Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


in the presence of bile enabling lactose to enter more
freely and be hydrolyzed. Thus, the mechanism
whereby cells of Lactobacillus acidophilus can allevi-
ate the problems of lactose maldigestion is similar to
that of yogurt. However, Lactobacillus acidophilus
has the added advantage that it can grow in the
presence of the bile, and so new cells can be produced
during its growth in the intestine, providing even
more of the enzyme necessary for hydrolyzing lactose.
When preparing a product to which cells Lactobacil-
lus acidophilus are added, it is important that the cells
have been grown in a medium containing lactose as a
sugar source to insure that cells contain adequate
levels of the enzyme b-galactosidase. If not, the
amount of enzyme probably will be too low to
produce the desired benefit with regard to lactose
maldigestion as rapidly as desired.
Hypocholesterolemic Actions
0013 The risk of coronary heart disease in hypercholes-
terolemic persons can be significantly reduced by
lowering serum cholesterol levels. Germ-free animals
on an elevated cholesterol diet accumulate approxi-
mately twice as much cholesterol in the blood as
conventional animals on a similar diet. This suggests
the possibility that microorganisms in the intestinal
tract can influence serum cholesterol levels. Con-
sumption of organisms such as Lactobacillus acid-
ophilus can aid in the control of serum cholesterol
levels in humans. Two studies published in the 1970s
showed this to occur. In one of the studies, infants fed
a formula supplemented with Lactobacillus acido-
philus exhibited reduced serum cholesterol levels as-
sociated with increased numbers of lactobacilli in the
feces, compared with infants receiving the control
formula. This suggests that the lactobacilli may be
associated with the reduction of serum cholesterol
levels. In another study, a group of men fed milk
fermented by the natural flora in raw milk exhibited
unexpected reduced serum cholesterol levels. The fer-
mentation apparently included what was described as
a culture of Lactobacillus, although the organism was
not identified.
0014 Because of these studies, considerable research has
focused on the potential for Lactobacillus acidophilus
or related bacteria to exert beneficial influences on
serum cholesterol levels. Lactobacillus acidophilus
growing in a laboratory medium supplemented with
cholesterol and bile salts under anaerobic conditions
will assimilate cholesterol. This ability to assimilate
cholesterol varies tremendously among strains of this
species of bacteria. Results from a feeding trial using
pigs on a high-cholesterol diet as an animal model
revealed that supplementation of the diet with a strain
of Lactobacillus acidophilus that assimilated choles-
terol during growth in a laboratory medium had a
significant beneficial effect on serum cholesterol,
whereas a strain that did not assimilate cholesterol
during growth in a laboratory medium did not. This
suggests the importance of screening cultures for use
as a probiotic to produce a beneficial effect on serum
cholesterol levels. In another feeding trial involving
pigs with diet-induced hypercholesterolemia, animals
receiving cells of Lactobacillus acidophilus exhibited
a significant positive correlation between the reduc-
tion of serum cholesterol levels and the concentration
of cholic acid in the serum. The decline in serum
cholesterol was initiated more rapidly and reached a
significantly lower level in the animals fed the
lactobacilli than in those not fed the lactobacilli.
Such data suggest the possibility that the lactobacilli
interfered with the enterohepatic circulation of bile
acids, which can be an important factor in reducing
serum cholesterol levels. This also suggests another
activity of the lactobacilli, which may be important in
controlling serum cholesterol levels. Lactobacillus
acidophilus has the ability to deconjugate bile acids
through the action of bile salt hydrolase. Free bile
acids being less well absorbed from the small intestine
than conjugated acids are more likely to be excreted
from the body. Replacement of these excreted bile
acids involves the synthesis of new bile acids from
cholesterol in the body. This has a tendency to lower
the cholesterol pool in the body. This represents the
major pathway for removing cholesterol from the
human body. An additional advantage for the decon-
jugation is that free bile acids do not support the
absorption of cholesterol for the intestinal tract as
well as conjugated acids.
0015The mechanism whereby consumption of fer-
mented milk products containing cells of Lactobacil-
lus acidophilus or other probiotics may reduce levels
of serum cholesterol appears to include their ability
to assimilate cholesterol and/or deconjugate bile
acids (Table 4). Some of the cholesterol assimilated
by Lactobacillus acidophilus is incorporated in the
cellular membranes of the organisms. Bifidobacter-
ium longum also can incorporate some cholesterol
into its membrane, but Lactobacillus casei does not.
All three species have the ability to deconjugate bile
salts.
tbl0004Table 4 Activities of probiotic cultures important for imparting
a hypocholesterolemic effect
. Assimilation of cholesterol
. Deconjugation of bile salts
PROBIOTICS 4795

Control of Colon Cancer
0016 Some lactic acid bacteria can produce anticarcino-
genic or antimutagenic compounds, which can result
in the inhibition of some types of tumor cells in
animals. For example, rats that had been implanted
with ascites tumor cells and fed milk fermented with
Lactobacillus acidophilus exhibited a lower prolifer-
ation of tumor cells than those in the control group.
The conclusion was that the Lactobacillus species
produced substances during growth in the intestine
that was antagonistic toward the proliferation of these
tumor cells.
0017 Other studies have shown that certain fermented
milks have a beneficial influence on the proliferation
of colon tumor cells in rats. Feeding rats cells of
Lactobacillus reduced the activity of three enzymes
capable of converting procarcinogens into carcino-
gens in the intestinal tract. Similar results have been
shown in human studies. These enzymes were b-
glucuronidase, azoreductase, and nitroreductase. Re-
duction of the level of activity of these enzymes in the
intestinal tract can reduce the chances of carcinogens
being formed. The enzymes apparently are of micro-
bial origin. Thus, the potential anticarcinogenic ac-
tivity of feeding Lactobacillus acidophilus may, in
this case, be related to the control of undesirable
microorganisms in the intestinal tract. In other stud-
ies, the consumption of milk containing cells of
Lactobacillus casei activated macrophages in mice.
This observation was based on increases in enzyme
activity associated with macrophages. This suggests
another potential means, whereby the consumption
of a selected probiotic culture may influence prolifer-
ation of tumor cells in the body.
