Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


chlorine, thus explaining why it is common for both
alkali metals to appear in the form of their correspond-
ing chloride salts. Given this relationship with cellular
liquids, potassium, sodium, and chlorine are also
known as electrolytes (ions with greater proportions
in the composition of organic fluids).
0005 The K
þ
ion is the main intracellular cation, with an
approximate [K
þ
] of 5.6 gl
1
in cellular fluids, ap-
proximately 30 times more concentrated than in
plasma or interstitial liquid (0.15–0.20 gl
1
). Its
high intracellular concentration is regulated by the
cell membrane through the sodium–potassium pump.
The intracellular K
þ
regulates the catalytic action of
numerous enzymes, through the attachment of the
cation to active locations with negative charges of
the enzymatic proteins, modifying the conformation
of the molecule and its activity, as well as participat-
ing in cellular division processes.
0006 The small percentage of extracellular potassium
(2% of body potassium) is of great physiological
importance, since it is a critical determinant of neuro-
muscular excitability (nervous impulse and contrac-
tion of bone muscles). At the cellular membrane level,
the transport and permeability of energy-dependent
potassium, with simultaneous excretion of sodium
linked to the Na/K enzyme ATPase, is essential for
generating the potentials of membranes required for
the proper functioning of nervous and muscular cells.
It also helps to maintain the acid–base equilibrium
and blood pressure.
0007 Homeostasis of potassium is still the subject of
research, although it is known that 90% of dietetic
potassium is absorbed in the small intestine and that
body potassium (1.6–2 g per kilogram of body
weight) is regulated by renal glomerular filtration
and tubular secretion, potassium being lost on a
daily basis through urine, gastrointestinal secretions
(ileum and colon) and, to a lesser degree, sweat. Pro-
vided that renal function is normal, it is practically
impossible to reach an excessive level of potassium
with normal dietary intake, since the kidney is capable
of excreting more potassium than it can filter. (See
Potassium: Physiology; Sodium: Physiology.)
0008 The effect of potassium on blood pressure has been
discussed in recent reports on metaanalysis; these
have confirmed that increases in doses of potassium
from 60 to 80 mmol per day (2.3–3.1 g per day) may
prompt a decrease of 4 mmHg in systolic blood pres-
sure and possibly reduce the number of deaths related
to high blood pressure by 25%.
0009 The use of potassium chloride salts as substitutes
for sodium chloride, in individuals in whom the
intake of sodium is restricted because of problems
relating to hypertension, is a questionable alternative,
since cardiac arrythmias have been reported in
association with the excessive intake of salt substitutes
containing potassium chloride. In this connection,
hyperkalemia is recommended in healthy individuals
for intakes exceeding 17.5 g per day (acute toxicity
limit), highly unlikely in normal diets, and certain
risks for individuals with renal dysfunctions not
detected with intakes of potassium exceeding 5.9 g
per day (safest maximum dose). Cardiovascular or
neuromuscular complications arising from situations
of hypo- and hyperkalemias are resolved favorably by
correcting the plasmatic potassium levels. (See Hyper-
tension: Hypertension and Diet.)
0010The mean potassium intake in Western populations
ranges between 1.6–5.9 g per day and the required
dietary intakes of this are met without any problem,
thanks to the ubiquitous presence of potassium in
both vegetable- and animal-based foods. The Scien-
tific Committee for Food of the European Commu-
nity recognizes that the Minimum Recommended
Intake of potassium is 1600 mg d
1
, and the Refer-
ence Intake for the population 3100 g d
1
.
Analysis of Potassium
0011Potassium can be analyzed using a number of differ-
ent methods, although many of these are not com-
monly used for routine analysis of potassium in food
and biological samples. In the case of potassium,
there has been a shift from the old gravimetric
methods, based on the precipitation of potassium
using chloroplatinate or tetraphenylboron, to the
spectroscopic methods, mainly flame emission and
flame absorbance spectroscopy and which, according
to the scientific community, are the most commonly
used.
0012However, there are other techniques for analyzing
potassium that are not as ‘popular’ as those men-
tioned above, and equally applicable to other mineral
elements. These include selective electrodes, nuclear
magnetic resonance spectroscopy, X-ray analysis,
helium glow photometry, inductively coupled plasma
optical emission, inductively coupled plasma atomic
fluorescence, ion-scattering spectrometry, and other
methods. Another procedure can be used – namely,
radioactive dilution of potassium isotopes – when
determining both potassium content and its distribu-
tion in extra- and intracellular compartments.
0013Emission and absorbance spectrometry have been
the most widely used techniques for analyzing trace
elements in biological and food samples. Their wide-
spread use is justified by their analytical specificity,
good detection limits, excellent accuracy, and rela-
tively low cost. Both techniques are based on energy
modifications of the electronic orbital structure of the
atoms of mineral elements in response to certain
POTASSIUM/Properties and Determination 4645
stimuli (flame emission spectrometry and incidence of
a beam of light of specific wavelength for absorption).
(See Sodium: Properties and Determination.)
0014 The determination of potassium by spectroscopy
requires a stage of preparation of samples for analysis
that entails the drying, grinding, and destruction of
the organic matter of the sample. There are two alter-
natives for destroying the organic matter of biological
and food samples, both with different variants: wet
oxidation and dry ashing. Both techniques yield rea-
sonably comparable results, and it is often the analyst
who decides on which technique to employ. (See Cad-
mium: Properties and Determination; Spectroscopy:
Atomic Emission and Absorption.)
Flame Emission Spectroscopy
0015 The determination of potassium in biological and
food samples, as in the case of sodium, may be per-
formed using flame photometers and atomic absorp-
tion spectrophotometers (with the option of working
in emission conditions). A specific wavelength of
766.5 nm is used in order to determine potassium,
and the detector is adjusted to the response given to
the pattern of greatest concentration used. Efforts
must be made to avoid possible interference problems
owing to the matrix characteristics of the sample in
flame emission photometry; this can be achieved by
separating the element from the object of study, elim-
inating the ions responsible for the interferences, or
using a compensatory technique. For this purpose,
recommended practice for correcting interferences
includes the addition of lithium (as an internal stand-
ard) both to the samples and to the standards. It is
important to obtain a suitable calibration curve,
either linear by diluting the samples, or by logarith-
mic adjustment between emission values and potas-
sium concentration. (See Sodium: Properties and
Determination.)
