Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


microbial activity. Nitrosating agents can be formed
from certain compounds added to foods and as a
result of specific processing conditions. In most in-
stances, nitrosamines are found in foods in one of the
three following categories.
0016 Cured meats Nitrosamines are formed in cured
meats because nitrite, and sometimes nitrate, are
added to these products during processing. Nitrate is
reduced to nitrite by the enzyme nitrate reductase,
which occurs in a number of bacteria. As discussed
earlier, nitrite is converted to nitrosating agents which
subsequently react with amines in the meat during
processing, storage, and cooking to form nitros-
amines. (See Curing.)
0017 Nitrite and nitrate have been added to cured meats
for many years to prevent outgrowth and toxin for-
mation by Clostridium botulinum. Nitrite, in com-
bination with other curing ingredients such as sodium
chloride, is particularly effective in inhibiting forma-
tion of the deadly botulism toxin. In addition, nitrite
reacts with pigments in meat to impart the desirable
pink color of cured meats and it prevents the devel-
opment of off-flavors. (See Nitrates and Nitrites.)
0018 Although not all cured meats have detectable
amounts of nitrosamines, fried bacon has been
shown consistently to contain these compounds.
Typically, fried bacon contains 1–20 mgkg
1
of
NPYR and 1–3 mgkg
1
of NDMA. The formation is
related to the relatively high internal temperature of
bacon during frying and the relatively low moisture
content of bacon as compared to other cured meat
products. When bacon is cooked by other methods,
particularly in a microwave oven, considerably lower
amounts of nitrosamines are found. The majority of
evidence suggests that the free amino acid proline is
first nitrosated and then decarboxylated to form
NPYR during frying. Neither the precise chemical
nature of the nitrosating agent nor the amine precursor
for NDMA in bacon is known with certainty. How-
ever, the evidence suggests that the nitrosating agent is
a reaction product of nitrite and lipids in the bacon.
0019 Dried foods and ingredients A variety of processes
and equipment are used to dry foods and food ingre-
dients. During the direct-fire process, air used to dry
the food is first heated by passing the air through the
flames of burners. As a result, products of combus-
tion, including oxides of nitrogen, are directly incorp-
orated into the hot air used to dry the food. The
oxides of nitrogen which include nitrosating agents
such as nitrous anhydride can then react with amines
in the food being dried to produce nitrosamines.
0020 In 1979, scientists in Europe reported the occur-
rence of NDMA in beer. Soon after, reports from a
number of countries confirmed that most beers
contained 1–5 mgkg
1
of NDMA. Although beer
was found to contain NDMA, the NDMA was not
formed during the brewing process. Investigation led
to the discovery that direct-fire-dried malted barley,
an ingredient used in the manufacture of beer, was the
source of the NDMA.
0021A number of investigators have attempted to
identify the amine precursors of NDMA in malted
barley. The evidence suggests that dimethylamine
and perhaps the tertiary amine alkaloids gramine
and hordenine serve as precursors for NDMA in
malted barley.
0022The discovery of nitrosamine formation in direct-
fire-dried malted barley has led to the investigation of
other dried foods. In the USA, certain dried dairy
products, notably nonfat dry milk, are manufactured
by the direct-fire drying process. Nonfat dry milk
manufactured by this process consistently contains
low amounts (less than 1 mgkg
1
) of NDMA. Since
the direct-fire drying process is not used commonly
in Europe for manufacture of nonfat dry milk, the
product does not contain NDMA.
0023Seafood can contain nitrosamines as a result of
either cooking or salt-drying. When fish is broiled
with a gas flame, the oxides of nitrogen produced
in the flame can cause nitrosamine formation in a
manner analogous to nitrosamine formation in
direct-fire drying. In certain areas of the world, par-
ticularly the Orient, fish is preserved by salt. Often
sea salt is used which contains appreciable amounts
of nitrate. The nitrate is reduced to nitrite with subse-
quent formation of nitrosating agents. In some cases,
reaction with amines in the fish produces relatively
large amounts of nitrosamines, principally NDMA.
The potential for NDMA formation in fish is consid-
erable since high levels of NDMA precursors, such as
dimethylamine and trimethylamine, can occur in fish.
0024Migration from surfaces which contact foods Vul-
canized rubber products, such as baby nursing
nipples, have been shown to contain nitrosamines. It
has been demonstrated that when baby bottle nipples
are stored inverted in milk, the nitrosamines partially
migrate from the nipples to the milk. Furthermore,
studies have shown that nitrosamines migrate from
rubber netting used to hold cured meats during the
smoking process. In addition to rubber, nitrosamines
in such substances as wax-treated wrapping paper
and paperboard-based materials have been shown to
migrate to foods.
0025Nonvolatile nitrosamines in foods The notion
that nonvolatile nitrosamines might form in foods
follows logically from the fact that the precursors,
4144 NITROSAMINES

nonvolatile amine and nitrosating agents, occur in
foods. Due to limitations in analytical methodology
for nonvolatile nitrosamines, less is known about
the occurrence of nonvolatile than volatile nitrosa-
mines in foods. Recently developed methods for
nonvolatile nitrosamines have been limited to the
detection of N-nitrosated amino acids and amino
acid derivatives. Most analyses have been on
cured meats and include reports of compounds
such as NPRO, N-nitrososarcosine (NSAR),
N-nitroso-4-hydroxyproline, N-nitrosothiazolidine-
4-carboxylic acid, N-nitroso-2-methylthiazolidine-4-
carboxylic acid, and N-nitroso-2-hydroxyl-
methylthiazolidine-4-carboxylic acid. Other than
NSAR, which is a weak carcinogen, all the other N-
nitrosated amino acids and amino acid derivatives
which have been tested have not shown a carcino-
genic response in animals.
0026 Scientists conjecture that other nonvolatile nitros-
amines such as nitrosated peptides and nitrosated
amides occur in foods. Definitive information in
this regard awaits further application of analytical
methodology for nonvolatile nitrosamines to foods.