Other Benefits
0018 One of the main interests in the livestock industry in
using probiotic bacteria as feed supplements is to
improve nutrient utilization resulting in improved
growth and/or feed efficiency of the livestock. While
there is great interest in this area, there is very little
evidence reported in the scientific literature to indi-
cate how such benefits might occur. It is possible that
such benefits can result from the action of certain
enzymes associated with the probiotic bacteria. This
could be similar, for instance, to the beneficial effect
of yogurt bacteria and Lactobacillus acidophilus on
lactose maldigestion in humans that results from
b-galactosidase associated with the bacterial cells.
Selection of a culture of Lactobacillus acidophilus
with a high level of amylase activity for inclusion as
a probiotic in the diet of young pigs can improve
starch utilization in the young animal. In a feeding
trial involving the use of such a culture, improved
growth and feed efficiency of weaning aged pigs
were observed as a result of including the selected
culture in the diet. The benefit was attributed to the
amylase activity of the culture. It is possible that other
enzymes that could improve the digestion of other
nutrients may be present in some bacteria used as
probiotics that could result in improved growth and
feed efficiency of the livestock. Of course, the im-
proved growth may relate to the control of undesir-
able microorganisms in the animal’s intestinal tract.
There is also interest in the livestock and pet-food
industries in using probiotics to help control intestinal
infections in animals.
Selection of Probiotic Culture
0019While there are a number of potential benefits from
the inclusion of a probiotic culture in the diet, it is
essential that a culture be properly selected for such
use. One culture, that is one strain of one species,
should not be expected to provide all of the potential
benefits that might be derived from probiotics. To
help insure that the culture is successful and has a
positive effect, certain requirements are to be met
(Table 5). Since, in most cases, the sight of action of
the probiotic organisms is the intestinal tract, it is
generally accepted that the organism should be a
normal inhabitant of the intestinal tract. There is
strong evidence in the literature indicating that
many of these probiotic bacteria exhibit host specifi-
city; for example, a strain of Lactobacillus acido-
philus originating in the intestinal tract of a calf
should not be expected to function equally well in
the intestinal tract of a pig or of a human. Thus, it is
desirable to use a selected strain of bacteria that ori-
ginated in the host species for which the product is to
be used. If it is to be used as a probiotic for humans,
it is highly desirable that the probiotic organism ori-
ginated in the human intestines. In order to avoid
problems with regulatory agencies, it is desirable
that the organism of choice has a history of having
been used for producing fermented food products.
tbl0005Table 5 Important factors to consider in selection of a probiotic
culture
. Normal inhabitant of intestine
. Host specificity
. Acid tolerance
. Bile tolerance
. Stability during production and delivery
. Production of desired health/nutritional benefit
. One strain would be unlikely to provide all possible benefits
4796 PROBIOTICS

The organism should be resistant to gastric acidity to
survive passage through the stomach. The main or-
ganisms considered for use as probiotics (i.e., lacto-
bacilli) are acid-tolerant. However, resistance to
acidity varies among strains and species, so it must
be considered. If the organism is expected to grow
and function in the intestinal tract, it must have a
certain degree of bile tolerance. The bile resistance
based on the ability of the organism to grow in the
presence of bile acids varies tremendously among
strains of each species of probiotic bacteria. The min-
imum level of bile tolerance required is not known,
but it is suggested that the most bile-resistant strain
that will provide the desired benefit be selected. The
probiotic culture must be stable during production
and storage of the food or feed containing it. This is
often the most challenging factor in providing a pro-
biotic organism that has adequate levels of viability at
the time of consumption. The most important char-
acteristic of course is that the probiotic culture pro-
duce the desired effect. Therefore, it is desirable that
some sort of laboratory screening test for the desired
effect be implemented. This is not always easy. How-
ever, if the organism for instance is to be used for
improving lactose utilization, it would be desirable
to select one having a high level of b-galactosidase
activity. If it is to be used for controlling serum chol-
esterol levels, it is desirable that the organism not only
assimilate cholesterol but also have a high degree of
ability to deconjugate bile acids. In order to develop
useful screening tests, it is of course essential that we
understand how the probiotic organism functions to
provide the desirable benefit. In many cases, we
simply do not know the answer to this mechanism
at this point.
0020 A major factor in selection of a specific organism
for use as a probiotic is to remember that one strain
should not be expected to provide all potential bene-
fits. Far more is required in the selection process than
to select a culture just because it is Lactobacillus
acidophilus, for example. There is a great variation
among strains of this bacterial species. Such variation
also occurs among strains of other species of probio-
tic bacteria.
Dietary Delivery of the Probiotic
0021 If the probiotic organism is expected to provide its
benefit during growth and action in the intestinal
tract, it is reasonable to assume that the organism
must be viable at the time of consumption. It is also
desirable and recommended that the probiotic be
consumed on a continuing basis. The numbers of
viable probiotic bacteria required in a food or feed
product to provide the benefit are not known. The
actual numbers required probably will vary with the
organism and the expected function. Some have sug-
gested, particularly with regard to milk products,
that the probiotic should be present at a level of one
to two million per milliliter; however, these numbers
are not based on scientific data but are based more on
an economic situation reflecting the cost of the or-
ganism. Research is needed to answer the question
with regard to the optimum numbers required. It is
essential that the organism be stable and that the
desired characteristics be maintained during the pro-
duction of the probiotic culture, during its storage,
and delivery in the food or feed. This is often the
greatest challenge in the use of probiotic bacteria.
The stability of the organism during storage and
delivery can be greatly influenced by the conditions
under which the bacteria are grown. For instance, the
growth of Lactobacillus acidophilus at pH 5 has
resulted in cultures that are more stable during stor-
age when added to refrigerated milk than the same
cultures grown at a higher pH. The storage stability,
especially during frozen and refrigerated storage,
varies among strains of a given bacterial species
used as a probiotic. Thus, this becomes a factor
to consider in selecting the organism for use as a
probiotic.
0022The use of probiotic bacteria such as Lactobacillus
acidophilus in conjunction with yogurt can be accom-
plished by either including the probiotic as part of the
starter culture or adding cells of the probiotic bacteria
after the product has been manufactured. Of course,
it would be desirable to use the probiotic as part of
the inoculum (i.e., starter culture) used in the manu-
facture of yogurt in order to yield higher numbers of
the probiotic bacteria during the incubation period
for the fermentation. However, most of the probiotic
bacteria do not grow well under such conditions, and
very little increase in numbers is achieved during the
incubation process involved in the manufacture of
yogurt. Research is needed to provide means of in-
creasing the numbers of probiotic bacteria that grow
during the manufacture of a product such as yogurt
when the organism is used as a part of the inoculum
for manufacturing the fermented milk. In summary,
once a desired probiotic culture has been selected, it is
important to develop procedures for producing the
culture, storing the culture, and delivering the culture
in the food or feed without damaging the desired
characteristics.