Flame Absorption Spectroscopy
0016 The determination of potassium using an atomic ab-
sorption spectrophotometer requires a light source
(wavelength for K ¼766.5 nm) and an atomization
source (flame). For the light source, arc discharge
lamps may be used for K, although hollow cathodes
are preferred. Standard working conditions for ana-
lyzing potassium by atomic absorption spectroscopy
are listed in Table 1.
0017 Comparing flame emission photometry and atomic
absorption spectroscopy, the detection limits for po-
tassium (mgml
1
) are 0.0005 and 0.005, respectively.
Using an atomic absorption spectrophotometer, the
accuracy and precision of potassium analysis by
flame emission can be obtained (Table 2).
0018Table 3 shows a series of official methods for de-
termining potassium, based on flame emission and
absorption spectrophotometry.
Inductively Coupled Plasma-atomic Emission
Spectrometry (ICP-AES)
0019There are numerous references to the use of this
powerful technique in multielement analysis by emis-
sion spectroscopy. ICP-AES enables samples with a
high degree of variability and minimum interferences
to be used. Samples can be prepared by either wet
oxidation or dry ashing, and a wide range of concen-
trations can be used without the need to dilute or
concentrate the sample. Also, many of the other min-
eral elements can be quantified, thus reducing analy-
sis time. These advantages, together with the high
speed and excellent instrument stability, make ICP-
AES highly attractive.
0020For potassium determined by ICP-AES in plant
tissues, a solution detection limit of 0.06 (mgml
1
)
and a sample detection limit of 2.0 (mgg
1
, based on
a 2-g sample diluted to 50 ml) are recommended.
There is also an AOAC Official Method (984.27)
for the determination of potassium in infant formula
by means of ICP-AES, which recommends the
following ICP emission spectrometer operating par-
ameters for potassium; wavelength (nm) ¼766.5, no
background correction and low standard ¼0 and
high standard ¼ 200 (mgml
1
). The AOAC also rec-
ommends an Official Method (985.01) for the analy-
sis of potassium in plants and pet foods by ICP
spectroscopy.
Potassium-selective Electrodes
0021Potassium-selective electrodes have developed from
the first ion-selective glass electrodes, with little ion
selectivity, to potassium-selective electrodes based
on valinomycin, a highly specific neutral carrier
compound for analyzing potassium, to potassium-
sensitive electrodes with ion-exchange compounds
dissolved in organic solvents that are not very specific
tbl0001Table 1 Standard conditions for potassium analysis by atomic
absorption spectrometry
Spectrometers setting Potassium
Wavelength/slit (nm) 766.5/0.2
Nebulizer Spoiler
Oxidant Air
Fuel C
2
H
2
Flame condition Oxidizing
Optimum concentration range in solution (mgml
1
) 1–10
Detection limit (mgml
1
) 0.005
Sensitivity 1% absorption (mgml
1
) 0.05
Interferences Ionization
4646 POTASSIUM/Properties and Determination
because of significant interference induced by the
presence of sodium and other cations in organic solu-
tions. Ion-exchange electrodes have faster response
times than those of organic solvent solution mem-
branes of valinomycin, although the latter show
fewer interferences from other ions. However, neither
type of electrode is effective in organic solvents. Fur-
thermore, electrodes measure ion activity and there-
fore do not detect ions linked to other molecules or
potassium that is not in solution.
0022 Ion-selective electrodes are also incorporated into
gasometric equipment for measuring electrolytes (Na,
K, Cl, and ionized Ca), in oxygenation, ventilation,
basic-acidic state, and electrolytic metabolism of pa-
tients with respiratory problems. In this context, it is
useful to quantify the exchange of gases and electro-
lytes through cellular membranes; for this purpose,
the so-called anion gap is calculated, which inter-
relates with sodium (anion gap Na), potassium
(anion gap K), bicarbonate, and chlorine. This par-
ameter provides information on electrolytic alter-
ations and the presence of toxins in blood, and also
helps to control quality in laboratories by interrelat-
ing informed values (in normal individuals, not very
high values).
0023 Some developments in the field of potassium-
selective electrodes have focused on the appearance
of novel potassium-selective valinomycins in the
development of ion-selective sensors based on new
technologies and on the combination of these ion-
selective electrodes with other analytical methodolo-
gies, as shown in Table 4.
Nuclear Magnetic Resonance (NMR)
0024NMR is used to study the relationship between metab-
olism and function in living systems as simple as cells in
culture and as complex as human subjects (e.g., the
effect of temperature on the sodium/potassium pump
in red blood cells). As a noninvasive, nondestructive
spectroscopic technique, NMR offers a powerful ap-
proach to the study of ion balance in intact biological
systems. The aims are to validate new NMR methods,
to elucidate tissue-specific mechanisms, and to draw
conclusions with respect to basic concepts and design in
tissue metabolism and function.
0025The possibility of determining potassium by NMR
is due, on the one hand, to the relatively high concen-
trations of the cation in cellular tissue and, on the
other hand, to the natural abundance of
39
K nuclide
(NMR sensitivity is about 2000 times less than that of
one hydrogen atom) and the short longitudinal relax-
ation time. The concentrations of intracellular and
extracellular potassium in tissues may be quantified
by applying impermeable cell-membrane chemical
shift reagents.
0026However, this quantification by NMR measurement
depends on the NMR invisibility of the potassium
actually present, in accordance with other techniques.
