein conformations are given in
Figure 5, which shows the sweet proteins monellin
and thaumatin.
Denaturation
0031 The term ‘denaturation‘ denotes a reversible or
irreversible change of native conformation (tertiary
structure) without cleavage of covalent bonds (except
for disulfide bridges). Denaturation is possible with
any treatment that cleaves hydrogen bridges, or ionic
or hydrophobic bonds. This can be accomplished by
changing the temperature, adjusting the pH, increas-
ing the interfacial area, or adding organic solvents,
salts, urea, guanidine hydrochloride, or detergents
such as sodium dodecyl sulfate. Denaturation is gen-
erally reversible when the peptide chain is stabilized
in its unfolded state by the denaturing agent and
the native conformation can be reestablished after
removal of the agent. Irreversible denaturation occurs
when the unfolded peptide chain is stabilized by inter-
action with other chains (as occurs for instance with
egg proteins during boiling). During unfolding, react-
ive groups, such as thiol groups, that were buried or
blocked may be exposed. Their participation in the
formation of disulfide bonds may also cause an irre-
versible denaturation. Denaturation of biologically
active proteins is usually associated with loss of activ-
ity. The fact that denatured proteins are more readily
digested by proteolytic enzymes is also of interest.
Physical Properties
Dissociation
0032Proteins, like amino acids, are amphoteric. Depending
on pH, they can exist as polyvalent cations, anions, or
zwitterions. Since a-carboxyl and a-amino groups are
linked together by peptide bonds, the uptake or re-
lease of protons is limited to free terminal groups, and
mostly to side chains. In contrast to free amino acids,
the pK
a
values fluctuate greatly for proteins since the
dissociation is influenced by neighboring groups in
the macromolecule. For example, in lysozyme the
g-carboxyl group of Glu(35) has a pK
a
of 6–6.5,
SER
CYS
MET
ARG
PRO
THR
CYS
ARG
PRO
LYS
ASP
ASP
ALA
LEU
LYS
CYS
CYS
CYS
CYS
ASP
SER
SER
SER
SER
PRO
SER
SER
GLU
ASP
PRO
LEU
CYS
CYS
SER
ASN
CYS
SER
ASP
ASN
GLU
PHE
GLN
GLN
GLN
THR
TYR
HIS
PHE
ILE
GLU
TYR
PRO
PRO
LYS
ALA
CYS
CYS
ASP
LYS
CYS
CYS
CYS
ALA
ALA
ASP
VAL
ASP
LYS
ASN
GLU
I
L
E
1
20
40
60
30
70
50
10
fig0004 Figure 4 Bowman–Birk proteinase inhibitor from soya beans. Solid black lines, disulfide bridges between half-cystine residues;
asterisks, trypsin (Lys-Ser) and chymotrypsin (Leu-Ser) reactive sites. Adapted from Ikenaka T, Odani S, and Koide T (1974) Chemical
structure and inhibitory activities of soybean proteinase inhibitors. In: Fritz H, Tschesche H, Greene LJ and Truscheit E (eds) Bayer-
Symposium V Proteinase Inhibitors, pp. 325–343. Berlin: Springer-Verlag, with permission.
PROTEIN/Chemistry 4813

while the pK
a
of the b-carboxyl group of Asp(66)
is 1.5–2, of Asp(52) is 3–4.6, and of Asp(101) is
4.2–4.7. The total charge of a protein, which is the
absolute sum of all positive and negative charges, is
differentiated from the so-called net charge which,
depending on the pH, may be positive, zero, or nega-
tive. By definition the net charge is zero and the total
charge in general is maximal at the isoelectric point.
Lowering or raising the pH tends to increase the net
charge toward its maximum, whereas the total charge
becomes less than at the isolectric point.
0033 Since proteins interact not only with protons but
also with other ions, there is a further differentiation
between an isoionic and an isoelectric point. The
isoionic point is defined as the pH of a protein solu-
tion at infinite dilution, with no other ions present
except for H
þ
and OH
. Such a protein solution can
be acquired by extensive dialysis (or, better, electro-
dialysis) against distilled water. The isoionic point is
constant for a given substance whereas the isoelectric
point is variable, depending on the ions present and
their concentration. In the presence of salts, e.g.,
when binding of anions is stronger than that of
cations, the isoelectric point is lower than the isoionic
point. The reverse is true when cation binding is
dominant. In most cases the shift in pH is consistently
positive, i.e., the protein binds more anions than
cations. At its isoelectric point a protein is the least
soluble and the most likely to precipitate (isoelectric
precipitation) and it is at its maximal crystallization
capacity. The viscosity of solubilized proteins and
the swelling power of insoluble proteins are at a
minimum at the isoelectric point.
Optical Activity
0034The optical activity of proteins is due not only to
asymmetry of amino acids but also to the chirality
resulting from the arrangement of the peptide chain.
Information on the conformation of proteins can be
obtained from recording the optical rotatory disper-
sion (ORD) or the circular dichroism (CD), especially
in the range of peptide bond absorption wavelengths
(190–200 nm). The Cotton effect occurs in this range
and reveals quantitative information on secondary
structure. An a-helix or a b-structure gives a negative
Cotton effect, with absorption maxima at 199 and
205 nm, respectively, whereas a random-coiled
conformation shifts the maximum to shorter wave-
lengths, i.e., results in a positive Cotton effect.
Solubility, Hydration, and Swelling Power
0035Protein solubility is variable and is influenced by the
number of polar and apolar groups and their arrange-
ment along the molecule. Generally, proteins are sol-
uble only in strongly polar solvents such as water,
glycerol, formamide, dimethylformamide, or formic
acid. In a less polar solvent such as ethanol, few
proteins have appreciable solubility (e.g., prolamins).
The solubility in water is dependent on pH and
on salt concentration. At low ionic strength, the
solubility rises with increase in ionic strength and
the solubility minimum (isoelectric point) is shifted
to a somewhat lower pH. This shift is due to prefer-
ential binding of anions to the protein. As a rule,
neutral salts have a twofold effect on protein
solubility. At low concentrations they increase the
solubility (‘salting-in‘ effect) by suppressing the elec-
trostatic protein–protein interaction (binding forces).
C
B
N
A
N
B
C
A
C
N
(b)
(a)
fig0005 Figure 5 Conformation of the peptide chains of the sweet pro-
teins (a) monellin and (b) thaumatin. b-sheet, |!; a-helix,
;
b-turn, ; N
A
, N
B
, C
A
, C
B
denote the N- and C-termini of the A and
B chains. Adapted from Ogata C, Hatada M, Tomlinson G, Shin
WC and Kim S-H (1987) Crystal structure of the intensely sweet
protein monellin. Nature 328: 739–742, with permission.
4814 PROTEIN/Chemistry