See also: Bifidobacteria in Foods; Cholesterol:
Properties and Determination; Role of Cholesterol in
Heart Disease; Colon: Cancer of the Colon; Immunology
of Food; Lactic Acid Bacteria; Lactose; Salmonella:
Salmonellosis; Staphylococcus: Food Poisoning
PROBIOTICS 4797
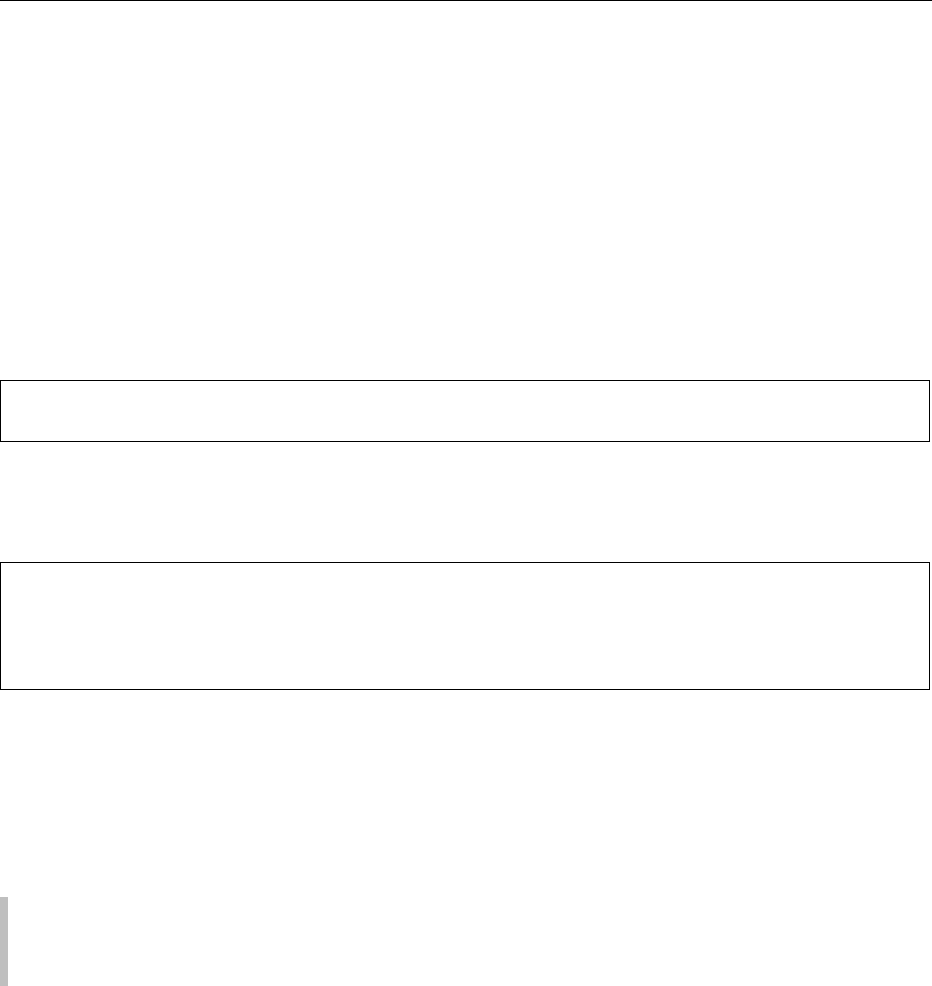
Further Reading
Bezkorovainy A and Catchpole RM (1989) Biochemistry and
Physiology of Bifidobacteria. Boca Raton,FL: CRC Press.
Fuller R (ed.) (1992) Probiotics the Scientific Basis. New
York: Chapman & Hall.
Gilliland SE (1990) Health and nutritional benefits from
lactic acid bacteria. FEMS Microbiology Reviews 87:
175–188.
Klein G, Pack A, Bonaparte C and Renter G (1998) Tax-
onomy and physiology of probiotic lactic acid bacteria.
International Journalof FoodMicrobiology41:103–125.
Ouwehand AC, Kirjavainen PV, Shortt C and Salminen S
(1999) Probiotics: mechanisms and established effects.
International Dairy Journal 9: 43–52.
Robinson RK (ed.) (1991) Therapeutic Properties of Fer-
mented Milks. Barking, UK: Elsevier Science.
Sissons JW (1989) Potential of probiotic organisms to pre-
vent diarrhoea and promote digestion in farm animals –
a review. Journal of the Science of Food and Agriculture
49: 1–13.
Tamine AY and Robinson RK (1999) Yoghurt Science and
Technology,2
nd
edn. Boca Raton, FL: CRC Press.
Process Control See Plant Design: Basic Principles; Designing for Hygienic Operation; Process Control
and Automation; Instrumentation and Process Control
Processed Cheese See Cheeses: Types of Cheese; Starter Cultures Employed in Cheese-making;
Chemistry and Microbiology of Maturation; Manufacture of Extra-hard Cheeses; Manufacture of Hard and Semi-
hard Varieties of Cheese; Cheeses with ‘Eyes‘; Soft and Special Varieties; White Brined Varieties; Quarg and
Fromage Frais; Processed Cheese; Dietary Importance; Mold-ripened Cheeses: Stilton and Related Varieties;
Surface Mold-ripened Cheese Varieties; Risk Assessment
PROSTAGLANDINS AND LEUKOTRIENES
S Katayama, Saitama Medical School, Iruma-gun,
Saitama, Japan
J B Lee, State University of New York, New York, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Prostaglandins (PGs) were discovered in the 1930s
and were first chemically isolated and identified in
sheep seminal vesicles and renal medulla in the 1960s.