Several previous studies have shown that only approxi-
mately 20% of cardiac intracellular potassium is visible
with current NMR techniques. This NMR sensitivity
of potassium (about 60%) represents a serious obstacle
to its proper quantification in biological tissues. (See
Spectroscopy: Nuclear Magnetic Resonance.)
tbl0003 Table 3 Official methods recognized for potassium determination by flame emission and absorption spectrophotometry
Officialmethods
Sodium and Potassium in Seafood Flame Photometric Method, AOAC Official Method 969.23
Potassium in Fruits and Fruit Products Rapid Flame Photometric Method, AOAC Official Method 965.30
Potassium and Sodium in Wines Flame Spectrophotometric Method, AOAC Official Method 963.13
Potassium in Distilled Liquors Flame Photometric Method, AOAC Official Method 963.08
Potassium in Beer Atomic Absorption Spectrophotometric Method, AOAC Official Method 987.02
Potassium in Water Atomic Absorption Spectrophotometric Method, AOAC Official Method 973.53
Potassium in Infant Formula, Enteral Products and
Pet Foods
Atomic Absorption Spectrophotometric Method, AOAC Official Method 985.35
tbl0002 Table 2 Accuracy and precision of the potassium analysis by flame emission
Accuracy Certified (g kg
1
)Found(gkg
1
)IC(95%)
a
rec (RSD)
b
Citrus leaves SRM-1572 18.2 + 0.6 17.7 + 0.5 16.8–18.7 97 (3)
Nonfat milk powder 16.9 + 0.30 16.3 + 0.41 15.5–17.1 96 (5)
NIST-1549
Precision RSD
Plant food 1.35
Dairy food 2.55
a
Intervals of confidence (95%).
b
Recovery percentages and relative standard deviation (RSD).
POTASSIUM/Properties and Determination 4647

0027 A study of factors affecting
39
K NMR detectability
in rat thigh muscle showed that the signal may be
substantially higher (up to 100% of total tissue po-
tassium) than values previously reported of around
40%, these signals presenting two superimposed
components – one broad and one narrow – and in-
volving improvements in spectral parameters (signal-
to-noise ratio and baseline roll), together with
computer simulations of spectra that enable a spec-
trum quality with a major effect on the amount of
signal detected, largely owing to the loss of detect-
ability of the broad signal component.
0028 Biological and biochemical cellular research in
connection with the quantification of ions in intracel-
lular and extracellular compartments, transport and
ionic metabolism at the level of different cells
and biological tissues, are based on the use of
39
K
NMR spectroscopy, together with
23
Na and
31
P
NMR studies.
0029 Proper ion equilibrium between intra- and extracel-
lular compartments is required for normal physio-
logical function. Conversely, alterations in membrane
ion transport occur in numerous pathological states. By
introducing an anionic paramagnetic shift reagent into
the medium, NMR signals of intra- and extracellular
Na
þ
and K
þ
can be resolved, enabling ion transport
processes to be studied by NMR. Unfortunately, rare
NMR active nuclides that are isotopes of the 100%
naturally abundant
23
Na
þ
and
39
K
þ
are not available
for tracer kinetic studies of Na
þ
and K
þ
transport.
However, Cs is a biologically active analog of K
þ
,and
the 100% naturally abundant NMR active
133
Cs
þ
nu-
clide can be employed to examine K
þ
transport. Other
studies have shown the potential of
39
K NMR as a
useful tool in the study of protein–cation interactions
and the binding of K
þ
to double-helical DNA.
X-ray Analysis
0030X-ray analysis is another nondestructive, noninva-
sive, in-vivo technique for determining mineral elem-
ents – including potassium – in foods and biological
samples. X-ray analysis is based on bombarding a
small area of the powdered sample with high-energy
X-rays, measuring the Ka line of the element using
one of several different detectors according to the
wavelength of the emitted radiation. The limitations
of this technique are related to scope, in terms of
detected elements and their concentration range.
X-ray analysis is combined with the use of an elec-
tronic microscope for quantifying and locating the
mineral element in the sample, even at the subnuclear
compartmental level. Another limitation of the X-ray
tbl0004 Table 4 Analytical methodologies with ion-selective electrodes for potassium analysis
Investigation Reference
A potassium-ion selective electrode with valinomycin-based
poly(vinyl chloride) membrane and a poly(vinyl ferrocene)
solid contact
Hauser PC, Chiang DWL. and Wright GA (1995) Analytica Chimica
Acta 302(2–3): 241–248.
Miniaturized ion-selective sensor chip for potassium
measurement in a biomedical application
Uhlig A, Dietrich F, Schnakenberg U, Hintsche R and Lindner E
(1996) Sensors and Actuators B: Chemical Abstract Export
34(1–3): 252–257.
Synthesis of novel potassium selective valinomycins Dawson JR, Dory YL, Mellor JM and McAleer JF et al. (1996)
Tetrahedron 52(4): 1361–1378.
Optical sensors for sodium, potassium, and ammonium ions
based on lipophilic fluorescein anionic dye and neutral
carriers
Wang E, Zhu L, Ma L and Patel H (1997) Analytica Chimica Acta
357: 85–90.
Recording of neuronal network properties with near-infrared
dark-field microscopy and microelectrodes
Holthoff K and Witte OW (1997) Electrochimica Acta 42(20–22):
3241–3246.
Flow injection analysis of potassium using an all-solid-state
potassium-selective electrode as a detector
Komaba S, Arakawa J, Seyama M, Osaka T, Satoh I and
Nakamura S (1998) Ta la n t a 46: 1293–1297.
Transferability of results obtained for sodium, potassium and
chloride ions with different analyzers
Rodriguez-Garcia J, Sogo T, Otero S and Paz JM (1998) Clinica
Chimica Acta 275: 151–162.
Plasticizer-free all-solid-state potassium-selective electrode
based on poly(3-octylthiophene) and valinomycin
Bobacka J, Ivaska A and Lewenstam A (1999) Analytica Chimica
Acta 385: 195–202.
Simultaneous detection of monovalent anions and cations using
all solid-state contact PVC membrane anion and cation-
selective electrodes as detectors in single column ion
chromatography
Isildak I and Asan A (1999) Ta l a n t a 48: 967–978.
