Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


not. As discussed in detail later, dietary purines are
degraded by a battery of enzymes in the intestinal
mucosa and enter the circulation as uric acid. By
contrast, dietary pyrimidines can be absorbed in the
nucleoside form and incorporated by salvage into the
nucleotide pool, as evidenced by the lifelong treat-
ment of patients with the pyrimidine de-novo salvage
defect, hereditary oroticaciduria, with oral uridine.
Nucleotides in infants
0006 Over the past two decades, there has been consider-
able interest in the role of dietary ribonucleotides
in infant feeds, in which significant effects have
been claimed. This debate stemmed from reports
that ribonucleotides were present in human milk,
but not in cows’ milk or infant formulas. However,
claims that nucleotide concentrations were much
lower in cows’ than in human milk were apparently
related to the state of lactation, with concentrations
higher during lactation. Infant formulas were con-
firmed as being devoid of nucleic acid.
0007 These findings stimulated investigations to deter-
mine: (1) whether the nucleotides found in human
milk result from degradation of nucleic acids, or are
actively secreted as a response to a nutritional
demand of the infant, and (2) the newborn infant’s
endogenous capacity to digest nucleic acids to ab-
sorbable products. Nucleotides were found in the
greatest quantity in human milk followed by nucleic
acids, nucleosides being a minor component. Import-
antly, the nucleotide/nucleosides were predominantly
pyrimidines, purines being present only as uric acid.
This specific profile must result from catalysis in
the breast. Enzymes capable of degrading nucleo-
tides, including xanthine dehydrogenase (XDH), are
known to be present in human milk. Evaluation of the
endogenous capability of infants to metabolize RNA
and nucleotides confirmed that the intestine of
infants, as in adults, digested RNA to cytidine, uri-
dine, and uric acid in vitro. This study confirmed the
extensive data derived from dietary purine loading in
adults that dietary purine nucleotides (if not already
degraded by gut bacteria) would be degraded to uric
acid in the intestinal mucosa. However, uridine and
cytidine would be absorbed (although the latter
would be degraded rapidly to uridine by cells in vivo).
0008 This research into the importance of human versus
cows’ milk, or formula feeding, was stimulated by
animal studies that had indicated that dietary nucle-
otides may be required for maintenance of normal
immune function in neonatal mice. A subsequent
study in healthy term infants demonstrated that in
those either breast-fed, or fed formula supplemented
with nucleotides, some indices of immune function
were indeed significantly higher (natural killer cell
cytotoxicity and interleukin-2 production by stimu-
lated mononuclear cells) compared with nonsupple-
mented formula groups. However, this was found
only in the newborn. The rate of growth and inci-
dence and severity of infections did not differ
significantly among these different dietary groups at
2 months. It was concluded that nucleotides may be a
component of human milk that contributes to the
enhanced immunity of the breast-fed infant. Another
study implied that allergic diseases develop during
feeding of cows’ milk, but not human milk. It may
be that the dearth of uridine nucleotides in cows’ milk
after 1 month of lactation could be implicated, since
these effects were independent of any difference in the
gut flora demonstrated in breast-fed, as distinct from
formula-fed, infants.
0009This debate and the problems addressed arose from
the use of animals. As indicated elsewhere in this
chapter, rodents have both XDH and uridine phos-
phorylase everywhere. By contrast, in humans, uri-
dine phosphorylase is confined to the liver, and XDH
is confined to the liver, intestinal mucosa, and breast
milk. This problem highlights the general lack of
awareness of the two curious and unexplained differ-
ences in the synthesis of nucleotides in humans. First,
pyrimidines derived from nucleotide degradation are
salvaged at the nucleoside (uridine) level, purines as
the base. Second, whereas purine nucleotides are de-
rived exclusively from endogenous sources in
humans, pyrimidine nucleotides can be formed from
uridine orcytidine ingested in the diet as well. These
important differences are underlined by the successful
treatment with oral uridine of patients with uridine
monophosphate synthase (UMPS) deficiency, which
presents generally as macrocytic anemia. A few
patients are also immunodeficient. UMPS is the
complex catalyzing the last two steps of pyrimidine
de-novo synthetic (DNS). Long-term follow-up of
such patients confirms that humans cannot only
survive without pyrimidine biosynthesis, but also
reproduce. Thus, uridine certainly can restore
immune function in humans as well.
Role of Endogenous Nucleotides, Nucleosides, and
Bases in Cellular Metabolism
0010Purines and pyrimidines are effectively anchored
inside the cell as the nucleotide by attachment to a
pentose linked to a mono-, di- or triphosphate group
(Figure 1a and 1b), principally the triphosphate. It
was originally assumed that all reactions of biological
significance took place intracellularly at the nucleo-
tide level. Attention has been focused recently on the
extracellular regulatory functions of purine nucleo-
sides (base plus pentose), or the bases themselves. The
pentose may be either ribose (ribonucleoside) or
4154 NUCLEIC ACIDS/Physiology

2
0
-deoxyribose (deoxyribonucleoside) bound by the
C1 atom through a glycosidic linkage to the N9
atom of the purine group, or to the N2 of the pyrimi-
dine group (Figure 1).
0011 The importance of purine and pyrimidine nucleo-
tides in cellular metabolism is twofold. In addition to
their role in the storage, transmission and translation
of genetic information (as the polynucleotides DNA
and RNA), as mononucleotides, they play an equally
vital role in lipid and membrane synthesis (in the
form of purine and pyrimidine sugars or lipids),
signal transduction, and translation (in the form of
guanosine triphosphate, cyclic adenosine monopho-
sphate, and cyclic guanosine monophosphate), as
well as providing the energy (adenosine triphos-
phate (ATP)) that drives many cellular reactions
and forms the basis of the coenzymes (nicotinamide
adenine dinucleotide, nicotinamide adenine dinucleo-
tide phosphate, flavin adenine dinucleotide, etc.) (See
Coenzymes.)
0012 All cells require a balanced supply of purine and
pyrimidine nucleotides for growth and survival, but
this may vary from cell to cell, depending on function.