Although together with thromboxanes (TXs) and
leukotrienes (LTs), the PGs possess the most potent
and most divergent biological activities of any natur-
ally occurring compounds, their true physiological
role in many instances remains unknown. From a
pathophysiological viewpont, an absolute or relative
deficiency of PGs relative to TXs has been implicated
in the etiology of hypertension, thrombosis, and
atherogenesis for many years. Since PGs and TXs
are derived from essential fatty acids, the role of
dietary intake of these fatty acids in PG and TX
production, particularly in relation to cardiovascular
system, will be examined. The possible role of dietary
essential fatty acids on LT and PG production in rela-
tion to inflammation, the immune system, and gastric
function will also be discussed.
Chemistry
0002In the 1930s, it was reported that fresh human semen
causes rhythmic contractions of human endomet-
rium. The active substance was called ‘prosta-
glandin.‘ In the 1960s, PG was determined to be a
mixture of biologically active compounds, which
were isolated from sheep seminal vesicles and identi-
fied as PGE
1–3
and PGF
1–2
. These compounds were
independently isolated from extracts of rabbit kidney
4798 PROSTAGLANDINS AND LEUKOTRIENES

medulla and identified as PGA
2
, PGE
2
, and PGF
2a
.
Later, in 1975, the compound TXA
2
, which is a
potent vasoconstrictor and platelet aggregant, was
isolated, and in 1976, prostacyclin (PGI
2
) was dis-
covered. Prostacyclin is synthesized mainly by endo-
thelial cells, prevents platelet aggregation, and is
vasodilatory. Studies of the slow-reacting substance
of anaphylaxis (SRS-A) showed that it was composed
of certain LTs, which are products of leucocyte mem-
brane arachidonate.
0003 The PGs are composed of a basic 20-carbon fatty
acid chain containing a cyclopentane ring, the so-
called hypothetical prostanoic acid. The carbons are
numbered 1–20 from the carboxyl to the terminal
group. The designations of PGE
1
, PGE
2
, and PEG
3
refer only to the number of double bonds in the
aliphatic side-chains. The PG
2
s are the most abun-
dant naturally occurring class. For PG
1
s, the precur-
sor is 8,11,14-eicosatrienoic acid (dihomo-g-linolenic
acid), and for PG
2
s, the precursor is 5,8,11,14-
eicosatetraenoic acid (arachidonic acid). The PG
3
s
can be formed from 5,8,11,14,17-eicosapentaenoic
acid (EPA).
0004 The LTs were named because they were discovered
in leucocytes and because the common structural
feature is a conjugated triene. Various members of
the group have been designated alphabetically, and
the subscript denotes the number of double bonds.
Analysis of Prostaglandins and
Leukotrienes
0005 Prostaglandins and leukotrienes are present in many
different biological samples, including plasma. How-
ever, eicosanoid quantification in the peripheral
circulation does not accurately reflect in vitro biosyn-
thesis and disposition because of rapid turnover, low
concentration, metabolism by the lung and liver, and
sample processing problems. For example, plasma
concentrations of PGE
2
and TXB
2
determined by
gas chromatography have been reported to be 12
and several picograms per milliliter, respectively.
Urinary samples can be utilized to evaluate in vivo
PGs or LTs for the kidneys, whereas pleural fluids or
bronchial lavages can be used to evaluate pulmonary
production. The most accurate reflection of local
synthesis is obtained by incubations of slices or
homogenates of specific cells and/or tissues with
measurement of PG, TX, or LT production in vitro.
0006 The most precise method to determine PGs and LTs
is undoubtedly gas chromatography linked to mass
spectrometry. However, this type of analysis is not
widely available. Radioimmunoassay using a specific
antibody against various PGs or LTs and
3
H- or
125
I-
labeled tracer are available, highly specific, sensitive,
and relatively inexpensive, and have been widely
utilized for eicosanoid analysis.
0007Prior to radioimmunoassay, extraction of arachi-
donic acid metabolites from plasma, urine or tissue
homogenates may be required. The most common
method is to extract acidified aqueous solution with
an oraganic solvent such as diethylether or ethyl
acetate, followed by purification by column chroma-
tography with a octadecylsilyl (ODS) silica (SEP-PAK
C-18) cartridge, silicic acid or sometimes by high-
performance liquid chromatography.
Biosynthesis of Prostaglandins and
Leukotrienes
0008Arachidonic acid and its metabolite byproducuts (the
arachidonic acid cascade) are important mediators of
a number of physiological phenomena. As shown in
Figure 1, the rate-limiting step in the formation of the
metabolites of arachidonic acid seems to be the initial
step, i.e., the release of free arachidonic acid from the
cell membrane phospholipid pool mediated through
activation of phospholipase A
2
. Phospholipase C may
play another role in arachidonic acid release through
liberating a diglyceride, which is then hydrolyzed by
another lipase to yield arachidonic acid. Following
the release of arachidonic acid, enzymes are involved
in its subsequent metabolism. Prostaglandin synthase,
i.e., cyclooxygenase (COX), is a key enzyme in con-
trolling the extent of prostanoid biosynthessis. It pos-
sesses two enzymatic activities: COX activity, the
conversion of arachidonic acid (AA) to PGG
2
and
peroxidase activity, the conversion of PGG
2
to
PGH
2
catalyzing a two-electron reduction of PGG
2
to PGH
2
. PGH
2
is the common precursor for a
number of biologically important prostanoids, i.e.,
PGI
2
, TXA
2
, PGE
2
, PGD
2
, and PGF
2a
, the formation
of which occurs through the action of the respective
synthetase called PGI synthase, TXA synthase, PGE
synthase, PGD synthase, and PGF synthase. COX is
distributed in most mammalian tissues. Two isoforms
of COX have been identified and cloned. COX-1,
originally cloned from ram seminal vesicles and sub-
sequently from mouse tissues and human endothelial
cells and platelets, is considered to be constitutive,
whereas COX-2, cloned from chicken embryonic
fibroblasts and mouse 3T3 fibroblasts, is highly
inducible. COX-1 activity is inactivated by aspirin
through acetylation of the serine residue and in-
hibited by other nonsteroidal antiinflammatory
drugs (NSAIDs), whereas COX-2 is inhibited by glu-
cocorticoids. Various biological activities are due to
binding of prostanoids to the receptors on the cell
surface. With the advent of the molecular biology
technique, eight prostanoid receptors have been
PROSTAGLANDINS AND LEUKOTRIENES 4799
cloned so far; TP, IP, FP, DP and four classes of EPs
(EP
1
,EP
2
,EP
3
,andEP
4
). Prostanoid receptors have
seven transmembrene domains and are members of
the superfamily of the rhodopsin receptor. These re-
ceptors are classified into three groups; IP, DP, EP
2
,
and EP
4
are the stimulatory receptors, which generate
cAMP, resulting in vasodilatoion; TP, FP, and EP
1
are
the G protein-linked receptors that increase intracel-
lular Ca
2þ
concentration, resulting in vasoconstric-
tion; and EP
3
is an inhibitory receptor, which inhibits
the elevation of cAMP, resulting in vasodilation.