Novel sensors for potassium, calcium, and magnesium ions
based on a silicon transducer as a light-addressable
potentiometric sensor
Seki A, Motoya K, Watanabe S and Kubo I (1999) Analytica Chimica
Acta 382: 131–136.
Reference ranges of electrolyte and anion gap on the Beckman
E4A, Beckman Synchron CX5, Nova CRT, and Nova Stat Profile
Ultra
Lolekha PH, Vanavanan S, Teerakarnjana N and
Chaichanajarernkul U (2001) Clinica Chimica Acta 307: 87–93.
4648 POTASSIUM/Properties and Determination

technique relates to the complexity of the sample
preparation process for electronic microscopy.
0031 The bibliography describes different X-ray analysis
techniques for measuring potassium in food and bio-
logical samples, as indicated in Table 5.
0032 X-ray fluorescence (XRF) can be successfully used
for the qualitative and quantitative elemental analysis
of various agricultural products. Its simplicity, high
throughput and automation possibilities make it
useful for screening large numbers of samples. The
K content of tea samples has been determined by XRF
analysis (an uncommon method for mineral analysis
in food), and the findings have been compared with
the results obtained using atomic emission tech-
niques, with the conclusion that the XRF system can
be used effectively for quantitative analysis of the
K content of tea samples.
0033 The methods for preparing the specimens of liquid
media of the organism and XRF analysis are simple
and fast, entail no disintegration of the sample, and
allow measurements of elements at sigma 1 ¼0.02 at
concentrations of 12 mgml
1
to be obtained. The
method is preferable and promising for several basic
elements (P, S, Cl, K, Ca), which are difficult to meas-
ure using other methods.
0034 The use of total reflection X-ray fluorescence an-
alysis (TXRF) in life sciences is considered a powerful
analytical tool for simultaneous multielement deter-
mination. TXRF is basically an energy-dispersive
technique, with the sample being excitated in total
reflection geometry. This technique only requires
minute samples with simple preparation and involves
a large dynamic measuring scale. Simultaneous detec-
tion of almost all chemical elements and lower limits
of detection are achievable in optimized excitation
conditions. The preferred sample types are aqueous
and acidic solutions, particularly samples digested
with HNO
3
. Special preparation techniques are re-
quired for solids and other samples.
0035The application of the proton-induced X-ray-emis-
sion (PIXE) method has enabled us to determine not
only the concentrations of elemental composition,
including K, but also their localization in different
artery-wall regions. Furthermore, the usefulness of
the micro-PIXE method for studying biomedical ma-
terials has also been considered.
Radioactive Isotopes
0036The dilution of radioactive isotopes of potassium is
applied to establish the concentration and distribu-
tion of the amounts of potassium in the different
sample compartments. The dilution of radioactive
isotopes enables the volumes of the organic tissue
compartments to be determined. The differences in
potassium concentrations between the extra- and
intracellular spaces may be studied by diluting the
radioactive isotopes of potassium.
0037For this purpose, it is important to take into ac-
count that animal and plant tissues present different
potassium compartments in which the ion is accumu-
lated, and this conditions the radioactive isotope
dilution process. In animal tissue, three cellular com-
partments (plasma þextracellular fluid, cells, and
spaces where ions are closely fixed or form com-
plexes) are described, whereas in plant tissue, only
two spaces (cytoplasm and vacuoles) must be con-
sidered, since the extracellular space is small in size.
This compartmental distribution conditions the pro-
cess of radioactive isotope dilution.
0038In animal tissue, the method involves incorporating
a known amount of the tracer isotope of potassium
into the extracellular compartment and monitoring
the changes in plasma isotope concentration until
equilibrium is reached with all the compartments.
This entails estimating the distribution of potassium
(1) in extracellular fluid, using tracer polymers (inulin
or polyethylene glycols) that cannot penetrate cell
membranes, (2) in cellular volume, by subtracting
the extracellular volume from the total distribution
space (determined using labeled water, tritiated or
deuterated), and (3) in the space where the ion is
strongly fixed or forms complexes (this is calculated
by subtracting the amount of extracellular potassium
þcellular potassium from the amount of potassium
isotope injected).
0039In plant tissue, the dilution of radioactive isotopes of
potassium presents limitations owing to the difficulty
involved in terms of separating vacuolar volume and
cytoplasmic volume. For this reason, more appropriate
methods than radioactive dilution methods have been
tbl0005 Table 5 X-ray techniques for potassium analysis in food and
biological samples
Samples X-ray analysis
Meat and meat products X-ray fluorescent analysis
Serum and peripheral blood cells
Fresh green tea, black tea, and
tea residue
Leaves of lettuce Electron microprobe X-ray
analysis
Walls of the mosaic virus-infected
wheat leaf cells
Energy-dispersive X-ray
analysis
Perisperm tissues of seeds
Cancerous and normal tissues Total reflection X-ray
fluorescence analysis
In vegetable foodstuffs and their
respective cell fractions
Cancerous breast tissue
Human brains Proton-induced X-ray
emission analysis
Human atherosclerotic artery wall
POTASSIUM/Properties and Determination 4649

developed for determining cytoplasmic and vacuolar
potassium concentrations, namely ion-selective micro-
electrodes or X-ray microprobe analysis.
See also: Cadmium: Properties and Determination;
Hypertension: Hypertension and Diet; Potassium:
Physiology; Sodium: Properties and Determination;
Physiology; Spectroscopy: Atomic Emission and
Absorption; Nuclear Magnetic Resonance
Further Reading
Ammon D (1986) Ion Selective Microelectrodes. New
York: Springer-Verlag.
Benton Jones J, Jr. (1984) Developments in the measure-
ment of trace metal constituents in foods. In: Gilbert J
(ed.) Analysis of Food Contaminants, pp. 157–202,
Barking, UK: Elsevier.
Cappuccio FP and MacGregor GA (1991) Does potassium
supplementation lower blood pressure? A meta-analysis
of published trials. Journal of Hypertension 9: 465–473.
Commission of the European Communities (1993) Nutrient
and Energy Intakes for the European Community.