Liver, for example, has a very complex nucleotide
profile, compared with heart. These nucleotides may
be built up by one of two routes: the energetically
expensive multistep synthetic route, or the single-step
so-called ‘salvage’ pathway. In normal circumstances,
salvage predominates over synthesis. Figure 2 illus-
trates the different metabolic pathways involved in
the de-novo synthesis of these nucleotides, as well as
the efficient recycling of the nucleotides or bases de-
rived from them during the wear and tear of daily life
(muscle work, wound healing, erythrocyte senes-
cence, protein glycosylation, providing essential
nourishment for the brain, etc.). Interestingly, whilst
this recycling takes place at the base level for purines,
it is the pyrimidine nucleosides that are actively re-
cycled in humans, with only a small proportion being
degraded further in either case (Figure 2). The import-
ance of these different pathways to the overall control
of nucleotide concentrations in the body is the subject
of many excellent reviews and for this reason is sum-
marized only briefly here.
Nucleotide Triphosphate Production and Nucleic
Acid Synthesis
0013 Purine and pyrimidine ribomononucleotides built
up by either the de-novo or synthetic routes are
phosphorylated via the correponding diphosphate to
the triphosphate, which is the active intracellular
form of most mononucleotides. In addition to the
variety of vital individual cellular functions described
above, these ribomononucleotides are essential inter-
mediates in the synthesis of the polynucleotides RNA
and DNA respectively (Figure 2), being incorporated
into DNA following the formation of the deoxyribo-
nucleotide from the corresponding diphosphate by
the enzyme ribonucleotide reductase. The latter is an
allosteric enzyme; its activity and specificity are con-
trolled in a complex manner by both purine and
pyrimidine ribo and deoxyribonucleotides. This pro-
cess is particularly active in cells and tissues with a
high rate of turnover (e.g., lymphocytes, gut epithe-
lium, skin, bone marrow, etc.). There is approxi-
mately five times as much RNA and DNA in the body.
Breakdown of Nucleic Acids
0014The polynucleotides DNA and RNA, although rela-
tively stable in most tissues, turn over rapidly in
dividing cells. Both DNA and RNA first must be
degraded to the constituent mononucleotides, which
are themselves degraded further. A variety of enzymes
capable of hydrolyzing the phosphodiester bonds
have been described and include ribonucleases spe-
cific for RNA and deoxyribonucleases for DNA as
well as nonspecific nucleases, phosphorylases and
phosphomonoesterases. The mononucleotides have
the highest turnover rate, DNA the lowest. Further
catabolism of the resulting monophosphate will differ
depending on whether it is a pyrimidine or purine
ribonucleotide of deoxyribonucleotide. For example,
whereas adenine ribonucleotides are predominantly
deaminated at the nucleotide level in humans by
AMP-deaminase, deoxy-AMP is not a substrate for
this enzyme and must first be degraded to deoxyade-
nosine and deaminated at the deoxynucleoside level
(Figure 2).
0015Purine and pyrimidine (deoxy) nucleotides are de-
graded to the corresponding (deoxy) nucleosides by
specific 5
0
nucleotidases. Different purine endo- or
ecto-5
0
nucleotidases have been identified with
different substrate specificities and may be of particu-
lar importance in providing bases for nucleotide
resynthesis in tissues where there is rapid cell turnover
and massive cell death (e.g., thymus, spleen, bone
marrow). As mentioned above, whereas the normal
metabolic route for pyrimidines is salvage at the nu-
cleoside level, that for purine nucleosides and deoxy-
nucleosides is degradation to the corresponding base
by purine nucleoside phosphorylase, prior to salvage.
This degradation is favored by the high intracellular
inorganic phosphate and low ribose 1-phosphate
levels in most tissues. Interestingly, these phosphory-
lases are not reactive toward either adenosine or
cytidine, or their analogs, in human cells and first
must be deaminated at the (deoxy) nucleoside (or
nucleotide) level.
0016Salvage is an active process for both pyrimidines
and purines. Consequently, only a small fraction of
NUCLEIC ACIDS/Physiology 4155
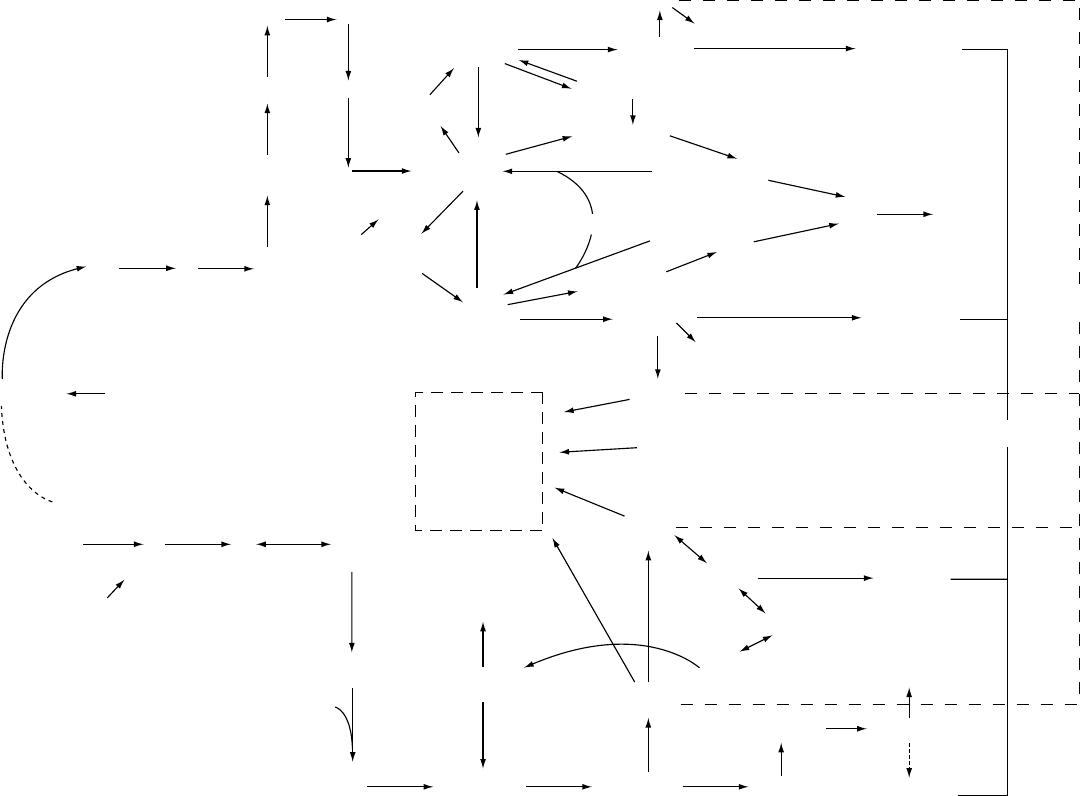
GDP-sugars
dCDP-lipids
CDP-lipids
UDP-sugars
UMP UDP dUDP dTTP
dTMPdUMP
cytidine
CMP
thymidine
dCTPCDP
CTP
d-cytidine
GTP
GDP
guanosine
guanine
GMP
XMP
IMPDH
p53
cGMP
dGTP
DNA
uridine
PPribP
PPribP
PPribP
ribose-5-p
+ ATP
p53
CPS II
1
1
2
2
3
3
4
4
5
6
8
9
10
AMPS
AMP
IMP
inosine
hypoxanthine
xanthine uric acid
adenosine
ADP
ATP
cAMP
dATP
7
5
6
HCO
3
+
ATP
UTP
uracil
Pyrimidine de novo synthesis Purine de novo synthesis
RNA
−
Figure 2 Multistep purine and pyrimidine nucleotide de novo synthetic (DNS) routes and single-step salvage (hypoxanthine, guanine, uridine, cytidine, D-cytidine) pathways, indicating the