0009 In the second pathway of arachidonic acid metab-
olism, 5-lipoxygenase forms a series of products
named LTs, as illustrated in Figure 1. 5-Hydroper-
oxyeicosatetraenoicacid (5-HPETE) may be enzyma-
tically dehydrated to LTA
4
(5(S)-trans-oxido-7,
9-trans-11, 14-cis-eicosa-tetraenoic acid). Subsequent
enzymatic hydrolysis of LTA
4
results in LTB
4
(5(S)-
12(R)-dihydroxy-6-cis-8, 10-trans-14-cis-eicosa-
tetraenoic acid). LTA
4
also reacts with sulfydryl com-
pounds by addition of gluthathione (Glu-Gys-Gly) by
glutathione-(S)-transferase, yielding LTC
4
(5(S)-glu-
tathionyl-7, 9-trans-11, 14-cis-eicosatetraenoic
acid). LTD
4
can be then produced from LTC
4
through
elimination of glutamyl residue by a g-glutamyl trans-
peptidase. The remaining peptide bond in LTD
4
is
hydrolyzed to give LTE
4
by a dipeptidase.
Physiological Action of PGs and LTs
0010The relative amounts of compound formed depend on
the tissue or cell being studied. When platelets are
activated by collagen or thrombin, unstable TXA
2
is
predominant, resulting in a rapid and irreversible
aggregation of platelets and contraction of vascular
smooth muscle cells. However, vascular endothelial
CH
2
O
CH
2
O
C
COOH
COOH
COOH
COOH
COOH
COOH
COOH
OOH
OOH
O
OCH
C
O
O
P O X
O
−
O
LTA
4
LTC
4
LTB
4
COOH
HO
S Cys Gly
Glu
OOH
OOH
15-HPETE
5-HPETE
OH OH
12-HPETE
arachidonic acid
5, 15-diHPETE
HOO
Phospholipids
COOH
11-epi-PGF
2α
OH
OH
OH
COOH
PGG
2
PGD
2
O
OOH
O
COOH
COOH
OH
OH
O
PGE
2
COOH
OH
OH
O
COOH
PCH
2
O
OH
O
O
O
TXA
2
OH
COOH
PGF
2
a
OH
OH
O
COOH
PGI
2
O
OH
O
LTD
4
COOH
HO
S Cys Gly
1
8
8
9
8
2
11
12
10
12
2
3
4
5
5
6
7
`
`
fig0001 Figure 1 Metabolic pathways of prostaglandin (PG) and leukotriene (LT) from arachidonic acid. The name of enzyme that catalyzes
each step is as follows: 1, phospholipase A
2
; 2, cyclooxygenase; 3, PGD synthase; 4, PGE synthase; 5, PGF synthase; 6, PGI synthase; 7,
TXA synthase; 8, 5-lipoxygenase; 8
0
, dehydrase; 9, hydolase; 10, glutathione-S-transferase; 11, 12-lipoxygenase; 12, 15-lipoxygenase.
4800 PROSTAGLANDINS AND LEUKOTRIENES

cells form unstable PGI
2
, which has a potent
vasodilating and antiaggregatory action similar to
stable PGE
2
, Neutrophils as well as monocytes or
macrophages produce PGE
2
, whereas PGD
2
is
formed in mast cells and basophils. In kidneys, renal
interstitial cells and collecting duct cells synthesize
PGE
2
, collecting dust cells synthesize PGE
2
, the
renovasculature endothelia synthesize PGI
2
, and
glomerular mesangial endothelia from PGF
2a
.In
addition to PGE
2
, other PGs are also produced in
the gastrointestinal tract. PGE
1
, PGE
2
, and their
analogs, administered exogenously, suppress gastric
acid secretion and possess the property of protecting
the gastric mucosa against necrotizing agents such as
absolute ethanol or hydrochloric acid (cytoprotec-
tion). This is the reason why NSAIDs cause a variety
of problems in the gastrointestinal tract including
irritation and ulceration of the stomach. Since
COX-1 appears to play a major role, treatment with
COX-2 selective inhibitors appears to be associated
with less gastrointestinal damage than conventional
NSAIDs.
0011 Prostaglandins play many physiological roles in
human reproduction, e.g., menstrual regulation,
pregnancy, and induction of labor. PGE
1
, PGE
2
,or
their analogs are now widely used clinically for in-
duction of labor or abortion. PGE
2
and PGI
2
produce
or augment vasodilation and enhance bradykinin or
serotonin-induced pain, resulting in redness, heat,
swelling, and pain in the inflammatory processes.
PGs such as PGE
2
and PGI
2
have also been shown
to increase during pyrogen-induced fever. In fact,
PGE
2
administered into the hypothalamus increases
body temperature, whereas PGD
2
deceases body tem-
perature, indicating a possible role of PGs in human
thermoregulation. PGs, TXs, and some monohy-
droxy derivatives of arachidonic acid (hydroxyeico-
satetraenoic acids) also have chemotactic effects on
polymophonuclear leucocytes. LTB
4
is chemotactic
not only for neutrophils and eosinophils but also for
monocytic macrophages. These phenomena are con-
sidered to be the initial events of the inflammatory
response. Polymorphonuclear leucocytes only survive
for hours in the inflammatory area, whereas mono-
cytes may stay for weeks and finally may be trans-
formed to fibroblasts, initiating the repair process of
the wounds. Furthermore, monocytes can present
antigens to cells capable of producing antibodies
and can synthesize all the members of the arachidonic
acid cascade. Monocytes are also capable of forming
interleukin, interferon, complement, and protease, all
of which participate in tissue disruption. The lip-
oxygenase system is more important in subacute and
chronic inflammation, whereas the COX system
produces or modulates acute inflammation. The
antiinflammatory action of aspirin, indomethacin,
and other nonsteroidal antiinflammatory agents is
produced almost entirely by PG synthesis inhibition,
as shown by studies revealing inhibition of PG-
cyclooxygenase with aspirin.