Reports of the Scientific Committee for Food, Thirty-
first Series, pp. 174–178.
Gunther K and von Bohlen A (1990) Simultaneous multi-
element determination in vegetable foodstuffs and their
respective cell fractions by total-reflection X-ray fluor-
escence (TXRF). Zeitschrift fu
¨
r Lebensmitteluntersu-
chung und -forschung 190(4): 331–335.
Intersalt Cooperative Research Group (1988) Intersalt: a
international study of electrolyte excretion and blood
pressure. Results for 24 hour urinary sodium and potas-
sium excretion. British Medical Journal 297: 319–328.
Luft FC (1990) Sodium, chloride and potassium. In: Brown
M (ed.) Present Knowledge in Nutrition, 6th edn, pp.
233–240. Washington, DC: International Life Sciences
Institute Nutrition Foundation.
Springer C (1987) Measurement of metal cation compart-
mentalization in tissue by high-resolution metal cation
NMR. Annual Review of Biophysics and Biochemistry
375–399.
Physiology
M P Navarro, Unidad de Nutricio
´
n, Estacio
´
n
Experimental del Zaidin, CSIS Granada, Spain
M P Vaquero, Ciudad Universitaria, Madrid, Spain
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Potassium is the most abundant intracellular cation in
the human body and plays an important role in a
variety of cell functions. This review summarizes the
main aspects of potassium physiology and nutrition,
including absorption, transport, distribution, storage,
and excretion, as well as homeostatic mechanisms of
K balance.
Role in the Body
0002Potassium is essential for the muscular, cardiovascu-
lar, nervous, endocrine, respiratory, digestive, and
renal systems. Cell metabolism depends on the main-
tenance of a high intracellular K
þ
concentration.
Many of the body functions of potassium are due to
its ionic character: it generates gradients of concen-
tration, potential, and pressure. Potassium is the pre-
dominant osmotically active species inside the cell.
Together with other ions such as sodium and chloride,
which are characteristic of the extracellular fluid,
potassium determines osmolarity and plays a major
role in the distribution of fluids inside and outside the
cell and hence in the maintenance of cellular volume.
In addition, potassium participates in the regulation
of the acid–base balance and is involved in cellular
growth and division, energy transduction, glyco-
genesis, protein synthesis, hormone secretion, etc.
Consequently, its deficiency causes growth retard-
ation, with a pronounced decrease in circulating
levels of growth hormone and somatomedin C and
inhibition of protein synthesis.
0003Cell excitability and muscle contraction depend on
potassium. The transmembrane electrical potential is
determined by the ratio of the intracellular to extra-
cellular potassium and sodium concentrations, in par-
ticular that of potassium. Differences in K
þ
and Na
þ
concentrations across cell membranes are maintained
by the specific permeability to each of these ions and
by K
þ
/Na
þ
-ATPase activity (K
þ
/Na
þ
-pump). Thus,
K
þ
is critical for the excitability of nerve and muscle
cells.
0004During vigorous exercise, potassium is released
from muscle cells, leading to an increase in extracel-
lular potassium concentrations which facilitates on-
going muscle contraction and induces vasodilatation,
increasing local blood flow. However, liberation of
potassium also leads to muscular fatigue. Training
reduces the exercise-induced rise in plasma K
þ
con-
centration and also increases the total activity of Na-
K pumps in muscle. The potassium internal balance
helps to delay the onset of fatigue during exercise and
to restore homeostasis during recovery.
0005Potassium can be defined as a cardioprotector
nutrient. It acts directly on the heart, regulating its
mechanical and electrical properties. Epidemiological
data suggest that potassium intake and blood pressure
are inversely correlated. The greatest hypotensive
4650 POTASSIUM/Physiology

effect of potassium occurs in hypertensive patients
and in subjects with a high sodium intake. The mech-
anisms involved include: enhancing natriuresis,
baroreflex sensitivity, direct vasodilatation, cate-
cholaminergic functions, improvement of glucose
tolerance, and effects on the central nervous and the
renin–angiotensin–aldosterone systems.
0006 Potassium also protects against stroke, which is the
third leading cause of death worldwide (after coron-
ary artery disease and cancer). This electrolyte may
decrease ischemic as well as hemorrhagic stroke risk
through its effect on blood pressure on one hand, and
by inhibiting the formation of free radicals at the
endothelial cell level, thus affecting vasomotion, on
the other hand. Moreover, potassium inhibits the pro-
liferation of smooth muscle cells, platelet aggrega-
tion, and arterial thrombosis. Potassium may also
prevent the death of cerebellar neurons.
0007 Interrelationships exist between potassium and
other nutrients. Potassium and sodium are strongly
metabolically interrelated, principally due to K
þ
/
Na
þ
-ATPase. This enzyme also provides the driving
force for the transport of other solutes, such as amino
acids, phosphate, vitamins, and glucose. Potassium
depletion, which is more intense if there is a simultan-
eous excess of sodium, enhances urinary loss of
calcium. This interaction may have adverse effects
on bone and blood pressure.
Requirements and Daily Intakes
0008 To date, no recommended potassium intake has been
unanimously established. The UK report on dietary
reference values established a reference nutrient
intake of 3500 mg day
1
for adults. Lower amounts
were recommended for infants (mg day
1
): 800 and
850, 0–3 months and 4–6 months, respectively; 700,
7–12 months; 800, 1–3 years; 1100, 4–6 years; 2000,
7–10 years; and 3100, 11–14 years of age. No specific
levels were recommended during pregnancy, lacta-
tion, or for the elderly. The Nutrition Working
Group of the European International Life Sciences
Institute (ILSI) suggested a higher recommended
daily intake (3900 mg). This recommendation was
aimed at promoting a potassium intake similar to
that of sodium, in molar terms, i.e., an intake of
100 mmol day
1
of each cation (2300 mg and
3900 mg for Na and K, respectively). However,
more recently a Na:K ratio below 1 (1600 mg and
3500 mg for Na and K, respectively) has been recom-
mended to prevent hypertension, stroke, and cardio-
vascular disease.