enzymes inosine monophosphate dehydrogenase (IMPDH) and carbamoyl phosphate synthetase 11 (CPS 11) targeted by analogs which deplete nucleotide pools, reportedly associated with
a p53-dependent Go/G1 arrest. Note the involvement of 5-phosphoribosyl pyrophosphate (PPribP) in both purine DNS and salvage as well as in pyrimidine DNS. The role of ATP, GTP, UTP,
and CTP in DNA and RNA synthesis, of ATP and GTP in 2nd messenger synthesis, of UTP, CTP, and GTP in the synthesis of the glycoconjugates (dashed box) essential for membrane
synthesis, structure, and function and protein glycosylation, is also evident.
fig0002

the nucleotides turned over daily is actually degraded
and lost to the body. The pyrimidine bases, uracil and
thymine, derived from nucleosides not recycled are
degraded further to the b-amino acids, and there is
thus no measurable end product. However, such loss
is probably comparable with that for purines, the
normal end product of which in humans is uric acid,
formed from the precursor purine bases xanthine and
hypoxanthine by the action of xanthine dehydrogen-
ase (Figure 2).
Rates of Nucleotide Synthesis and Degradation in
Different Cells
0017 It has become apparent that the original concept of
endogenous metabolism and its overall control, in-
volving a complex interplay between de-novo synthe-
sis and salvage, does not apply to all cells but is
governed by a tissue- or cell-specific complement of
enzymes and/or controls on them, depending on the
function of that cell or tissue. The human erythrocyte,
for instance, is anucleate and lacks the ability to use
either salvage or de-novo synthesis to maintain its
ATP levels, being dependent on adenosine scavenged
from other tissues for this. In addition, the pyrimidine
nucleotides found in nucleated cells are absent from
mature erythrocytes, the only pyrimidines normally
present being in the form of uridine diphosphate
(UDP) sugars. ATP is also the most important purine
in both skeletal and heart muscle, adenine nucleotides
making up 95 and 90% of the total nucleotide com-
plement, respectively. Although DNA in most tissues
is considered relatively stable, it is evident from the
two inherited disorders associated with immunodefi-
ciency that cell death and rapid turnover of cells of
the hemopoietic system (e.g., extrusion of the nucleus
during erythrocyte maturation, or mounting an
immune response) normally produces significant
amounts of deoxyribonucleosides, as well as ribonu-
cleosides, which must be degraded further. These
disorders have highlighted the fact that removal of
metabolic waste from DNA catabolism is vital to the
normal immune response; failure to do so can result
in the accumulation of deoxy-ATP and deoxy-GTP.
These deoxynucleotide triphosphates are extremely
toxic to T-lineage stem cells, resulting in severe
combined immunodeficiency affecting both T- and
B-cells in subjects deficient in adenosine deaminase,
or a T-cell-specific immunodeficiency, in purine nu-
cleoside phosphorylase deficiency (Figure 2).
Endogenous Nucleic Acid Synthesis in the Gut
0018 It was originally reported from studies in guinea-pigs
that the gastrointestinal tract was incapable of
de-novo synthesis. However, subsequent workers
have shown significant synthetic activity in rat intes-
tine, as evaluated by the incorporation of radio-
labeled glycine into RNA. The nucleic acid content
of intestinal mucosa is high, as is the rate of cell
turnover in the luminal villi, and it has been calcu-
lated in rat that about 30 mg of endogenous nucleic
acid enters the lumen daily. This implies a consider-
able loss of both purines and pyrimidines, which can
only be replaced by de-novo synthesis. Studies of
nucleotide concentrations in rat intestine have shown
both pyrimidine and purine nucleotides at concentra-
tions equivalent to those in liver, supporting active
nucleotide metabolism in the intestine.
0019Intestinal absorption is obviously related to intes-
tinal motility and local blood flow. The presence of
adenosine receptors on rat jejunal mucosal cells may
be particularly important in the regulation of fluid
and electrolyte absorption as well as the active trans-
port of nutrients. Such adenosine may be generated
slowly from nucleotides by nucleotidases physically
or functionally coupled to the adenosine membrane
translocator.
0020It is evident from the above that the pathways
leading to the formation and degradation of nucleic
acids are complex, and that many factors determine
the origin as well as amount of endogenous pyrimi-
dine or purine nucleotides turned over daily. More-
over, this will differ depending on the cell or tissue.
Role and Fate of Dietary Nucleic Acids
0021The metabolism of nucleic acids ingested in the diet
difers from that of endogenous nucleic acids. The
intestinal mucosa plays an important role in this.