0027 Reduction of nitrosamine formation Considerable
efforts have been expended during the past 25 years
to reduce nitrosamine formation in foods. Scientists
have looked critically at the use of nitrite and nitrate
in the manufacture of cured meats. Since nitrite is
considered essential to guard against outgrowth and
toxin production by C. botulinum, its use in the
manufacture of cured meats has been retained. How-
ever, in many countries, permissible levels of nitrite
have been reduced to the minimum necessary for
control of botulism. Generally, nitrate is only permit-
ted in a few fermented cured meat products where
long-term inhibition of C. botulinum is required.
0028 The nitrosation inhibitors ascorbic acid and a-
tocopheral are either required or extensively used in
processing cured meats. Alternatives and/or partial
substitutes for nitrite in cured meats include use of
lactic acid-producing organisms, potassium sorbate,
sodium hypophosphite, fumarate esters, and ionizing
radiation. To date, no adequate alternative for nitrite
has been found. Consequently, nitrosamines continue
to be found in cured meats, especially fried bacon, but
generally at lower levels than occurred a number of
years ago.
0029 Efforts to reduce nitrosamine levels in barley malt,
and hence in beer, have been very successful. This
is largely due to conversion of direct-fired kilns to
indirect-fired kilns for the manufacture of barley
malt. With indirect-fired kilns, the products of com-
bustion are not incorporated into the drying air and,
therefore, nitrosamine formation in the barley malt is
greatly reduced. Interestingly, use of an indirect-fired
kiln does not completely inhibit nitrosation, probably
because ambient air which is drawn into the kiln
usually contains trace levels of oxides of nitrogen
and, therefore, some nitrosation occurs. In addition
to the use of indirect-fired kilns, sulfur dioxide, which
has been shown to be a nitrosation inhibitor, is some-
times used with both direct-fired kilns and indirect-
fired kilns to reduce nitrosamine formation.
0030Due to these changes in processing, the NDMA
levels in beer have been markedly reduced. In a recent
survey, Canadian and US beers were found to contain
on average 0.07 mgkg
1
of NDMA whereas, prior
to 1980, beer commonly contained 1–5 mgkg
1
of
NDMA.
0031Similarly, nitrosamine formation in rubber prod-
ucts, including baby nursing nipples, has been
reduced. This has been accomplished by substituting
nitrosatable with nonnitrosatable vulcanization
accelerator compounds in the manufacture of rubber
products.
0032Products other than food A variety of industrial,
agricultural, and consumer items have been shown to
contain nitrosamines. These include cosmetics, tobacco
products, industrial cutting fluids, vehicle tires, and
pesticides. In each case, the formation can be traced
to use of amines and contact with nitrosating agents
during product formulation, manufacture, or use.
0033Tobacco products are especially important in terms
of nitrosamine occurrence and subsequent human
exposure. During growth, tobacco plants biosynthe-
size a variety of alkaloids such as nicotine and nor-
nicotine. These compounds are either secondary or
tertiary amines. During curing, fermentation, and
aging the alkaloids react with nitrosating agents
formed from nitrate to produce a group of com-
pounds commonly referred to as tobacco-specific ni-
trosamines. Tobacco-specific nitrosamines have been
shown to occur in a wide range of tobacco products
such as cigarettes, cigars, pipe tobacco, chewing
tobacco, snuff, masheri, zarda, and nass. It is note-
worthy that the tobacco-specific nitrosamines fre-
quently occur in tobacco products at several orders
of magnitude higher than volatile nitrosamine occur-
rence in foods and beverages.
Endogenous Formation
0034Convincing evidence exists for endogenous formation
of nitrosamines in humans. Based on our knowledge
of nitrosation in acidic, aqueous media, it is not
surprising that nitrosamine formation has been dem-
onstrated to occur in the stomach. Estimation of
endogenous nitrosation in humans and experimental
animals has been accomplished by measuring urinary
NITROSAMINES 4145

NPRO following oral administration of proline and
nitrate. Inhibition of nitrosamine formation has been
accomplished by the administration of nitrosation
inhibitors such as ascorbic acid. Gastric nitrosation
proceeds through reaction between amines and nitro-
sating agents derived from the diet. Nitrate, which
occurs in substantial amounts in certain vegetables,
is partially reduced to nitrite by nitrate reductase-
containing bacteria in the oral cavity.
0035 Recently, the existence of endogenous nitrosation
pathways other than gastric nitrosation has been
recognized. Evidence exists for mammalian cellular
enzymatic conversion of arginine to nitric oxide,
which in turn affects nitrosation. Scientists believe
that this process occurs in several cell types, including
macrophages. The extent and relevance of cellular
nitrosation are yet to be determined.
Estimates of Exposure to Nitrosamines
from Foods
0036 Several groups have made estimates of human expos-
ure to volatile nitrosamines from food and beverage
consumption. Most of the estimates relate to expos-
ure from consumption of foods and beverages in
western Europe. Volatile nitrosamine exposure was
predominately from NDMA and NPYR.
0037 The estimation of daily exposure to volatile nitros-
amines in most reports ranged from 0.1 to 1.0 mg per
person. In comparison, a US National Academy of
Sciences report in 1981 estimated exposure to nitros-
amines from cigarette smoking to be 17 mg per person
per day.
0038 Several recent reviews reported an estimated daily
exposure to nitrosamines of 10–120 mg per person
from diet. Estimates in this range include, in addition
to volatile nitrosamines, N-nitrosated amino acids
and amino acid derivatives and some include appar-
ent total N-nitroso compounds as well. It should be
recognized that most N-nitrosated amino acids and
amino acid derivates have failed to elicit a carcino-
genic response in animals and the identity and
carcinogenicity of the apparent total N-nitroso com-
pounds are unknown.
0039 Some cautionary comments are needed regarding
estimates of exposure to nitrosamines. First, estimates
should be based on nitrosamines with known identity
and known carcinogenicity in animals. Second, a
number of the estimates were conducted a decade
ago or are based on reports of the volatile nitrosamine
content of foods from over a decade ago. As discussed
previously, the volatile nitrosamine content of foods,
e.g., NDMA in beer, has been dramatically reduced in
recent years. Therefore, some of the estimates in the
literature may be higher than reflected by current
levels of volatile nitrosamines. Third, information
currently available does not allow reliable estimation
of exposure from currently undetected carcinogenic
nonvolatile nitrosamines, of nitrosamines formed
endogenously, and of other N-nitroso compounds
formed exogenously and endogenously. A fourth limi-
tation of estimates is that they are based on average
food consumption for relatively large populations.