0012LTC
4
,LTD
4
,LTE
4
, and their trans isomers do not
have any effects on chemotaxis, enzyme release, or
leucocyte aggregation. However, they possess potent
biological activities, which were formerly attributed
to SRS-A release from sensitized lungs treated with a
specific antigen. These compounds may be important
mediators in asthma and other acute hypersensitivity
reactions. Contraction of guinea-pig ileum and other
smooth muscle by LTs exhibits a slow onset and
relaxation, which is the basis of the original designa-
tion as SRS-A. This distinguishes these substances
from histamine, bradykinin, and PGF
2a
.LTC
4
and
LTD
4
are respectively about 200-fold and 20 000-
fold more potent than histamine in promoting small
airway contraction. In addition, these LTs cause rapid
arteriolar contraction and promote plasma leakage in
postcapillary venules. They also slow the rate of
mucus clearance from the airways of patients with
asthma after the inhalation of antigen and increase
the amount of mucus glycoprotein synthesis in the
airways.
0013With the advent of cloning of various prostanoid
synthetases and their receptors, vast numbers of pros-
tanoids analogs synthesized so far will be retested.
Diverse physiological actions of various prostanoids
will be shortly reevaluated and reclassified, based on
their specific receptors using a specific agonist and
antagonist. For example, inhibition of vasopressin-
induced water reabsorption in kidney or histamine-
induced gastric acid secretion can be explained by
EP
3
-mediated inhibition of adenylate cyclase. Fur-
thermore, genetic engineering such as prostanoid
receptor knockout mice may be able to clarify a
specific physiological role of PGs and LTs.
Effects of Diet on PG Formation
Polyunsaturated Fatty Acids
0014It was first shown in 1974 that dietary deficiency of
essential fatty acids in animals results in hyperten-
sion, which depends on a high sodium intake, thus
being very similar to renoprival hypertension or
experimental ‘salt sensitive‘ hypertension. This was
later confirmed in 1983, and the blood-pressure
response was attributed to a dietary deficiency of
linoleic acid. However, it would appear unlikely that
this is a cause of human hypertension, since selective
deficiency of essential fatty acids in the human diet is
rare.
PROSTAGLANDINS AND LEUKOTRIENES 4801

0015 A low prevalence of atherosclerosis and a low mor-
tality from myocardial infarction among Greenland
Inuit, despite a diet high in fat and cholesterol, sug-
gested that dietary fish may have some properties that
could prevent coronary artery disease. The Inuit con-
sume 5–10 g daily of the long-chain n-3 polyunsatur-
ated fatty acids EPA (C20; 5n-3) (Figure 2)and
docosahexaenoic acid (C22; 6n-3), which are present
in fish oils. When n-3 fatty acids are included in the
diet, EPA inhibits conversion of linoleic acid and
competes with arachidonic acid for the 2-acyl pos-
ition in membrane phospholipids, reducing plasma
and cellular levels of arachidonic acid. In addition,
EPA competes with arachidonic acid as the substrate
for cyclooxygenase. The platelets produce biologi-
cally inactive TXA
3
instead of TXA
2
. However,
PGI
2
synthesis in endothelial cells is not markedly
inhibited, and any newly synthesized PGI
3
has the
same potency as PGI
2
. Thus, a diet abundant in EPA
may inhibit platelet aggregation and cause vascular
dilatation, resulting in blood-pressure reduction. In
fact, a metaanalysis including 1356 subjects from
31 placebo-controlled trials confirmed the blood-
pressure-lowering effects of dietary fish oils. The
hypotensive effect was dose-dependent; there was
little change in blood pressure at doses lower than
3 g per day (corresponding to 250 g of salmon), but
above this, a 0.66 mmHg fall in systolic blood pres-
sure was demonstrated per 1.0-g increase in n-3 poly-
unsaturated fatty acid. The hypotensive effect
appeared greater in patients with hypertension,
hypercholesterolemia, or coronary heart disease.
Mechanisms by which n-3 polyunsaturated fatty
acids may act might be attributed to an attenuated
constrictor responses to vasoactive agents, such as
noradrenaline and angiotensin II, an attenuated sym-
patho-adrenal stimulation and/or modulated pro-
duction or release of nitric oxide from vascular
endothelial cells. However, the safety and long-term
benefits of this treatment have been questioned
because PGE
2
synthesis is inhibited and replaced
by PEG
3
from EPA in the fish oils. Since renal
blood flow is dependent on PGE
2
in renal disease
and during volume contraction, any decrease in
PGE
2
might result in more severely compromised
renal function.
0016The other merit of dietary fish or fish oil supple-
ments may be its lowering effect on plasma lipid. The
principal effect has been found to be a reduced pro-
duction of triacyglycerols and very-low-density lipo-
protein (VLDL) in the liver. Because VLDL is a
precursor of low-density lipoprotein (LDL), a second-
ary reduction in LDL cholesterol is also seen. Such
conditions are considered to reduce the atherogeni-
city of lipoprotein particles. Futhermore, n-3 fatty
acids have been reported to improve insulin sensitiv-
ity in skeletal muscle, possibly through a decrease in
triacylglycerol accumulation. However, fish oil sup-
plementation to diabetic patients resulted in no
change in glycemic control in one study and an actual
deterioration in glycemic control in another study.
0017Increased intake of polyunsaturated fatty acids has
also been reported to result in reduced gastric acid
secretion and an increase in gastric mucosal protec-
tion. Diets enriched in fish oil or dihomo-g -linolenic
acid also reduce LTB
4
formation in leucocytes or
monocytes, suggesting a possible role for fish oil
diets in suppressing inflammation in such disorders
as rheumatoid arthritis or systemic lupus erythema-
tosus.
Carbohydrate
0018The increase in urinary excretion of TXB2 associated
with a decreased 6-keto-PGF
1a
has been demon-
strated in diabetic animals and humans. An overpro-
duction of PGE
2
has also been considered to be one of
the reasons for glomerular hyperfiltration. In fact,
enhanced growth of mesangial cells under high-
glucose conditions can be explained, at least in part,
by a decreased cyclic adenosine monophosphate pro-
duction via EP
4
, which augments EP
1
function in
conjunction with the overproduction of PGE
2
.