0009 During exercise potassium needs may be increased
owing to higher losses in sweat, especially in hot
climates and in unaccustomed individuals. These
needs should be satisfied by eating food rich in potas-
sium and exceptionally by potassium supplements.
0010Published values of nutrient consumption demon-
strate that in some populations, sodium intake is
higher than recommended, while potassium intake
should increase. In the UK, the 1991 report of the
National Food Survey Committee showed that the
average sodium intake was 170% of the reference
intake, while that of potassium was 80% of the refer-
ence value. In Spain, data from the same year revealed
that the estimated potassium intake was satisfactory
but that of sodium was 140% above the reference
value.
0011Potassium is ubiquitous in all kinds of food, but the
best sources are vegetables and fruits because they
combine a high potassium content with low sodium
concentration (Table 1). Potassium is an essential
element of all forms of life, whereas sodium and
tbl0001Table 1 Potassium and sodium content of various foods
(mg per 100 g of edible portion)
Potassium Sodium
Vegetables
Potatoes 320 11
Cabbage 275–310 7–12
Carrots 235 60–90
Spinach 500–633 65–140
Legumes
Red kidney beans 1370 18
Soya beans 1730 5
Lentils 940 12
Fruits
Banana 400 1
Melon 100–310 5–32
Avocado pear 400–600 2–4
Orange 195 2–3
Apple 120 2–3
Dried fruits
Raisins 1020 60
Figs 970 62
Almonds 780 14
Walnuts 450 7
Cereals
White bread 100 460–540
Brown bread 175–240 540–636
Rice 110 6
Meat and fish
Beef, veal, lamb 230–360 52–110
Chicken 320 81
Bacon 350 1860
Herring 320 120
Halibut 410 60
Tuna 400 47
Mussels 320 290
Milk
Whole cows’ milk 140 55
Various
Chocolate 300 11
Tomato ketchup 590 1120
POTASSIUM/Physiology 4651

chlorine are essential for animals but not for many
plants. Meat and fish contain important amounts of
potassium because they are highly cellular tissues, but
they also contain large amounts of sodium chloride,
due to the extracellular fluid present in animal food.
Raw foods are preferable to processed ones because
during food preparation salt is usually added to
enhance flavor and retard bacterial growth (e.g.,
bread, bacon, tomato ketchup, and cookies). Potas-
sium salts, however, are not palatable and are not
added to food during processing.
0012 Legumes represent a good source of potassium
because they provide at least 1 g potassium per
100 g portion and very little sodium. Dried fruits,
for example, apricots, figs, and prunes, should also
be mentioned, since they often exceed 0.5 and even
1 g potassium per 100 g portion. Nuts such as pista-
chios and almonds contain a considerable quantity of
potassium, but the addition of salt during roasting
upsets the ideal sodium-to-potassium ratio normally
present in these foods. Bananas are known to be one
of the fruits richest in potassium, together with coco-
nuts, melons, avocados, and kiwi fruit. Milk is the
largest dietary source of potassium for infants.
0013 All these data should be considered, taking into
account the amount of each food item consumed.
For example, a ration of legumes is approximately
80 g while those of meat and milk can easily reach
200 g. Although cereals are low in potassium, they
account for almost 75% of potassium ingestion in
western diets. There is a wide range of potassium
intake by western populations (1560–5850 mg). It is
higher in vegetarians and people who consume more
food from plant than animal sources. The Mediterra-
nean diet provides a mean daily intake of potassium
equivalent to the recommended value (3500 mg).
0014 Although thermal treatments have no effect on
dietary potassium, during processes such as boiling,
soaking, or canning it can be reduced as a result of
leaching, particularly if food is to be cut into pieces
and the liquid is not consumed.
0015 In order to protect against hypertension and stroke,
except in clinical hypokalemia, potassium from good
dietary sources should be encouraged rather than
potassium from supplements, because such supple-
ments may have severe adverse effects. Fruits and
vegetables also contain magnesium and fiber which
seem to have protective effects.
Absorption
0016 Normal subjects absorb about 90% of ingested po-
tassium in the intestinal tract. The net absorption
is the difference between fluxes from lumen to
blood and from blood to lumen. In the human small
intestine, K
þ
permeability is high and potassium ab-
sorption is carried out across the epithelium of duo-
denum, jejunum, and ileum by passive mechanisms in
response to electrochemical gradients and solvent
drag. In the proximal small bowel K
þ
is concentrated
through the absorption of water, providing a driving
force for the movement of this cation across the intes-
tinal mucosa, preferably through the tight junctions
between enterocytes. Duodenum and jejunum absorb
this ion even more rapidly than water. Indeed, shortly
after a meal, the K
þ
concentration [K
þ
] in jejunum
rapidly reaches plasma levels. In the ileum, the trans-
epithelial electrical potential difference strongly influ-
ences its movement. There is no evidence of active
potassium absorption in the small intestine, but the
existence of an apical membrane H
þ
/K
þ
-ATPase
could suggest active K
þ
transport.
0017In the colon K
þ
may be secreted or absorbed in
response to variations in potassium status: net secre-
tion occurs when the luminal concentration is less
than 20–25 mmol l
1
, while net K
þ
absorption takes
place when levels are above 25 mmol l
1
. There are
two mechanisms for K
þ
secretion; the major mechan-
ism involves potential-dependent, passive flux,
mostly via the tight union, or by facilitated transcel-
lular diffusion. The second is an active serosa-to-
mucosa secretory mechanism in the proximal and
distal colon which is the result of uptake across the
basolateral membrane mediated by Na
þ
/K
þ
-ATPase
and Na
þ
-K
þ
-Cl
cotransport and movement across
the apical membrane through potassium channels.
Active K
þ
absorption also occurs in the distal colon,
in which the K
þ
uptake appears to be an exchange for
H
þ
across the apical membrane. This process is ener-
gized by adenosine triphosphatases (ATPases) located
in that membrane. Passive potassium absorption may
also take place in the proximal colon.