Food consumption patterns for subgroups and indi-
viduals within populations vary widely and, there-
fore, so does exposure to nitrosamines in the diet.
Role of Nitrosamines and Other
N
-Nitroso
Compounds in Human Cancer
0040Based on current information on carcinogenicity in
experimental animals, and on other pertinent infor-
mation, most scientists believe that nitrosamines and
other N-nitroso compounds are capable of inducing
cancer in humans. However, at the present time we do
not know what exposure to the various nitrosamines
is required to induce cancer in a human population.
0041Convincing evidence exists for the role of tobacco-
specific nitrosamines in cancer induction in people
who use tobacco products. Particularly compelling
evidence exists for the causative role of tobacco-
specific nitrosamines in cancer of the oral cavity
for people who engage in snuff dipping and chewing
betel quid.
0042In order to assess more fully the part nitrosamines
and other N-nitroso compounds play in human
cancer, progress will be needed in the following
areas: improvement and application of analytical
methodology will be required for estimation of
exposure to nonvolatile nitrosamines and other
N-nitroso compounds in foods and in other materials;
second, a better understanding of formation and
exposure to endogenously formed nitrosamines and
other N-nitroso compounds is needed; and, finally,
methodology will need to be improved in order to
allow reliable estimation of human cancer incidence
from exposure to relatively low levels of carcinogens,
including nitrosamines.
See also: Amines; Cancer: Carcinogens in the Food
Chain; Carcinogens: Carcinogenic Substances in Food:
Mechanisms; Carcinogenicity Tests; Clostridium:
Occurrence of Clostridium botulinum; Botulism; Curing;
Drying: Equipment Used in Drying Foods; Nitrates and
Nitrites; Smoking, Diet, and Health
Further Reading
Forman D and Shuker D (eds) (1989) Cancer Surveys,
Advances and Prospects in Clinical, Epidemiological
and Laboratory Oncology. Nitrate, Nitrite and
4146 NITROSAMINES
Nitrosocompounds in Human Cancer, vol. 8, no. 2.
Oxford: Oxford University Press (published for the
Imperial Cancer Research Fund).
Gloria MBA, Barbour JF and Scanlan RA (1997) N-Nitro-
sodimethylamine in Brazilian, US domestic, and US
imported beers. Journal of Agriculture and Food
Chemistry 45: 814–816.
Gloria MBA, Barbour JF and Scanlan RA (1997) Volatile
nitrosamines in fried bacon. Journal of Agriculture and
Food Chemistry 45: 1816–1818.
Hill MJ and Giacosa A (eds) (1996) Proceedings of the
thirteenth annual ECP symposium N-Nitroso com-
pounds in human cancer: current status and future
trends. European Journal of Cancer Prevention 5 (sup-
plement 1): 1–163.
Hotchkiss JH (1989) Relative exposure to nitrite, nitrate
and N-nitroso compounds from endogenous and
exogenous sources. In: Taylor SL and Scanlan RA (eds)
Food Toxicology, a Perspective on the Relative Risks.
IFT Basic Symposium Series. New York: Marcel Dekker.
O’Neill IK, Chen J and Bartsch H (eds) (1991) Relevance to
Human Cancer of N-Nitroso Compounds, Tobacco
Smoke and Mycotoxins. IARC Scientific Publication,
no. 105. Lyon: International Agency for Research on
Cancer.
Scanlan RA and Tannenbaum SR (eds) (1981) N-Nitroso
Compounds. ACS Symposium Series, no. 174. Washing-
ton, DC: American Chemical Society.
Tricker AR (1997) N-nitroso compounds and man: sources
of exposure, endogenous formation and occurrence in
body fluids. European Journal of Cancer Prevention 6:
226–268.
Tricker AR and Preussmann R (1991) Carcinogenic N-
nitrosamines in the diet: occurrence, formation, mech-
anisms and carcinogenic potential. Mutation Research
259: 277–289.
NMR Spectroscopy See Spectroscopy: Overview; Infrared and Raman; Near-infrared; Fluorescence;
Atomic Emission and Absorption; Nuclear Magnetic Resonance; Visible Spectroscopy and Colorimetry
Nonstarch Polysaccharides See Dietary Fiber: Properties and Sources; Determination;
Physiological Effects; Effects of Fiber on Absorption; Bran; Energy Value
NUCLEIC ACIDS
Contents
Properties and Determination
Physiology
Properties and Determination
D W Gruenwedel, University of California at Davis,
Davis, CA, USA
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Presence of Nucleic Acids in Food
0001 Nucleic acids are natural constituents of all foods
derived from animal or plant sources, for they are
intrinsic components of the cells making up food
tissue. They occur usually in close association with
basic proteins (histones, protamines), forming nucleo-
proteins. In general, their effect on texture, nutritive
value, or sensory properties of foods is small in view
of their low tissue concentrations (typically < 200 mg
of nucleic acid phosphorus per 100 g of fresh tissue).
These low tissue concentrations normally also pre-
clude the occurrence of detrimental health effects.
However, health consequences may arise with per-
sons suffering from metabolic disorders such as im-
pairment of purine excretion. In these instances, the
dietary breakdown of the nucleic acids can generate
NUCLEIC ACIDS/Properties and Determination 4147

unacceptably high levels of uric acid in blood as well
as tissues, producing ultimately hyperuricemia, acute
and chronic arthritis, or other symptoms of gout.
