Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


cancer suggests that fatty acid synthesis helps pro-
mote tumor growth. This is in marked contrast to its
role as an anabolic energy storage pathway in liver
and adipose tissue, and fatty acid synthesis is now
associated with clinically aggressive tumor behavior
and tumor-cell growth and survival, and has become
a novel target pathway for chemotherapy develop-
ment.
See also: Adipose Tissue: Structure and Function of
White Adipose Tissue; Structure and Function of Brown
Adipose Tissue; Fats: Digestion, Absorption, and
Transport; Requirements; Classification; Fatty Acids:
Properties; Metabolism; Gamma-linolenic Acid; Analysis;
Dietary Importance; Hormones: Pancreatic Hormones;
Steroid Hormones; Triglycerides: Structures and
Properties; Characterization and Determination
Further Reading
Chirala SS, Jayakumar A, Gu A and Wakil SJ (2001)
Human fatty acid synthase: role of interdomain in the
formation of catalytically active synthase dimer. Pro-
ceedings of the National Academy of Sciences, USA 98:
3104–3108.
Coleman RA, Lewin T and Muoio DM (2000) Physiological
and nutritional regulation of enzymes of triacylglycerol
synthesis. Annual Review of Nutrition 20: 77–103.
Dutta-Roy AK (2000) Cellular uptake of long-chain fatty
acids: role of membrane-associated fatty-acid-binding/
transport proteins. Cellular and Molecular Life Science
57: 1360–1372.
Eaton S, Barlett K and Porfarzman M (1996) Mammalian
mitochondrial b-oxidation. Biochemical Journal 320:
345–357.
Girard J (1997) Mechanisms by which carbohydrates regu-
late expression of genes for glycolytic and lipogenic
enzymes. Annual Review of Nutrition 17: 325–352.
Holm C, Osterlund T, Laurell H and Contreras JA (2000)
Molecular mechanisms regulating hormone-sensitive
lipase and lipolysis. Annual Review of Nutrition 19:
365–393.
Hoppel CL (1976) Carnitine palmitoyltransferase and
transport of fatty acids. In: Martonosi A (ed.) The
Enzymes of Biological Membranes, vol. 2, pp. 119–
143. New York: Plenum.
Jump DB (1999) Regulation of gene expression by dietary
fat. Annual Review of Nutrition 19: 63–90.
Kerner J and Hoppel C (2000) Fatty acids import into
mitochondria. Biochimica et Biophysica Acta 1486:
1–17.
Kim K (1997) Regulation of mammalian acetyl-coenzyme A
carboxylase. Annual Review of Nutrition 17: 77–99.
Kuhajda FP (2000) Fatty-acid synthase and human cancer:
new perspectives on its role in tumor biology. Nutrition
16: 202–208.
Rasmussen BB and Wolfe RR (1999) Regulation of fatty
acid oxidation in skeletal muscle. Annual Review of
Nutrition 19: 463–484.
Shanklin J and Cahoon EB (1998) Desaturation and related
modifications of fatty acids. Annual Review of Plant
Molecular Biology 49: 611–641.
Smith S (1994) The animal fatty acid synthase: one gene,
one polypeptide, seven enzymes. FASEB Journal 8:
1248–1259.
Sprangers F, Romijn JA, Endert E, Ackermans MT and
Sauerwein HP (2001) The role of free fatty acids (FFA)
in the regulation of intrahepatic fluxes of glucose and
glycogen metabolism during short-term starvation in
healthy volunteers. Clinical Nutrition 20: 177–179.
Stanley H and Sherratt A (1994) Introduction: the regula-
tion of fatty acid oxidation in cells. Biochemical Society
Transactions 22: 421–422.
Storch J and Thumser AEA (2000) The fatty acid transport
function of fatty acid-binding proteins. Biochimica et
Biophysica Acta 1486: 28–44.
Subrahmanyamm SC, Jayakumar A, Gu A and Wakil SJ
(2001) Human fatty acid synthase: role of interdomain
in the formation of catalytically active synthase dimer.
Proceedings of the National Academy of Sciences, USA
98: 3104–3108.
Sul H and Wang D (1998) Nutritional and hormonal regu-
lation of enzymes in fat synthesis: studies of fatty acid
synthase and mitochondrial glycerol-3-phosphate acyl-
transferase gene transcription. Annual Review of Nutri-
tion 18: 331–351.
Towle HC and Kaytor EN (1997) Regulation of the expres-
sion of lipogenic enzyme genes by carbohydrate. Annual
Review of Nutrition 17: 405–433.
Wakil SJ (1989) Fatty acid synthase, a proficient multifunc-
tional enzyme. Biochemistry 28: 4523–4530.
Gamma-linolenic Acid
F D Gunstone, Scottish Crop Research Institute,
Invergowrie, Dundee, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Linolenic acid (GLA) is an important member of the
n-6 family of polyunsaturated fatty acids, being an
intermediate in the bioconversion of linoleic acid to
arachidonic acid. Since the formation of GLA is rate-
determining in this sequence of changes there are
several circumstances when it might be desirable to
add it to the diet. GLA is available in seed oils of
evening primrose, borage, and blackcurrant, and
methods of raising the concentration of this acid in
these sources have been described.