The above studies confirm that pyrimidine as well as
purine nucleotides in intestinal mucosal cells are de-
rived from endogenous sources. However, a growing
body of evidence supports a role for dietary nucleo-
tides derived from the gut in intestinal development,
turnover, and repair. Suggested effects include en-
hancement of the host mucosal defense system and
an influence on neonatal lipid metabolism and on
iron bioavailability, implying a novel role for nutri-
tion in the modulation of gut function.
0022Most of our knowledge relating to dietary nucleic
acid metabolism in the intestine is derived from studies
of the absorption of exogenous purine in different
species – mouse, dog, rat, pig, etc., as well as humans
– which date back as far as the latter half of the
nineteenth century. Investigations in animals have
shown that whereas the ribose attached to nucleosides
derived from RNA was further metabolized, the
phosphate was absorbed and excreted in the urine.
0023Recent studies using
13
C-labeled nucleic acid to
supplement the diets of rats and chickens have
NUCLEIC ACIDS/Physiology 4157

provided further evidence for the incorporation of
dietary pyrimidine nucleosides, but not purine
nucleosides, directly into hepatic RNA. The success-
ful lifelong treatment with uridine of patients with
hereditary oroticaciduria – a defect in pyrimidine
de-novo synthesis – confirms that dietary uridine is
certainly absorbed and salvaged into mono- as well as
polynucleotides in humans. The lack of effect of oral
uracil in this disorder also confirms that uracil is a
metabolic waste and that pyrimidine salvage occurs
at the nucleoside level in humans.
0024 Recent investigations have addressed the sup-
posedly beneficial effects of dietary CDP-choline
(citicholine) in patients with stroke or trauma, theor-
etically by increasing acetylcholine production in
cholinergic neurons, as well as the amount of cell
membrane. These studies confirmed that, when
taken orally, CDP-choline elevates plasma levels of
both choline and uridine (not cytidine) in humans,
but no benefit was demonstrated in clinical trials.
Metabolism of Dietary Purines by Gut Bacteria
0025 Animal studies have shown that the metabolism of
dietary nucleic acid and the corresponding purine
nucleotides, nucleosides, and bases in the gut is
rapid. Isotope studies demonstrated that up to 50%
of the radiolabeled purine is recovered as carbon
dioxide within 30 min. The role of gut bacteria in
this process was indicated by experiments using the
XDH inhibitor, allopurinol, concomitantly, when the
radiolabel was recovered in toto (in urine and feces
only), presumably relating to inhibition of bacterial
XDH. Allopurinol not only inhibited purine degrad-
ation but also decreased the absorption of dietary
purine, a seemingly beneficial effect that may explain
the reduction in total purine excretion noted in
human subjects on a normal diet taking allopurinol.
Intestinal Mucosa Degrades Dietary Purines to Uric
Acid
0026 Interestingly, the above radiolabeling studies in pigs
(where, as in humans, XDH activity is significant
only in intestinal mucosa and liver), confirmed a
lack of any radiolabel incorporation from dietary
purine into tissue nucleotides. These studies demon-
strated conclusively that dietary purine is degraded to
a nonreutilizable form by the intestinal mucosa. Stud-
ies in other animal species have confirmed this, purine
nucleotides, nucleosides, and bases absorbed from the
gut lumen being largely converted to uric acid during
passage across the mucosa and released as such in
serosal secretions, prior to further degradation by
urate oxidase (uricase) in the liver. Thus, in contrast
to pyrimidines, humans have no apparent require-
ment for dietary purines. The intestine thus serves as
an effective barrier through the activity of a battery of
enzymes capable of rapidly degrading purines already
partly processed by gut bacteria to the nonreutilizable
metabolic waste, uric acid. Such rapid degradation of
purines by the gut presumably reflects an important
evolutionary development to protect the integrity of
the human genome. The uric acid produced daily in
humans thus derives from two sources: catabolism of
exogenous as well as endogenous mono and polynu-
cleotides (Figure 3).
Excess Dietary Levels of Nucleic Acids
and their Clinical Consequences
0027As mentioned above, pyrimidines have no measurable
end product. The potential toxicity of dietary nucleic
acids thus relates predominantly to a single muta-
tional event that has resulted in the fact that the insol-
uble uric acid, and not the much more soluble
allantoin as in other mammalian species, is the only
measurable end product of nucleic acid degradation
in humans. Although it had long been accepted that
the enzyme uricase had been lost in the course of
human evolution, more recent studies have estab-
lished that the absence of uricase activity results
from a lack of gene transcription, rather than loss of
the gene itself. However, other factors play an equally
important role in determining the pathogenesis of
nucleic acids ingested in the diet.
Role of Exogenous Purine in Determining
Circulating Uric Acid Concentrations
0028The ingestion of food now known to be rich in
purines has been noted for millennia to be high
in subjects with what has been designated ‘primary’
gout, the gout affecting predominantly the middle-
aged male. This type of gout is a disorder of affluent
societies consuming diets rich in purines; it is rare
in women or children. During times of hardship,
uric acid concentrations in the population fell consid-
erably, and ‘primary’ gout almost vanished. The fact
that gout was extremely prevalent among wealthy
Englishmen for more than three centuries up to
World War I is not surprising when the dietary habits
of the day are examined. These affluent gentlemen
habitually consumed vast meals comprising many
courses and frequently including 16 different meats,
the majority of which were rich in nucleic acids and
other purines.
Type of Nucleic Acid and Toxicity
0029Detailed studies of dietary nucleic acid absorption in
humans were carried out by Zo
¨
llner and coworkers
in the late twentieth century and confirmed that
nucleic acids ingested in the diet exert their major
4158 NUCLEIC ACIDS/Physiology
effect on uric acid levels. These studies showed that
the nature of the nucleic acid ingested is equally im-
portant, with the effect of RNA being more than
twice that of DNA (Figure 4a). This effect is also
evident whether the purine is ingested in the form of
nucleic acids, mononucleotides, nucleosides, or bases.
Moreover, some forms of purine, e.g., guanosine – the
principal RNA degradation product in yeast-rich
beverages such as beer (especially real ale) – are
absorbed and catabolized more readily than others.
A controlled study of diet in primary gout patients
compared with control males of comparable age dem-
onstrated that the average daily intake of most
nutrients, including purine nitrogen, was similar.