Properties of Nucleic Acids
Chemical Properties
0002 Nucleic acids are macromolecules of high molecular
weight, made up of heterocyclic pyrimidine (Py) and
purine (Pu) bases, a five-membered (furanose-type)
sugar ring, and orthophosphate. Bases, sugar and
phosphate occur in equimolar ratios, i.e., Pu(Py):
sugar: phosphate of 1:1:1. Thus, schematically, their
chemical composition may be denoted as
½PuðPyÞpentose PO
4
n, ð1Þ
with n being a large number. Among naturally
occurring nucleic acids, n may range from 4.5 10
3
(polyoma virus) to 7.0 10
7
(Drosophila melanoga-
ster chromosome). Two broad categories exist, de-
oxyribonucleic acids (DNAs) and ribonucleic acids
(RNAs), depending on whether the sugar moiety
is b-d-2
0
-deoxyribose or b-d-ribose (note: primed
entities refer to sugar-binding sites). Their elemental
composition is roughly 15% nitrogen, 10% phos-
phorus, 36% carbon, 4% hydrogen, and 35%
oxygen. Cellular DNA is localized almost exclusively
in the nucleus, while RNA is found predominantly in
the cytoplasm.
0003 The principal bases found associated with DNAs
are the purine derivatives adenine (6-aminopurine,
Ade) and guanine (2-amino-6-hydroxypurine, Gua),
and the pyrimidine derivatives cytosine (2-hydroxy-4-
amino-pyrimidine, Cyt) and thymine (5-methyluracil,
Thy). Occasional base variations (e.g., 5-methylcyto-
sine or 5-hydroxymethylcytosine) are encountered.
In RNAs, the base Thy is replaced by uracil (2,4-
dihydroxypyrimidine, Ura). Certain RNAs, e.g.,
(transfer) tRNAs, show considerable base variations:
they may contain a wide variety of methylated bases,
including Thy, bases with thio groups, inosine, etc.
0004 The Pu bases are bound to the sugar in an
N9 !C1
0
-glycosidic linkage; with Py bases, bonding
occurs via Nl !Cl
0
. The resulting compounds are
N-glycosides; they are called nucleosides. Some
nucleosides are: adenosine (Ado or A), guanosine
(Guo or G), cytidine (Cyd or C), uridine (Urd or U),
and thymidine (dThd or dT). Attachment of ortho-
phosphate in positions O3
0
(or O5
0
) produces the
corresponding 3
0
(or 5
0
) (mono)nucleotides. 5
0
-Mono-
nucleotides are the actual building blocks of DNAs
and RNAs. Schematically, their polymerization
takes place by 5
0
!3
0
phosphodiester bond forma-
tion, e.g., the 5
0
-phosphate group of one nucleotide
links up with the free 3
0
-hydroxyl group of another,
thereby forming a dinucleotide. It is customary to
use short-hand notations such as 5
0
-pApCpGpT-3
0
or 5
0
-ApCpGpTp-3
0
to indicate 5
0
-terminal and 3
0
-
terminal phosphate, respectively.
0005In both DNA and RNA, in-vivo diester bond for-
mation proceeds solely in the 5
0
! 3
0
direction, pro-
ducing long-chain, unbranched structures (strands).
Branching in RNA molecules can occur transiently
during RNA splicing. Both DNA and RNA strands
display polarity, meaning that their primary structure
is to be read in the direction ! O3
0
! [PO
2
! O5
0
!
C5
0
! C4
0
! C3
0
! O3
0
] ! [PO
2
! O5
0
! C5
0
!
C4
0
! C3
0
! O3
0
], etc. (the brackets define a DNA/
RNA monomer subunit). The strand polarity is a
consequence of furanose pucker as well as associated
base stacking.
0006The chemical, i.e., base composition of nucleic
acids is variable and differs from one species to an-
other. Variation in base composition forms the basis
of the genetic information content of DNAs. How-
ever, in DNAs, certain regularities apply (Chargaff’s
rules): (1) ~Pu ¼~Py, (2) ~A ¼~T and ~G ¼~C,
(3) ~(A þ C) ¼~(G þ T). On occasion, 5-methyl-
cytosine needs to be taken into consideration, too, in
order to satisfy points (2) and (3). These rules hold
because of the double-stranded (complementary)
structure of DNAs brought about, in part, by
Watson–Crick hydrogen bonding between opposite
bases (A
—
—
T and G
—
—
—
C base pair formation; the
symbols
—
—
and
—
—
—
denote that base pairing between
A and T involves two hydrogen bonds, while three
hydrogen bonds are formed in the base pairing
between G and C). Chargaff’s rules do not apply to
single-stranded nucleic acids, whether they are RNAs
(usually) or DNAs (occasionally).
Physiochemical Properties
0007At physiological pH values, the orthophosphate
groups of the nucleic acids are negatively charged.
Outward charge neutralization occurs through posi-
tively charged counterions aligning themselves with
the negative charges on the polymers. Counterions
can be Na
þ
,Mg
2þ
, basic proteins, polyamines, etc.
Nucleic acids are therefore polyelectrolytes, a prop-
erty that determines their solution behavior: they are
extensively hydrated and form gel-like structures if
present in higher concentrations. Even in dilute solu-
tions they display non-Newtonian behavior, as noted,
for instance, by their shear-rate-dependent viscosity.
Once hydrated, they are readily soluble in water.
Isolation of nucleic acids from tissues usually yields
their sodium salt. How they really exist in vivo is
unknown, although it is assumed that Na
þ
also serves
there as the principal counterion.
4148 NUCLEIC ACIDS/Properties and Determination

0008 DNAs, with very few exceptions, are double-
stranded helices of opposite polarity. By contrast,
RNAs are usually single-stranded, although double-
(as well as triple-) stranded RNAs are known to exist.