Bioavailability
0018The availability of dietary potassium is high because
potassium salts are entirely soluble and few dietary
factors modify its digestive utilization. Olive oil favors
potassium uptake, while some fibers and certain ion
exchange resins may decrease K
þ
absorption.
0019Physiological status also affects the nutritive util-
ization of potassium. Infants absorb a greater propor-
tion of salts than adults. This may be due to the
increased permeability of the immature small intes-
tinal epithelium of newborns, and especially to a
higher activity of the K
þ
-absorptive pumps in the
colonic apical membrane, while K
þ
-secreting chan-
nels are less relevant. During the second half of preg-
nancy, fecal potassium decreases. Urinary K
þ
output
increases in parallel with Na
þ
retention; nevertheless,
4652 POTASSIUM/Physiology
enough potassium is retained to cover gestational
needs. Glomerular filtration rates fall with age, ac-
companied by limitations of K
þ
secretion and Na
þ
conservation. Many drugs used in the elderly alter
potassium homeostasis.
Transport
0020 Potassium, being an electrolyte, is transported mainly
under ionic form in the body fluids. Intracellular
potassium concentration is 110–160 mmol l
1
of cells
while plasma concentration averages 4–5mmoll
1
,
of which only 10–20% is bound to proteins.
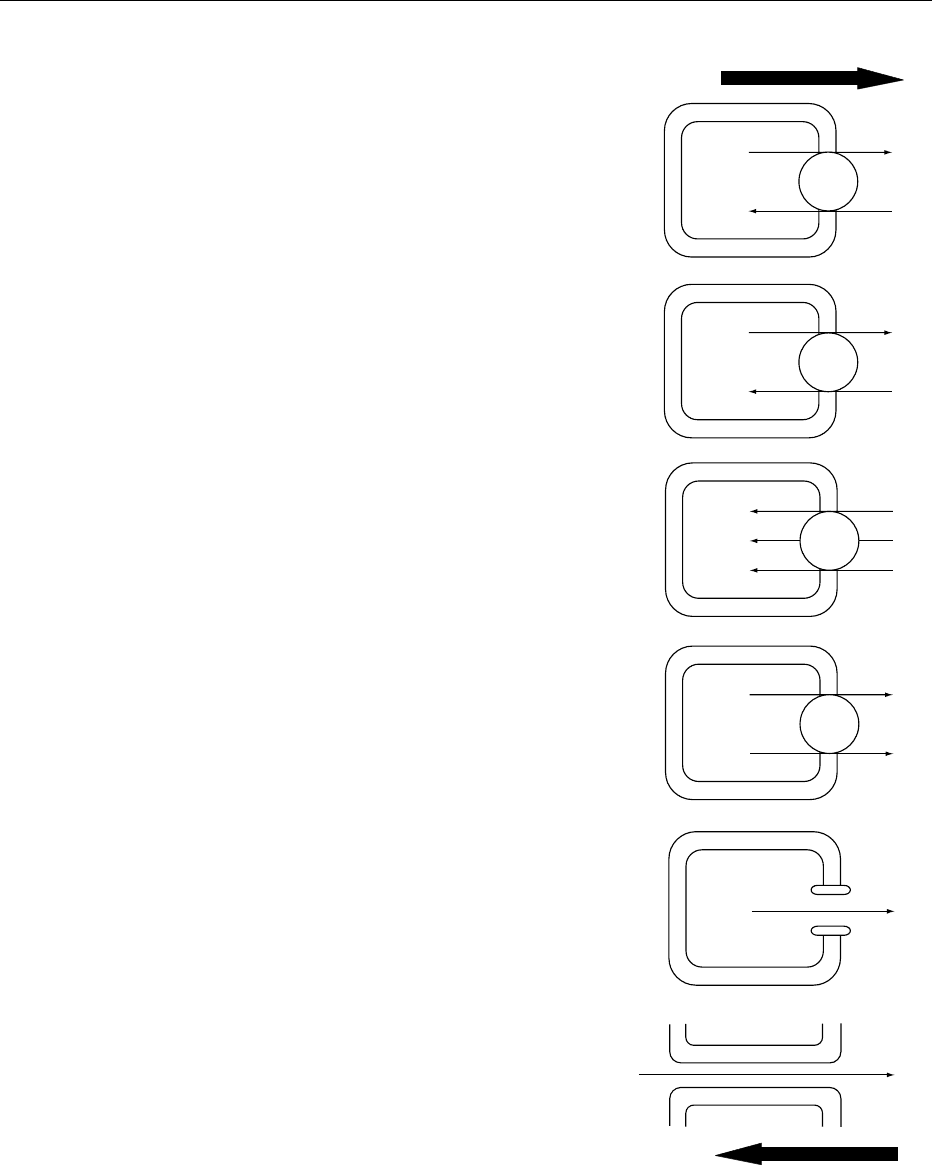
0021 Figure 1 schematically illustrates the intracellular
and paracellular mechanisms of potassium transport.
Different potassium transport mechanisms can oper-
ate simultaneously. They are usually interrelated and
are also linked to other ion transport systems. Each
name, except the last one, corresponds to an entire
family of transporters.
0022 Na
þ
/K
þ
-ATPase activity is responsible for the
maintenance of the extra- and intracellular Na
þ
and
K
þ
concentrations against electrochemical gradients.
This ATPase is found in the plasma membrane of
virtually all animal cells and represents an enormous
metabolic energy cost which increases the entropy of
the system. It is a carrier protein that pumps 2K
þ
in
and 3Na
þ
out of the cell during each cycle of con-
formational changes driven by adenosine triphos-
phate (ATP) hydrolysis. This process is electrogenic,
meaning that one net positive charge is removed from
the cell every pump cycle. Many hormones and vari-
ous neurotransmitters can modulate K
þ
/Na
þ
-ATPase
activity.
0023 Another active K
þ
transport is controlled by the
H
þ
/K
þ
-ATPase which ejects H
þ
in exchange for K
þ
.
This pump has an important role in some gastro-
intestinal cells and in the renal tubules.