However, gouty patients drank significantly more
alcohol, predominantly in the form of beer (60 g per
day, equivalent to 2.5 l of beer) and had a significantly
higher mean plasma uric acid (0.49 mmol l
1
com-
pared with 0.39 mmol l
1
).
Role of the Kidney or Drugs in the Genesis of
Toxicity
0030 The pathological changes that result from uric acid
being the exclusive end product of purine metabolism
in humans are due entirely to the insolubility of this
metabolic waste, coupled with the other peculiarity
that primates display, i.e., the renal tubule reabsorbs
around 90% of the filtered urate (Figure 3). Net
reabsorption is slightly higher in normal males
(92%) than in females (88%) and is lower in children
of either sex (80–85%). This explains the higher
plasma uric acid in adult males and makes them
more vulnerable to situations of overproduction,
or overingestion. Clearly, sex and age are equally
important contributory factors.
0031It is noteworthy that when normal subjects are fed
yeast RNA, the plasma urate rises little with increased
intake, the increase in the excretion of urate being
dramatic when the rise in plasma urate is modest
(Figure 4b). Thus, neither overingestion nor overpro-
duction (unless sudden and massive) is likely to raise
the concentration of uric acid in the plasma consider-
ably, if the renal response is normal. It is obviously
not so in the majority of gouty males with primary
gout, where the striking change is that, for any
plasma urate concentration, the excretion of urate is
consistently less than in normal subjects. This defect
in handling by the kidneys relates to a greatly reduced
Filtration
Body wear
and tear
Body
purine
synthesis
Plasma
uric acid
Excretion
Purines
in diet
Reabsorption
Secretion
8−18%
2
3
1
3
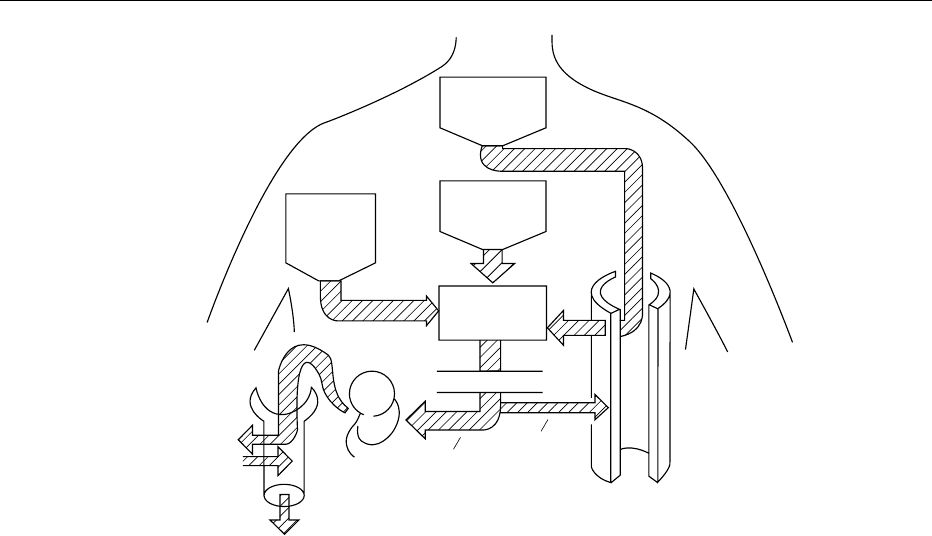
fig0003 Figure 3 Schematic diagram showing the factors influencing the plasma uric acid, the end product of endogenous (body synthesis
plus daily wear and tear) and exogenous (dietary) purine metabolism in humans. The different mechanisms by which this metabolic
waste is eliminated (two-thirds by the kidney, one-third by the gut) in normal individuals are also shown. At the bottom left is a
simplified version of the complex factors (involving filtration, reabsorption and secretion) interacting in the proximal tubule of the
human kidney and resulting in the urinary excretion of only 8–18% of the filtered load depending on age and sex. Reproduced from
Nucleic Acids: Physiology, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds),
1993, Academic Press.
NUCLEIC ACIDS/Physiology 4159
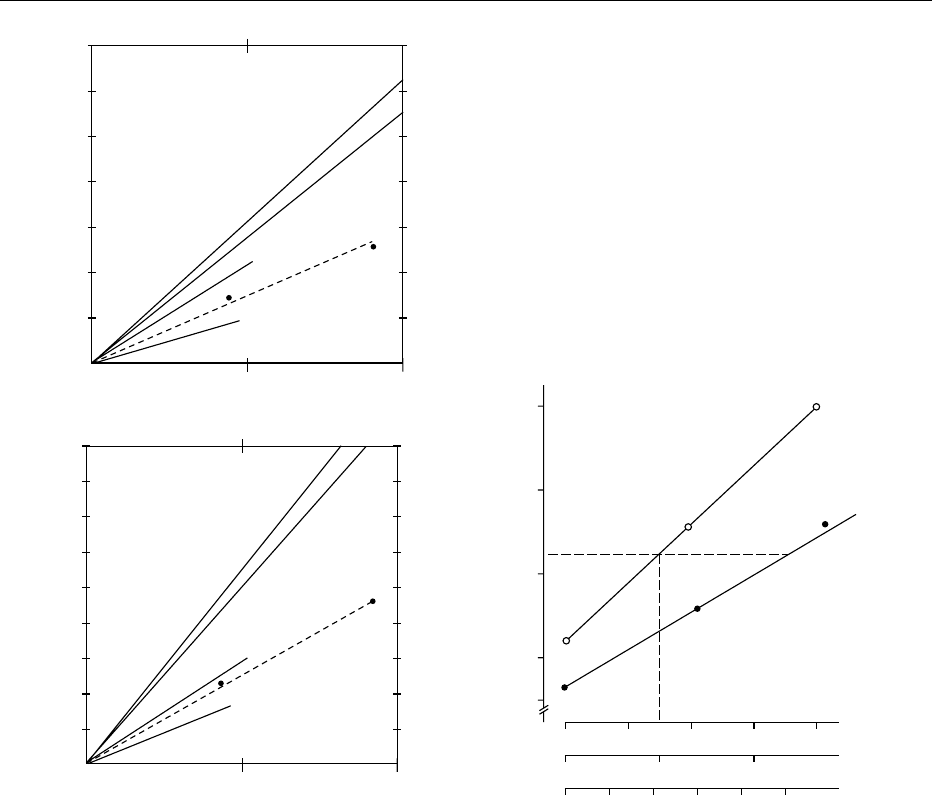
clearance of uric acid relative to the glomerular filtra-
tion rate (FE
UR
); FE
UR
is the fractional excretion of
uric acid. The mean is 5.4% in gouty males compared
with 8.1% for healthy males of comparable mean age
(52 years).