There are also duplex helices made up of DNA and
RNA single strands; they form, for example, in vivo
during transcription. DNA double-strandedness,
brought about by interstrand hydrogen bonding and
intrastrand base stacking, is termed DNA secondary
structure; this structure is salient to the preservation
of genetic information by DNA. Hydrophobic bond-
ing and London dispersion forces are the major
factors contributing to base stacking. DNA secondary
structure is quite stable over a wide range of pH
(3–12), ionic strength, I (0.001–5), and temperature
(up to 100
C). The ranges given are approximate: not
only are the effects of I and temperature interdepend-
ent (rule-of-thumb: the higher I is, the higher the
thermal stability), but base composition (GC-rich
DNAs are more heat-stable than AT-rich DNAs) con-
tributes heavily to the stability of DNA secondary
structure as well. Lastly, at elevated levels of I, indi-
vidual cation/anion effects, often destabilizing in
nature, also become noticeable. Transforming the
DNA double helix into two single strands of DNA,
a process occurring cooperatively, is called ‘denatur-
ation.’ Synonymous terms are ‘helix-to-random coil
transition’ or ‘order–disorder transition.’‘Denatur-
ation’ represents a change in DNA conformation
(conformational changes are those that rupture
hydrogen bonds and/or hydrophobic bonds but
leave covalent bonds intact). It is evident from the
above that variations in pH, I, and temperature can
be used to bring about changes in DNA conformation
experimentally. While much is known regarding the
subtleties of DNA secondary structure, very little in-
formation is available with respect to the structural
properties of the single-stranded states of DNA (or
RNA).
0009 Nucleic acids are optically active molecules, i.e.,
they rotate the plane of (linearly) polarized light
and, hence, display chirality. The major contribution
to optical activity comes from base stacking, for it
imparts molecular asymmetry. The optical activity of
the asymmetric carbon atoms of deoxyribose or
ribose does not contribute much to the overall effect.
Double-stranded DNAs are usually of right-handed
screwness or helicity. This information has been
obtained from X-ray diffraction analysis of DNA
fibers. Depending on the sign and magnitude of the
parameters defining the orientation of a base pair in
DNA relative to the helix axis (e.g., rotational twist,
t, tilt, y
T
, roll y
R
, propeller twist, y
p
, axial rise, h,
pitch, width, and depth of major and minor grooves),
one can distinguish between conformational families
such as A-, B-, C-, and Z-type DNA. They exist
among naturally occurring DNAs, i.e., DNAs with
random base sequences. Synthetic DNAs (poly-
nucleotides with nonrandom sequences) are charac-
terized by additional families such as B
0
,C
00
,D,E,
S, Z
0
. Z-type DNA is a left-handed double helix.
0010Until quite recently, the consensus was that
double-stranded DNA is rather inflexible (‘rod’ or
‘worm-like chain’) and that, apart from its exciting
base-pairing capabilities, the structure of the sugar–
phosphate backbone, in view of its seeming monot-
ony, is of little consequence to its biological function.
This perception was due to the fact that X-ray diffrac-
tion studies executed on DNA fibers gave structural
information only at low levels of resolution. How-
ever, experimental evidence has shown that double-
stranded DNA is surprisingly flexible locally, giving
rise to what has been termed DNA ‘polymorphism.’
DNA polymorphism refers to seemingly minor, quite
localized alterations in secondary structure that may
very well be of crucial importance in biological pro-
cesses such as replication and transcription. That
there is such a thing as DNA polymorphism has
been demonstrated by X-ray diffraction analysis of
oligonucleotide crystals. Oligonucleotide crystals
(formerly not available) yield high-resolution X-ray
data down to atomic dimensions. It was found that
DNA polymorphism resides in the nonplanar ring
structure of b-d-2
0
-deoxyribose (or b-d-ribose in
RNA) and that this structure is conformationally
quite flexible. Its two main conformations (called
sugar pucker) are envelope E (four atoms in a plane,
the fifth out of plane) and twist T (three atoms in a
plane, the two others out of plane on opposite sides).
Out-of-plane atoms on the same side of C5
0
are de-
noted endo, while those on the opposite side are
denoted exo. Transitions between E and T are facile,
giving rise to a pseudorotation cycle of the furanose
ring in nucleosides. The cycle contains 10 different
(endo/exo) T and E forms. In addition, O5
0
can
assume a number of orientations about the C4
0
–C5
0
bond axis; they are known as gauche, gauche
(þsynclinal); gauche, trans (antiperiplanar); and
trans, gauche (synclinal). Lastly, relative to the
sugar moiety, a Pu can adopt two major orientations
along the N—Cl
0
bond; anti (the bulk of the base
turns away from the sugar) and syn (it is over or
toward the sugar). All orientations influence each
other, giving rise to a multitude of sugar conform-
ations – and, hence, structural DNA families – with
important implications for biological function. Thus,
the binding of enzymes (e.g., DNase I, restriction
endonucleases) or regulatory proteins such as TFIIIA
(zinc fingers) to DNA is now known to be greatly
influenced by the geometry of the backbone. In fact,
NUCLEIC ACIDS/Properties and Determination 4149

it has been said that while base pairing may be viewed
as the ‘brain’ of DNA, the conformational flexibility
of the sugar–phosphate backbone constitutes its
‘heart.’
Determination and Characterization of
Nucleic Acids
0011 There exist literally hundreds of techniques that
permit the detection, quantitative evaluation, struc-
tural, and biological characterization of nucleic acids.
They are chemical, biochemical, physicochemical, or
physical in nature; however, their description is
beyond the scope of this article. ‘Determination of
nucleic acids’ is therefore understood here to mean
their detection (qualitatively as well as quantitatively)
in cells and tissues in particular, and the determin-
ation of their chemical composition (average base
composition) when in solution. Hence, techniques
that serve their physical characterization in terms of
structure, mass, or shape, or techniques used in DNA
sequencing, in the enzymatic manipulation of DNA
and RNA, in recombinant DNA research, in cloning,
etc., are not presented. Techniques relating to their
isolation from animal and plant tissues or from
microbial sources are also not discussed here.
Detection of Nucleic Acids in Cells and Tissues
0012 Histochemical approaches (in-situ staining) Most
advantageous are histochemical staining techniques.
Each constituent of DNA/RNA (cf. eqn (1)) can be
identified histochemically: acidic phosphate groups
are demonstrated with the aid of basic dyes, b-d-2
0
-
deoxyribose with the help of a triphenylmethane dye,
and Pu(Py) can be made visible via intercalation with
fluorescent dyes. The specificity of the techniques can
be improved further through judicious use of DNA
(deoxyribonuclease) and RNA (ribonuclease) degrad-
ing enzymes prior to staining. This eliminates possible
interference by the other polymer. Although staining
techniques are usually used to obtain qualitative
information, they can be adapted to furnish also
quantitative data.