Diabetic pregnancy is associated with growth
disturbances and malformations in the offspring.
Embryonic dysmorphogenesis induced by high con-
centration of glucose is protected by addition of ara-
chidonic acid or PGE
2
. COX inhibitors such as
indomethacin or aspirin yield embryonic dysmorpho-
gensesis similar to that caused by glucose, indicating
that this disturbance may be partly mediated via
altered metabolism of arachidonic acid.
Proteins
0019Protein intake has profound effect on renal hemody-
namics and excretory function. A high protein intake
has been shown to have deleterious effects on the
kidneys, especially in preexisting renal disease, indi-
cating a rationale for dietary protein restriction to
lessen renal parenchymal damage. However, not all
proteins are equipotent in relation to their renal
COOH
fig0002 Figure 2 Eicosapentaenoic acid (EPA).
4802 PROSTAGLANDINS AND LEUKOTRIENES
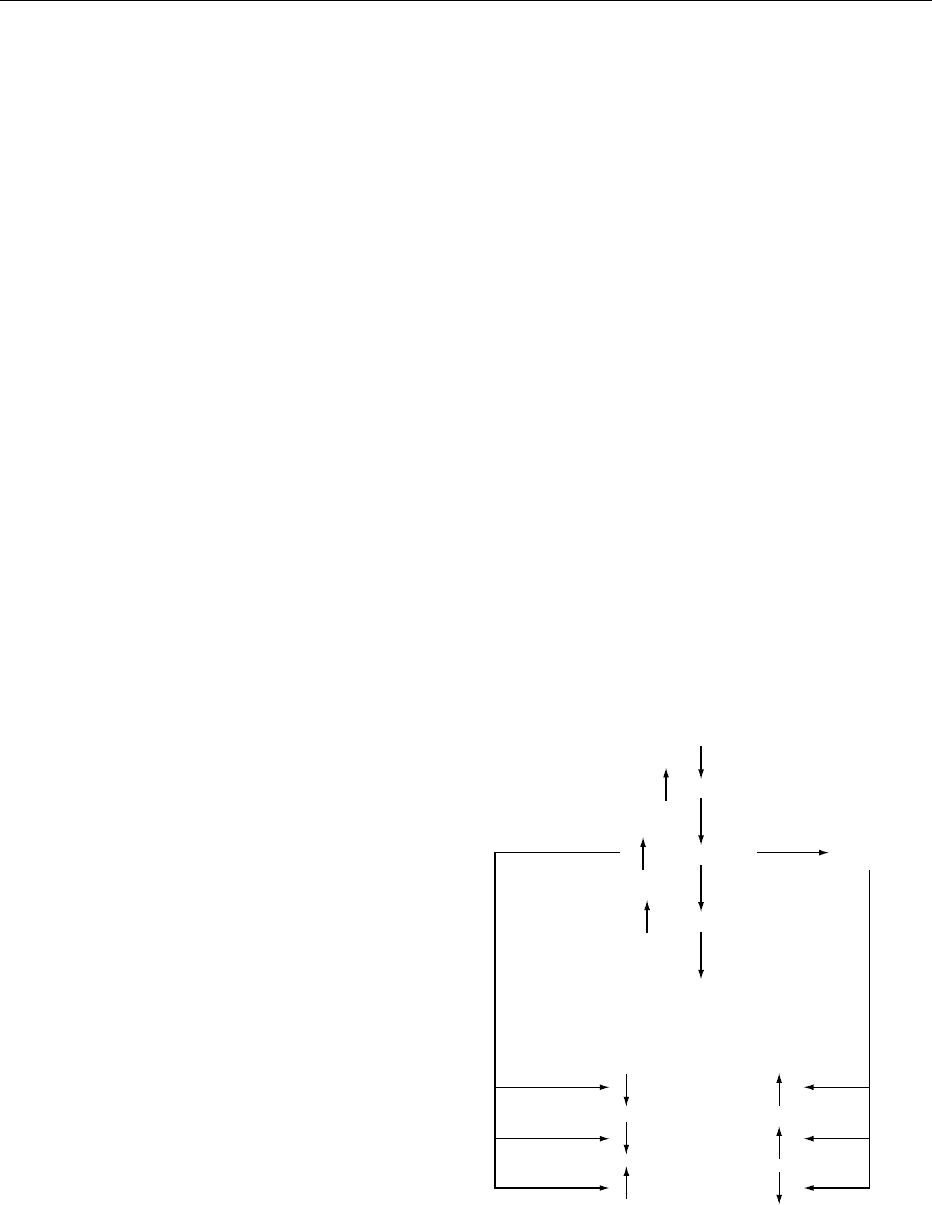
effects. A lower glomerular filtration rate and renal
plasma flow, as well as reduced excretion of albumin
and some proteins, have been reported in vegan and
lactovegetarian individuals compared with omnivo-
rous subjects. In fact, acute (more than 80 g) or
chronic loading (more than 1 g per kg of body weight
per day) with animal proteins has resulted in an in-
crease in renal plasma flow and glomerular filtration
associated with a higher clearance of albumin than
that on vegetable protein diets. Vasodilatory PGs such
as PGI
2
, which may be partly dependent on a rise in
glucagon secretion from the pancreas, have been pro-
posed as a possible mediator of such renal effects of
animal proteins, since the administration of indo-
methacin was found to abolish the renal response to
a meat meal.
Minerals
0020 The isolation and identification of renal PGE
2
and
PGA
2
between 1965 and 1967 provided biochemical
support for the renal anitihypertensive-endocrine
function. Numerous studies in humans and animals
thereafter have shown that PGE
2
has a potent vaso-
dilatory and natriuretic action and hence a pivotal
role in sodium homeostasis. In fact, PGE
2
adminis-
tration augments renal blood flow and urinary
sodium excretion. In addition, PGE
2
stimulates renal
renin secretion and inhibits vasopressin-mediated
water reabsorption. During a low sodium intake,
the renin–angiotensin axis is activated by volume
depletion, resulting in antinatiuresis and vasocon-
striction. Renal PGE
2
synthesis is stimulated by ele-
vated circulating angiotensin II, which then
counteracts the action of angiotensin II, leading to
normalization of renal blood flow, blood pressure,
and sodium excretion (Figure 3). This is the rationale
for the beneficial effects of a low-sodium diet on these
cardiovascular parameters. The same can be said for
diuretic therapy, since the renomedullary synthesis of
PGE
2
from arachidonic acid is directly stimulated by
furosemide. The natriurietic and antihypertensiv
effects of furosemide are mediated by this newly syn-
thesized PGE
2
, since both effects are inhibited by
indomethacin. While COX-1 has long been recog-
nized to be involved in normal kidney function,
COX-2 is now seen to have a distinct role. COX-2
has been localized in both the macula densa and the
interstitial cells of the medulla. Chronic sodium de-
privation was found to increase COX-2 levels in the
macula densa, and COX-2-generated prostanoids
may be important mediators of renin production
and tubuloglomerular feedback.