0024 Other potassium transport systems are driven by
the force of ion gradients. The free energy gained
during the movement of an inorganic ion down an
electrochemical gradient (sum of the concentration
gradient and the electrical potential difference) is
used as the driving force to pump other solutes
against their electrochemical gradient. Thus, the car-
rier protein acts as a coupled transporter and electro-
neutrality is maintained. Cotransport depends on
ATP only indirectly. Various Na
þ
-K
þ
-Cl
cotranspor-
ters, which carry 1Na
þ
,1K
þ
and 2Cl
inside the
cell, have been identified in salivary glands, gastro-
intestinal tract, and renal tubules. The K
þ
-Cl
cotransporter, related to the former ones, plays an
important role in erythrocytic volume. The first
cotransport mediates K
þ
influx while the second
mediates efflux.
ATP
ATP
3Na
+
H
+
2K
+
K
+
K
+
K
+
K
+
K
+
2Cl
−
Cl
−
Na
+
Na
+
/K
+
-ATPase
H
+
/K
+
-ATPase
Na
+
-K
+
-Cl cotransport
K
+
-Cl cotransport
K
+
channels
Paracellular diffusion
Electrochemical gradients for Na
+
and Cl
−
Electrochemical gradient for K
+
fig0001Figure 1 Potassium transport via intracellular and paracellular
pathways. ATP, adenosine triphosphate.
POTASSIUM/Physiology 4653

0025 Passive transport of K
þ
occurs via intracellular and
paracellular pathways. The intracellular mechanism
involves K
þ
channels, which are pores in the plasma
membrane made by a specific protein. Channels have
‘gates’ which open and close in response to specific
stimuli: voltage, stretch, ATP, Ca
2þ
, hormones,
neurotransmitters, etc. Various stimuli sometimes
act together on a channel. K
þ
channels exhibit great
diversity and participate in many cell functions.
Distribution and Storage
0026 Body potassium concentration is 45–55 mmol kg
1
of
body weight, thus a 70-kg adult man contains ap-
proximately 135 g potassium. Women contain 35–
40 mmol kg
1
of body weight, and during childhood
and in the elderly, body potassium concentration is
also lower than that for young men. These differences
are due to the lower muscle mass in these groups.
Almost 98% of the potassium in the body is inside
the cells, making it the major intracellular cation.
More than 95% of total potassium is exchangeable.
0027 Total potassium is an index of lean body mass
because potassium is only present in the fat-free com-
partments of the body. It can be measured with K
40
,a
natural isotope present as a small fraction of the total
potassium, which emits g-rays and can be detected by
a sensitive whole-body counter, although other iso-
topes and other techniques that are not based on total
potassium are also available.
0028 Studies of body composition in healthy humans
have demonstrated that the total body potassium/
total body nitrogen ratio is constant. However, in
chronic protein-energy malnutrition this ratio can
decrease owing to the reduction of muscle proteins
and loss of intracellular potassium. The cellular ex-
change of sodium and potassium is altered, leading to
potassium loss and increased intracellular sodium
and water. In contrast, exercise and training increase
total lean body mass, thereby causing a rise in potas-
sium body content. Increase in muscular mass is only
possible if proteins, as well as potassium, phosphorus
and magnesium, which are the main intracellular
elements, are present in the diet in adequate amounts.
0029 In the extracellular fluid (ECF) an increases of potas-
sium equivalent to 1% of total body potassium may
double the concentration of potassium in plasma,
resulting in muscle hypopolarization. However, if
such an increase is stored in the cells, only minor intra-
cellular changes would result, with little change to the
potassium concentration difference across the cell
membranes. To accomplish this buffer function, the
major reservoir of potassium is the muscle, followed
by the liver and erythrocytes, although each cell pos-
sesses the capacity for accumulating potassium.
Excretion
0030About 90% of daily potassium intake is excreted
in the urine and 10% in the stool, although this
last percentage can be somewhat higher in cases of
diarrhea. When dietary potassium is severely re-
stricted, its fecal loss decreases to approximately
3.5 mmol day
1
. This presumably represents obliga-
tory potassium losses related to K
þ
digestive secre-
tions (salivary, gastric, biliary, and pancreatic), cell
desquamation, and mucus secretion. Potassium con-
centration in sweat is about 10 mmol l
1
, so loss
through perspiration is small, provided that the
climate and exercise conditions are not extreme.
Renal Potassium Handling
0031The kidney plays the main role in potassium excre-
tion. Renal potassium excretion shows a circadian
rhythm, characterized by peak output during the
subject’s activity.
0032K
þ
is freely filtered by the glomerulus. Usually,
urinary potassium excretion is 5–15% of the amount
filtered, which indicates the existence of tubular po-
tassium reabsorption. The renal tubules are capable
of reabsorbing and secreting potassium in response to
various stimuli.
0033Proximal tubule The proximal tubule is responsible
for the reabsorption of approximately 60% of previ-
ously filtered potassium. Solvent drag and diffusion
have been proposed as the major driving forces of this
process, and K
þ
is reabsorbed largely via the paracel-
lular pathway. However, some observations suggest
participation of a transcellular route of potassium re-
absorption by an active mechanism. The fractional
rates of K
þ
reabsorption are similar to those of sodium
and water along the proximal tubule and changes in
fluid and potassium transport are closely coupled.
0034In this region the basolateral K
þ
channels have
higher conductance than the apical ones, and this is
essential to maintain a negative intracellular potential
and hence promote reabsorption of positively
charged carriers (Na
þ
-glucose cotransport, etc.)
0035Loop of Henle The concentration of K
þ
increases as
the filtrate passes through the loop of Henle, where
permeability for potassium is very high. At the end of
the descending limb the amount of K
þ
present usually
exceeds that of the glomerular filtrate. There is a net
passive secretory entry of potassium into the prox-
imal straight tubule and the descending thin limb of
Henle, which arises from reabsorption in the collect-
ing tubule and partly in the ascending limb. These
pathways of K
þ
transport constitute potassium
recycling in the renal medulla.
4654 POTASSIUM/Physiology