0013 Techniques detecting DNA
Feulgen nucleal reaction DNA in tissue is depuri-
nated by strong acid treatment. The resulting apurinic
acid releases b-d-2
0
deoxyribose, which in turn is
converted to its open-chain aldehyde form. The
sugar aldehyde reacts with the colorless triphenyl-
methane derivative leucofuchsin (Schiff’s reagent) to
give a magenta color (fuchsin). The chemistry of the
reaction is known. Since the ribose of RNA cannot be
changed to an open-chain aldehyde form, RNA will
not be stained; hence, this technique is very specific.
0014Kurnick’s methyl green method Methyl green, a tri-
phenylmethane dye possessing two positively charged
amine groups, adds on to double-stranded DNA.
Details of the process remain obscure but appear to
be related to the secondary structure of DNA: the two
positively charged amine groups of the dye are said to
have just the correct distance to add on to phosphate
groups separated from one another by one turn of the
DNA double helix. DNA stains green or green-blue.
0015Fluorescence flow cytometry A number of macro-
cyclic dyes (acridine orange (derivative of acridines),
Hoechst 33342, Hoechst 33258 (derivatives of
piperazine), ethidium, propidium (derivatives of phe-
nanthridine), etc.) bind to DNA bases. Some of them
(e.g., acridine orange) will also react with RNA. Their
commonality resides in the fact that they are planar,
highly aromatic molecules that fluoresce when irradi-
ated. Because of their flatness, they can bind to DNA
through intercalation, i.e., they can sandwich them-
selves between bases. Intercalation increases their
basic fluorescence, which makes the nucleic acids
visible. Excitation sources are mercury arc lamp,
argon ion laser, or krypton ion laser. Since DNA–
dye emission spectra differ usually from those of the
RNA–dye complexes, distinction between the two
types of nucleic acids is possible. Determination of
DNA (or RNA) is quantitative. The technique is used
most frequently in cell-culture work or in studies in
which free-flowing cellular or subcellular particles
are available (e.g., red blood cells).
0016Techniques detecting DNA and RNA
Methyl green–pyronin method If proper experimen-
tal conditions are maintained, nuclei (DNA) will be
stained green or blue (methyl green), and nucleoli
and cytoplasm (RNA) red (pyronin). Since pyronin,
a xanthene derivative, is also a fluorescent dye, the
technique can be adopted for flow-cytometric
determination of RNA.
0017Acridine orange method The dye acridine orange
binds to DNA and RNA. The two dye–polymer com-
plexes exhibit different fluorescence emission spectra:
when illuminated with purple light, DNA fluoresces
green–yellow to bright yellow and RNA reddish
brown to orange; when illuminated with ultraviolet
light, DNA fluoresces greenish yellow and RNA crim-
son red. (See Spectroscopy: Fluorescence.)
0018Nonhistochemical Approaches
Radioactive labeling method Nucleic acids can be
further identified qualitatively or quantitatively in
cells or tissues by labeling them radioactively. Fre-
quently used radionuclides are
31
P,
14
C, and
3
H.
4150 NUCLEIC ACIDS/Properties and Determination

They are administered experimentally in the form of
labeled precursors of DNA or RNA (e.g., [
3
H]dT
for DNA, [
3
H]U for RNA, [ a-
32
P]dATP for DNA,
[a-
32
P]ATP for RNA, etc.) and are incorporated
into the polymers enzymatically during macromol-
ecular synthesis. Labeling can be performed in vitro
or in vivo. The presence of the radionuclides and,
hence, of the nucleic acids is noted by the radioactive
decay of the nuclides in the form of a or b particles
or g rays.
0019 Burton assay for DNA This assay is a colorimetric
procedure for measuring the deoxyribose moiety of
diphenylamine DNA. Color is produced by a second-
ary amine (phenylamine) reacting with the sugar.
The assay is relatively specific, although RNA in
high concentrations, or sucrose, should be absent.
DNA does not have to be present in purified form;
thus, determinations can be undertaken in crude cel-
lular extracts. DNA is usually extracted from tissue or
cells through treatment with strong acids (perchloric
acid, trichloroacetic acid), although alkaline extrac-
tions have also been used. Extraction is undertaken to
remove interfering substances, particularly proteins.
Color readings are taken at 595 and 650 nm. A stand-
ard curve is prepared by using samples of pure DNA
(commercially available). A number of modifications
of the basic procedure exist. (See Spectroscopy:
Visible Spectroscopy and Colorimetry.)
0020 3,5-Diaminobenzoic acid fluorescence assay DNA
is extracted from cellular material with acid treat-
ment (usually perchloric acid) and the sample ex-
posed to hydrochloric acid (1 N) for about 45 min
at 55–57
C. This produces apurinic acid and free
b-d-2
0
-deoxyribose (see section Feulgen nucleal reac-
tion). The C1
0
and C2
0
carbons of the sugar then react
with 3,5-diaminobenzoic acid to produce a strongly
fluorescent compound. Excitation occurs at 410 nm;
fluorescence is measured at 510 nm. Calibration with
pure DNA is required. Submicrogram quantities of
DNA can be determined.
Detection of Nucleic Acids in Solution
0021 These techniques assume DNA as well as RNA to
exist in solution in essentially pure form, i.e., extrac-
tion from tissues or cells and purification occurring
prior to analysis.
0022 Spectrophotometric methods
Spectrophotometry in the ultraviolet (concentration
determinations) The presence of stacked Pu(Py)
bases in DNA/RNA makes the polymers absorb radi-
ation in the ultraviolet region. With duplex DNA,
absorption starts at wavelengths below 300 nm and
reaches a maximum near 260 nm and a minimum
around 230 nm. The presence of a second peak around
200 nm has been suggested; however, its demonstra-
tion is not simple because most spectrophotometers
cease functioning around 200 nm. Concentration
determinations are usually performed at 260 nm by
applying the Bouguer–Lambert–Beer law:
A
260
¼ e
260
cd, ð2Þ
where A
260
is the absorbance of DNA at 260 nm, e
260
the DNA molar absorptivity (1 (mol phosphate)
1
cm
1
), c its molar concentration in solution (mol
phosphate l
1
), and d the optical pathlength (cm).