0021 Dietary potassium deficiency leads to hypokalemia
and impairment of maximal urinary concentrating
ability. Since PGE
2
antagonizes vasopressin-induced
water reabsorption, an increase in renomedullary
PGE
2
production was postulated to underlie this con-
centrating defect. However, during chronic dietary
potassium depletion secondary to dietary potassium
deprivation in animals, PGE
2
synthesis by the renal
medulla is markedly reduced, and thus current evi-
dence does not support a role for PGs in hypokalemic
polyuria.
0022Oral calcium supplementation has been reported to
decrease blood pressure in some patients with essen-
tial hypertension and hypertensive rats. However, in
other studies, calcium supplementation produced no
effect on blood pressure in normotensive or hyperten-
sive subjects, Although calcium supplementation has
been shown to increase urinary PGE
2
excretion in
normotensive women, there was no associated fall
in blood pressure.
Spices
0023Spices have been consumed by many cultures for
thousands of years. In fact, in classical Indian (Ayur-
vedism), Chinese, or Graeco-Arabic systems of medi-
cine, some spice-containing plant materials were used
to prevent or treat some diseases. Some spices have
Sodium restriction
Renin
Angiotensin II PG release
Aldosterone
Sodium retention
Sodium excretion
Blood pressure
Renal blood flow
fig0003Figure 3 Scheme whereby volume depletion secondary to
dietary sodium restriction leads to an increase in PG release
from the renal medulla and resultant physiological actions of
angiotensin II. From Lee JB (1980) Prostaglandins and the
renin–angiotensin axis. Clinical Nephrology 14: 159–163, with
permission.
PROSTAGLANDINS AND LEUKOTRIENES 4803

been shown to inhibit platelet aggregation. These
include onion, garlic, ginger, cloves, omum, cumin,
and turmeric. Extracts from these spices were found
to inhibit platelet COX, allowing less TXA
2
to be
produced. As COX is inhibited, more substrate (ara-
chidonic acid) becomes available to the lipoxygenase
pathway, as shown by the observation that 12-
HPETE increases in platelets treated with extracts
from ginger, cloves, cumin, and turmeric. However,
onion extracts have been demonstrated to inhibit the
5- or 12-lipoxygenase in platelets. This might at least
partially explain the observation that crude ethanolic
extracts of onion prevented allergen-induced bron-
choconstriction in animals and humans. Ginger,
which is demonstrated to have both antihistaminic
and antioxidant action, also inhibits both the COX
and 5-lipoxygenase pathway. Garlic extracts have
also been demonstrated to inhibit COX activity and
LTC4 formation.
See also: Calcium: Physiology; Fatty Acids: Metabolism;
Dietary Importance; Tr a n s -fatty Acids: Health Effects;
Fish: Dietary Importance of Fish and Shellfish;
Hypertension: Physiology; Nutrition in the Diabetic
Hypertensive; Potassium: Properties and Determination;
Physiology; Renal Function and Disorders: Nutritional
Management of Renal Disorders; Sodium: Physiology
Further Reading
Bergstrom S, Ryhage R, Samuelsson B and Sjo
¨
vall J (1962)
The structure of prostaglandin E
1
,F
1
and F
2
. Acta
Chemica Scandinavica 16: 501–507.
Chin J and Dart AM (1995) How do fish oils affect vascular
function? Clinical and Experimental Pharmacology and
Physiology 22: 71–81.
Fiorelto P, Trevisan R, Valeho A et al. (1990) Impaired renal
response to a meat meal in insulin-dependent diabetes:
role of glucagon and prostaglandins. American Journal
of Physiology 258: F675–683.
Katayama S, Maruno Y, Inaba M et al. (1991) Effect
of dietary calcium on renal prostaglandins. Post-
aglandins, Leukotrienes and Essential Fatty Acids 42:
197–200.
Knapp HR and Fitzgerald GA (1989) The antihypertensive
effects of fish oil. New England Journal of Medicine
320: 1037–1043.
Kontessis P, Jones S, Dodds R et al. (1990) Metabolic and
hormonal responses to ingestion of animal and vegetable
proteins. Kidney International 38: 136–144.
Lee JB and Katayama S (1985) Prostaglandins, thrombox-
anes and leukotrienes. In: Wilson JD and Foster DW
(eds) Williams Textbook of Endocrinology. Phila-
delphia, PA: WB Saunders.
Moncada S, Gryglewski R, Bunting S and Vane JR (1976)
An enzyme isolated from arteries transforms prosta-
glandin an unstable substance that inhibits platelet
aggregation. Nature 263: 633–665.
Morris MC, Sacks F and Rosner B (1993) Does fish oil
lower bood pressure? A meta-analysis. Circulation 88:
523–533.
Narumiya S, Sugimoto Y and Ushikubi F (1999) Prosta-
noids receptors: Structures, properties, and functions.
Physiological Reviews 79: 1193–1226.
Oates JA, Fitzgerald GA, Branoh RA et al. (1988) Clnical
implications of prostaglandin and thromboxane A
2
for-
mation. New England Journal of Medicine 319:
689–698, 761–767.
Samuelsson B (1983) Leukotrienes: Mediators of immedi-
ate hyper-sensitivity reaction and inflammation. Science
220: 568–575.
Vane JR, Bakhle S and Botting RM (1998) Cyclooxygenase
1 and 2. Annual Review of Pharmacology and Toxicol-
ogy 38: 97–120.
Wentzel P and Ericksson UJ (1998) Antioxidants diminish
developmental damage induced by high glucose and
cyclooxygenase inhibitors in rat embryos in vitro.
Diabetes 47: 677–684.
4804 PROSTAGLANDINS AND LEUKOTRIENES