0032 The exact nature of this difference is not yet
clear, since studies must be reevaluated in the light
of recent knowledge of how complex the renal
tubular handling of urate is. However, there is no
doubt that it involves the transport of uric acid,
which occurs predominantly in the proximal tubule,
is bidirectional, and has both a secretory and
reabsorptive component (Figure 3). Current opinion
suggests that the majority of gouty patients under-
excrete urate because of a defect in tubular secretion.
Confirmation must await identification at the
molecular level of the different transporters involved
in urate reabsorption and secretion. Clearly, a com-
bination of events is needed to produce hyperurice-
mia and the clinical syndrome of gout. These are a
large intake of readily absorbed purine coupled with a
defect in the renal handling of uric acid at the kidney
level, which means that the kidney cannot respond
to a purine load without an abnormal rise in
plasma urate concentration. (See Renal Function
and Disorders: Kidney: Structure and Function.)
0033However, diet alone may not be the only culprit.
Numerous other physiological and pathological
agents such as lead are also capable of reducing
urate excretion, and hence exacerbate the rise in
7
6
5
4
3
Plasma uric acid rise (mg per day)
Purine derivate (mmol per 70 kg bodyweight)
Purine derivate (mmol per 70 kg bodyweight)
Plasma uric acid (mg per 100 ml)
2
1
4
8
AMP
GMP
Hypoxanthine
RNA
DNA
Increase of urinary uric acid
excretion (mg per day)
200
400
600
800
4
8
AMP
GMP
Hypoxanthine
RNA
DNA
0
12
0.5(b)(a)
0.5
1.0
34
3
4
6
8
10
RNA (g)
Uric acid (g)
Purine N (g)
y = 3.25 + 0.9 x
y = 4.4 + 1.46 x
fig0004 Figure 4 (a) Increase in plasma and urine uric acid (mg per day) in response to a purine load from the different sources indicated
on a molar basis. (b) Influence of graded additions of RNA to a purine-free formula diet on plasma uric acid levels of either
normouricemic (-
.
-) or hyperuricemic (-
s
-) individuals (some of whom had a positive family history of gout). Reproduced from Nucleic
Acids: Physiology, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and Sadler MJ (eds), 1993,
Academic Press.
4160 NUCLEIC ACIDS/Physiology
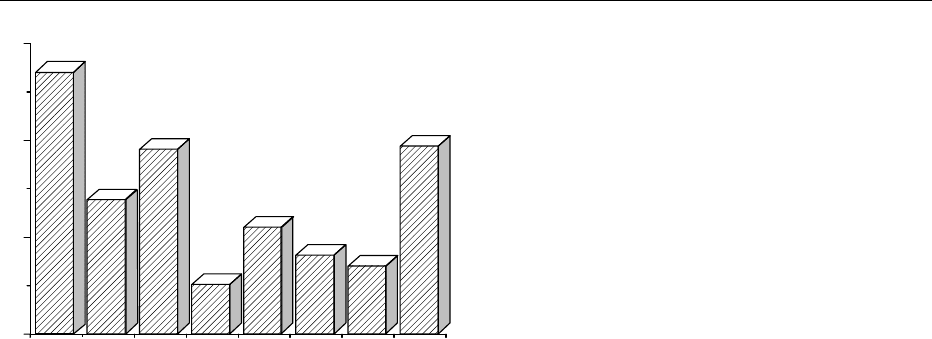
plasma urate caused by diet. Any of these may lead to
an acute attack of gout in a susceptible individual
whose plasma urate is already elevated. The best
known of the physiological substances are organic
acids such as lactate; their overproduction may ex-
plain in part the hyperuricemia associated with exces-
sive alcohol consumption coupled with inadequate
food intake. Diuretics cause plasma volume contrac-
tion, and fractional urate reabsorption increases
under these circumstances. The appearance of gout
in patients treated with hypotensive agents over long
periods currently accounts for over 50% of new pre-
sentations in gout clinics. Unusually, the number of
elderly females in this group is high, and the mode of
clinical presentation is frequently atypical.
0034 Diet may also be important in drawing attention to
an unusual subset of patients with juvenile onset of
gout; a disorder affecting young males and, unusually,
young females and children of either sex equally.
Unlike primary gout, where renal function is normal
for age, unrecognized, this dominantly inherited dis-
order is associated with progressive renal disease.
Considerable variation in presentation has existed,
and there has been no consensus as to whether the
gout or the renal disease is the primary factor. How-
ever, it is now evident that there are two hallmarks for
this disease: the first is hyperuricemia disproportion-
ate to the degree of renal dysfunction; the second is
that the degree of renal hypoexcretion of urate is
generally extreme, which explains the associated ten-
dency to gout. Moreover, the mean FE
UR
in this group
is only 5.1%, irrespective of age or sex: lower than
that of the middle-aged gouty male (5.4%), and even
more remarkable considering the high percentage of
children and young women in this group. This univer-
sally low clearance explains the added susceptibility
to dietary purine leading to the isolated attack of gout
in children as well as young adults, which has drawn
attention to families with ‘familial renal disease,’
hitherto leading to death in the 30s. Recognition of
the correct nature of the familial disorder has under-
lined the need to measure urate clearance in all
kindred members and enabled early diagnosis and
treatment of 42% of seemingly healthy children.
0035This is important because long-term follow-up
(>20 years) in these patients treated with plasma
uric acid lowering agents has shown that early recog-
nition and treatment ameliorates the progression of
the renal disease. These studies implicate uric acid in
the etiology of the renal disease, but this has been
disputed by others. However, very recent studies sug-
gest that a reappraisal of the pathogenesis and
consequences of hyperuricemia in hypertension, car-
diovascular disease, and renal disease is warranted,
which adds credence to the above hypothesis.