By measuring A
260
, one can readily compute c, the
desired quantity, e
260
, and these are available from the
literature for almost all DNAs. They are usually
around 6.6 10
3
l (mol phosphate)
1
cm
1
. Because
of the 1:1:1 molar relationship between phosphate,
sugar, and base, c can also be expressed in terms of
moles of base per liter or moles of monomer per liter,
etc. Since the average molecular weight of a nucleic
acid monomer unit is 330, it is easily derived from
eqn (2) that for duplex DNA:
1 A
260
unit ¼ 50 mg DNA ml
1
, ð3Þ
while for single-stranded DNA or for RNA
1 A
260
unit ¼ 40 mg RNA ml
1
: ð4Þ
0023Spectrophotometric methods in the ultraviolet (base
composition determinations) The secondary struc-
ture of DNA can be used for analytical purposes.
Heating duplex DNA at a given pH and ionic strength
ultimately yields single-stranded DNA (‘denatur-
ation’). The process can be followed spectrophotome-
trically since single-stranded DNA has a higher
absorbance (by about 25% at room temperature) at,
say, 260 nm than double-stranded DNA. The increase
in absorbance is called ‘hyperchromicity’ (H) and is
defined as follows:
H
260
¼ A
260
t
A
260
0
=A
260
0
, ð5Þ
where A
260
t
is the absorbance of DNA at 260 nm at a
given temperature, t, while A
0
is its absorbance prior
to heating (usually room temperature). H is thus the
normalized absorbance increase of DNA due to heat-
induced denaturation. Plotting H
260
against tempera-
ture produces the so-called DNA ‘melting curve.’ The
expression ‘melting’ refers to the fact that DNA
‘helix-to-random coil transitions’ resemble the infin-
itely sharp temperature-dependent phase transitions
occurring in ice during melting. DNA ‘melting curves’
are of sigmoidal shape. The limiting value of A
260
t
at
NUCLEIC ACIDS/Properties and Determination 4151

T
max
is about 40% higher than A
260
0
. The temperature
at which H
260
amounts to 50% of its limiting value
H
260
max
is called the ‘melting temperature’ (T
m
in
C).
T
m
is linearly related to the (average) base compos-
ition of DNA. For the ‘standard saline citrate’
medium (0.15 M sodium chloride and 0.0015 M
sodium citrate, pH 7.0):
%GC ¼ 2:44 ðT
m
69 :3 Þ:ð6Þ
Since % GC ¼ 100X
GC
, with X
GC
, representing the
‘mole fraction’ of GC base pairs in DNA, and since
X
AT
¼ 1 X
GC
, eqn (6) furnishes equally well infor-
mation on %AT. Equations similar to eqn (6) have
been established for other solvents. In conclusion,
heating native DNA under controlled conditions
(constant pH and ionic strength, application of a
linear temperature gradient, automatic recording of
hyperchromicity) enables the rapid determination
of its chemical (base) composition. It is best to work
at A
260
0
1( 50 mg DNA ml
1
).
Seealso: Spectroscopy:Fluorescence;Visible
SpectroscopyandColorimetry
Further Reading
Adams RLP, Knowler JT and Leader DP (1986) The
Biochemistry of the Nucleic Acids, 10th edn. London:
Chapman & Hall.
Alberts B, Bray D, Lewis J et al. (1989) Molecular Biology
of the Cell, 2nd edn. New York: Garland.
Ausubel FM, Brent R, Kingston RE et al. (1989) Current
Protocols in Molecular Biology, vols 1 and 2. New York:
Greene, Wiley Interscience.
Chayen J and Bitensky L (1991) Practical Histochemistry,
2nd edn. Chichester, UK: John Wiley.
Saenger W (1984) Principles of Nucleic Acid Structure.
New York: Springer-Verlag.
Shapiro HM (1985) Practical Flow Cytometry. New York:
Alan R. Liss.
Walker JM (ed.) (1984) Methods in Molecular Biology,
vol. 2. Nucleic Acids. Clifton: Humana Press.
Physiology
H A Simmonds,PurineResearchUnit,Guy’sHospital,
London,UK
Copyright2003,ElsevierScienceLtd.AllRightsReserved.
Physiology
000 1 Nucleic acids are essential components of all cells and
consequently are found in many foods. The term
‘nucleic acid’ commemorates the first isolation of
this vital cell constituent, ‘nuclein,’ from the sperm-
atic fluid of Rhine salmon and the nuclei of pus cells,
by Miescher in 1868. Some 20 years later, it was
demonstrated that uric acid, which had been recog-
nized by the Swedish chemist Scheele in 1776 as a
constituent of human urine and kidney stones, arose
from nucleic acid degradation. Fischer and his school
in Germany at the end of the nineteenth century
(1895–1899), established the first chemical structure
for uric acid, as well as that of other purine and
pyrimidine bases. It was demonstrated subsequently
that nucleic acids consisted of chains of these purine
and pyrimidine bases linked to a pentose sugar, esteri-
fied with phosphoric acid (Figure 1b). Two types of
nucleic acid have been identified – deoxyribonucleic
acid (DNA) and ribonucleic acid (RNA).
Physiological Roles
0002The purine and pyrimidine bases of DNA carry the
genetic information of all prokaryotic and eukaryotic
organisms, with the sugar and phosphate groups per-
forming a structural role. The human genome is con-
sidered to contain between 50 000 and 100 000 genes,
each of which is composed of a linear polymer of
DNA of varying length. In viruses, genes are made
of either DNA or RNA. The infinite variation in
genetic information is achieved by the sequence of
the four bases that constitute DNA (Figure 1b) – the
purines, adenine and guanine, and the pyrimidines,
thymine and cytosine. DNA is double-stranded, and
each nucleotide of the chain in one strand is linked by
hydrogen bonding to a complementary nucleotide in
the other. Complementary pairs of nucleotides are
adenine and thymine, and guanine and cytosine.
DNA is principally found in the nucleus and is con-
sidered to be relatively stable in most cell types. (See
Viruses.)
0003Ribonucleic acid is essential for the transmission of
the genetic message in the form of protein synthesis
and must first be synthesized from DNA. In the case
of RNA, one of the four bases differs from that in
DNA – uracil replaces the pyrimidine base thymine –
and the molecule is single-stranded, except in some
viruses. In contrast to DNA, most of the RNA is in the
cytoplasm. Cells contain three types of RNA: messen-
ger RNA (mRNA; 5% of total RNA) provides the
template for protein synthesis and is relatively labile;
transfer RNA (tRNA; 15%) carries the message in
the form of activated amino acids to the ribosome
for the synthesis of specific polypeptides, as deter-
mined by the particular mRNA template; and
ribosomal RNA (rRNA), the major RNA component
(80%), is metabolically stable. (See Protein: Synthesis
and Turnover.)
4152 NUCLEIC ACIDS/Physiology
0004 The important physiological roles played by
the metabolic pathways responsible for sustaining
these different nucleic acid pools in humans is
demonstrated by the clinical manifestations when
different steps in the synthesis, degradation, and
repair of the constituent mononucleotides are
defective or absent.
Nucleic Acid Metabolism in Humans
0005The building blocks of the nucleic acids, the purine
and pyrimidine ribonucleotides, are of central
importance to virtually all biological processes.
Whereas cellular purines are derived exclusively
from endogenous sources, cellular pyrimidines are
1
6
5
4
N
N
N
8
9
N
7
3
NH
2
2
O
1'
2'
3'
OH OH
4'
5'
OOOP
O
OH
O
OH
O
(a)
(b)
OH
PPHO
α
βγ
Adenosine
Adenosine 5' monophosphate (AMP)
Adenosine 5' diphosphate (ADP)
Adenosine 5' triphosphate (ATP)
O
H
HO
O
OH
NH
2
O
PO
CH
2
O
NH
2
H
H
O
O
POOCH
2
O
OH
H
H
O
O
P
O
O
CH
2
O
H
HO
O
POO
CH
2
OH
CH
3
NH
2
OH
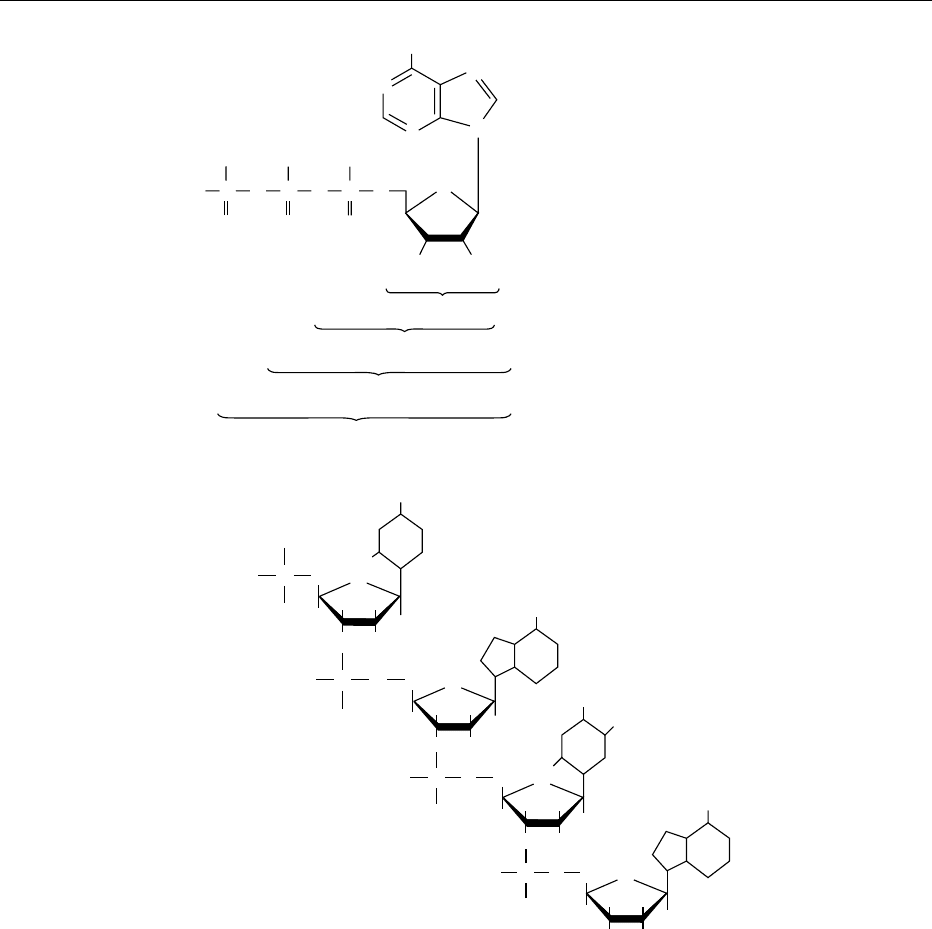
fig0001 Figure 1 (a) Structural formula of adenosine 5
0
-triphosphate (ATP) indicating the numbering of the atoms on the ribose, as well as
the purine ring, which consists of a six-membered pyrimidine ring fused to a five-membered imidazole ring. The position of attachment
of the phosphate groupings for AMP, ADP, and ATP is also indicated. (b) Schematic representation of the structure of part of a single
DNA strand made up of a sequence of the four bases, the pyrimidine cytosine, the purine adenine, the pyrimidine thymine, and purine
guanine respectively, showing that the deoxyribose has an H group at the 2
0
position on the pentose ring, instead of the OH group of
ribose. These bases are linked via the 3
0
-OH group of the deoxyribose-phosphate moiety to the 5
0
-OH group of the next deoxyribose.
Reproduced from Nucleic Acids: Physiology, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK and
Sadler MJ (eds), 1993, Academic Press.
NUCLEIC ACIDS/Physiology 4153