Role of Dietary Nucleic Acid in the Genesis of
Urolithiasis
0036Although overingestion of purines by subjects with a
normal renal response does not precipitate gout, it
can predispose to uric acid lithiasis. Uric acid stones
are relatively common in Australasia and similar
countries, where the consumption of purine-rich bev-
erages and food (e.g., beer, seafood, and meat) is high,
and in individuals addicted to health foods such as
yeast tablets. Vitamin C is also uricosuric, and acute
uric acid nephropathy and sometimes urolithiasis
have also been reported following therapy with a
variety of uricosuric drugs. Perhaps less well recog-
nized is the uricosuric effect of a high-protein diet and
the fact that the intake of purine-rich foods also pre-
disposes to calcium stone formation.
0037Some foods rich in nucleic acid – vegetarian diets
consisting of pulses and grains and yeast extracts –
have a particularly high adenine content. Although
this is not a problem in the normal population, such
diets can be potentially lethal to a rare group of
subjects with a genetic defect leading to the inabi-
lity to recycle adenine, overexcretion of adenine,
and the even more insoluble uric acid analog
2,8-dihydroxyadenine (2,8-DHA). Patients with this
defect generally present in childhood with kidney
stones composed of 2,8-dihydroxyadenine. One
such case from a commune consuming a macrobiotic
diet presented in acute renal failure with severe renal
damage, progressing to dialysis and transplantation.
Diet can thus be an important precipitating factor in
the pathogenesis of inherited disorders associated
% males with asymptomatic hyperuricemia
Maori NZ France Japan USA Finland UK Germany
0
10
20
30
fig0005 Figure 5 Percentage of Caucasian males, compared with a
Polynesian race (the Maori), with asymptomatic hyperuricemia
(defined as a plasma uric acid in excess of 0.42 mmol l
1
,or
7.0 mg dl
1
) in four EU countries, North America, Japan, and
New Zealand (NZ).
NUCLEIC ACIDS/Physiology 4161

with the overexcretion of insoluble purines. (See
Macrobiotic Diets; Vegetarian Diets.)
Reducing Dietary Levels of Nucleic Acids
0038 Diet varies considerably in different countries and
must be taken into account when investigating pa-
tients for suspected disorders of purine metabolism.
Normal ranges for uric acid in plasma and urine differ
greatly in the healthy population depending on
the country (Figure 5). For example (and in contrast
to the nineteenth century), until recently, the majority
of subjects in the UK ingested a low purine diet con-
sisting of one meat meal a day, and urinary uric acid
excretion above 3.5 mmol per day was considered
abnormal. By contrast, the upper limit of normal in
Australia has been given as 7.00 mmol per day. How-
ever, the increasing affluence of some societies is now
leading to an increase in gout, not only in adult Cau-
casian males, but also in countries such as Japan,
where gout was once rare.
Turnover of Exogenous and Endogenous Uric Acid
0039 A useful guide to the dietary purine consumption in
different countries can be obtained from the percent-
age of males with asymptomatic hyperuricaemia.
Using this yardstick, the highest consumption in the
EU 20 years ago was in France (lovers of pa
ˆ
te
´
and
seafoods), the lowest in the UK (Figure 5). The statis-
tics for Germany were from Bavaria where, as in New
Zealand and Australia, beer consumption is high.
Although similar statistics for diet-related differences
are unavailable today, plasma uric acid will have risen
along with the increase in obesity and blood pressure,
especially in the UK. Race also plays a part, and
Polynesians have a genetically low urate clearance
compared with Caucasians, as exemplified by the
New Zealand Maori (FE
UR
4.9% in normouricaemic
males, 3.9% in asymptomatic hyperuricemic males).
0040 As we have seen, the body pool of urate, and hence
the plasma urate concentration, is the result of a
balance between production, ingestion, and excre-
tion. The method for assessing the contribution of
diet is to evaluate de-novo production of purines by
placing the subject on a purine-free diet for 5–7 days
and measuring the urinary excretion of urate, which
will equal endogenous production. In this way, less
than 1–5% of subjects in any country have been
found to excrete abnormally large amounts of urate
(>3 mmol per day). In such rare cases, an underlying
genetic metabolic defect can be generally demon-
strated in which the normal feedback controls on
de-novo nucleotide synthesis and thus endogenous
purine production are overridden, resulting in gross
uric acid overproduction. Two such defects – both of
which are X-linked and generally present in child-
hood, or adolescence, but sometimes neonatally –
have been identified.
Purine Content of Foods
0041A knowledge of the purine content of specific foods
is essential if dietary effects are to be reduced to a
minimum. Until recently, such data have been diffi-
cult to find but are now available on the web. Most
tables give only purine nitrogen, which, as demons-
trated by the purine loading studies mentioned earlier,
is not always a good guide because of the variation in
absorption. Pa
ˆ
te
´
is a particular culprit, as is most
offal and organ meat (liver, kidney, heart, brains,
sweetbreads), game (venison, pheasant, partridge,
grouse), and the nucleic-acid-rich fish and seafoods
– herring, kippers, sardines, smelts, sprats, anchovies,
salmon, trout, mackerel, crustaceans (crab, lobster,
prawns), shellfish (scallops, mussels), and caviar or
roe. Purine nitrogen varies and ranges from 50 mg per
100 g in beef steak to 234 mg per 100 g in sardines.
Many fresh vegetables, e.g., spinach, peas, beans,
lentils, mushrooms, asparagus, and cauliflower, also
have a considerable purine content, as have soya and
other pulses and grains (porridge and oats, wheat and
rye cereals). All meat extracts (Bovril, Oxo) or yeast
extracts (Barmene, Tastex) are very rich in purine.
0042However, many studies have established that
humans addicted to diets rich in nucleic acids are
generally very reluctant to alter their dietary habits
despite the strongest advice to do so. Consequently,
therapy to reduce the pathological effects of dietary
nucleic acids, namely the elevated uric acid levels,
becomes essential.
Therapeutic Approaches to Reducing Uric Acid
Levels
0043Numerous plasma uric acid-lowering drugs are
in current use. Some act by increasing the renal elim-
ination of uric acid (uricosuric drugs, e.g., benzbro-
marone), or restrict its formation (e.g., allopurinol).
Allopurinol reduces urine uric acid levels as well. As
mentioned earlier, studies in both humans and
animals have shown that allopurinol has an add-
itional beneficial effect in reducing dietary purine
absorption. Allopurinol is usually a safe drug, but in
rare instances, mostly in renal disease, other factors
must be considered. The active metabolite of allopur-
inol, oxypurinol, is handled by the kidney in a fashion
akin to uric acid and thus, even normally, has a long
half-life. Since excretion of oxipurinol is reduced in
renal failure, the allopurinol dose must be reduced to
lower the plasma oxipurinol and minimize the risk
of bone marrow depression, or other undesirable
side-effects, which include epidermal necrolysis and
4162 NUCLEIC ACIDS/Physiology

hepatotoxicity. A recent study pinpointed the poor
response to allopurinol in heavy drinkers and related
this to the combined effect of ethanol in impairing
urate excretion and increasing production. In rare
instances, patients have had a severe allergic reaction
to allopurinol. In such cases, the potent uricosuric
drug, benzbromarone has proved beneficial, even in
renal failure patients with kidney function as low as
25% of normal.
Allopurinol and Acute Renal Failure
0044 It is important to note that in patients with genetic
uric acid overproduction, allopurinol should be used
with care. Its use to avert gout (or uric acid nephrop-
athy) has precipitated acute or chronic renal failure
due to xanthine nephropathy and sometimes xan-
thine stones instead. Xanthine is even more insoluble
than uric acid, and as with uric acid, solubility cannot
be improved by alkalinization of the urine. Xanthine
nephropathy also may occur during massive endogen-
ous nucleic acid breakdown in patients given allopur-
inol during aggressive therapy with cytotoxic drugs
for malignant disorders. In such cases, much lower
doses of allopurinol should be coupled with adequate
hydration and alkalinization of the urine.
Role in Chemical Carcinogenesis
0045 Substantial evidence from microorganisms and mam-
malian cells has implicated mutagenic events caused
by damage to endogenous DNA as the initiating
factor in carcinogenesis. Damage to critical regions
of the genome of somatic cells can be produced by a
variety of environmental mutagens. Examples of en-
vironmental factors affecting humans are cigarette
smoke, asbestos, and ultraviolet (UV) irradiation.
Recent putative additions to the list are radiation
from microwaves and mobile phones.
0046 A high correlation invariably exists between the
mutagenic activity of different chemicals and their
carcinogenic activity. Epidemiological evidence indi-
cates a relationship between exposure to benzene and
nonlymphocytic leukemia in humans, but the signifi-
cance of DNA adduct formation in this is not clear.
Normally, DNA possesses active repair systems to
protect against such damage, which can be caused
by a variety of chemical and physical agents, includ-
ing ionizing, radiation, and UV light. Strands may
become cross-linked, bases can be altered or lost,
and phosphodiester bonds may be broken. UV
light induces the formation of pyrimidine dimers,
which are recognized and cleaved by specific endo-
nucleases. Failure to repair the damage before DNA
replication occurs results in the damaged region be-
coming the site of somatic mutations, chromosomal
rearrangements or amplifications, and aberrant DNA
methylation.
0047In this context, it is of interest that the P53 gene is
altered in many human cancers. Recent studies using
analogs that target specific enzymes of either purine
or pyrimidine nucleotide biosynthesis have identified
P53 as a cellular regulator of both nucleotide syn-
thetic pathways (Figure 2). These studies have led to
the proposal that P53 is not only a sensor of DNA
damage, inducing apoptosis in response to DNA
strand breaks, but rather a sensor of nucleotide deple-
tion. Upregulation of P53 expression, activated by
nucleotide depletion or related processes, prevents
cells entering the S phase when precursor nucleotide
pools are low, thus avoiding replication of damaged
DNA through a prolonged, but reversible, G0/G1
arrest. The significance of these findings is that
elongation of the S phase during nucleotide depletion
is known to be associated with increased chromo-
some breakage. (See Carcinogens: Carcinogenic Sub-
stances in Food: Mechanisms; Carcinogenicity Tests;
Mutagens.)
See also: Carcinogens: Carcinogenic Substances in
Food: Mechanisms; Carcinogenicity Tests; Coenzymes;
Infant Foods: Milk Formulas; Macrobiotic Diets;
Mutagens; Protein: Synthesis and Turnover; Renal
Function and Disorders: Kidney: Structure and Function;
Vegetarian Diets; Viruses
Further Reading
Diem K and Lentner C (eds) (1956 and 1970) Scientific
Tables – Chemical Composition of Foodstuffs, 7th edn,
pp. 230–243. Basle: Geigy.
Grahame RG, Simmonds HA and Carrey EA (eds) (2002)
Gont – an at your fingertips guide. London: Class Pub-
lishing.
Grimble GK (1994) Dietary nucleotides and gut mucosal
defence. Gut 35: S46–S51.
Henderson JF and Paterson ARP (1973) Nucleotide Metab-
olism: An Introduction. New York: Academic Press.
Linke SP, Clarkin KC, Di Leonardo A, Tson A and Wahl
GM (1996) A reversible, p53-dependent G0/G1 cell
cycle arrest induced by rNTP depletion in the absence
of DNA damage. Genes and Development 10: 934–947.
Scriver CR, Beaudet AL, Sly WS and Valle D (eds) (2001)
The Metabolic and Molecular Basis of Inherited Dis-
ease, 8th edn. New York: McGraw-Hill.
Secades JJ and Frontera G (1995) CDP-choline: Pharmaco-
logical and clinical review. Methods and Findings in
Experimental and Clinical Pharmacology 17: 1–54.
Stone TW and Simmonds HA (1991) Purines: Basic and
Clinical Aspects. London: Kluwer.
Zo
¨
llner N and Gresser U (eds) (1991) Urate Deposition in
Man and its Clinical Consequences. Berlin: Springer-
Verlag.
NUCLEIC ACIDS/Physiology 4163