Structure
0002g-Linolenic acid (GLA) is all-cis-6,9,12-octadecatri-
enoic acid and may also be designated as 18:3 (n-6).
2308 FATTY ACIDS/Gamma-linolenic Acid

This later term indicates an acid with 18 carbon
atoms and three double bonds starting on the sixth
carbon from the methyl group. It is understood that, in
the absence of other indicators, the double bonds have
a cis configuration and are methylene-interrupted, i.e.,
the unsaturated centers are separated from each other
by one CH
2
group.
CH
3
CH
2
CH
CHCH
2
cis
CH
CHCH
2
cis
CH
CHðCH
2
Þ
7
cis
COOH:
There are two important families of polyunsaturated
fatty acids: the n-6 family based on linoleic acid, and
the n-3 family, based on linolenic acid. Since animals
– including humans – cannot produce linoleic or lino-
lenic acids themselves, these acids are essential parts
of the diet, obtainable from vegetable sources or from
animals that have already derived these acids from
a plant source. Once ingested, the two C18 acids can
be metabolized to important C20 and C22 acids
through a series of changes involving desaturation
and elongation. The same enzymes are required for
both families of acids, and there is competition for
these. The most important acids in these sequences
are arachidonic acid (20:4) in the n-6 series, and
eicosapentaenoic acid (n-3 20:5) and docosahexa-
enoic acid (22:6) in the n-3 series. These acids are
important components of phospholipid membranes,
and the two C20 acids are also precursors of prosta-
glandins and other eicosanoids.
0003 GLA is the first intermediate in the metabolic
conversion of linoleic acid to arachidonic acid. The
change involves introduction of a (third) double bond
at the D6 position under the catalytic influence of
the 6 desaturase enzyme. This step is believed to be
the rate-limiting stage in the metabolic pathway.
For humans in good health, this presents no problem,
but there are some conditions such as aging, smoking,
diabetes, high alcohol intake, stress-related hormones,
and viral infections that inhibit the rate-determining
step and where it is considered wise to supplement the
intake of GLA. Nutritional factors can also influence
this metabolic pathway.
0004 Table 1 shows the series of changes by which lino-
leic and linolenic acids are converted to the long-
chain polyunsaturated fatty acids.
Occurrence of GLA in Seed Oils and
Microorganisms
0005 As an intermediate in the conversion of linoleic acid
to arachidonic acid, it is not surprising that GLA
is found at low levels in many animal fats. It is present
in cow milk fat (*0.1%) and human milk fat
(0.35–1.0%), and its presence in the latter has often
been cited as an indication of its importance. How-
ever, richer sources of this acid are found in the seed
oils of some less common plants and are also pro-
duced by some microorganisms.
0006There are three commercial sources of GLA from
seed oils: evening primrose (Oenothera biennis and
O. lamarckiana), borage (Borago officinalis), and
blackcurrant (Ribes nigrum). Typical fatty acid pro-
files for these three oils are given in Table 2. Ucciani
found GLA in 164 higher plant families belonging to
10 botanical families.
0007Evening primrose oil was the first to be developed
and used as a source of GLA. It contains 6–14%
(generally 9–10%) of this acid and is accompanied
by high levels of linoleic acid. Borage (starflower) is a
richer source of GLA in terms of oil content of the
seed and level of GLA in the oil, but it has less linoleic
acid than evening primrose oil and also contains a
tbl0001Table 1 n-6 and n-3 families of polyunsaturated fatty acids
n-6 n-3
18:2 (9,12) Linoleic 18:3 (9,12,15) a-Linolenic
# a # a
18:3 (6,9,12) g-Linolenic 18:4 (6,9,12,15) Stearidonic
# b # b
20:3 (8,11,14) 20:4 (8,11,14,17)
# c # c
20:4 (5,8,11,14) Arachidonic 20:5 (5,8,11,14,17)
Eicosapentaenoic
# b
22:5 (7,10,13,16,19)
# b
24:5 (9,12,15,18,21)
# a
24:6 (6,9,12,15,18,21)
# d
22:6 (4,7,10,13,16,19)
Docosahexaenoic
a, 6-desaturase; b, elongase; c, 5-desaturase; d, b-oxidation.
tbl0002Table 2 Fatty acid composition of evening primrose oil, borage
oil, and blackcurrant oil and of oils from Mortierella isabellina and
Mucor javanicus
epo
a
bor
a,b
bck
a
Mortierella Mucor
Oil (%) 16–26 27–35 22–26
16:0 7–10 10 6–7 27 22–25
18:0 1.5–3.5 4 1.5 6 5–8
18:1 6–11 18 9–11 44 38–41
18:2 65–80 37 46–49 12 10–12
18:3 (n-6) 8–14
c
22 14–16 8 15–18
18:3 (n-3) 13–14
18:4 (n-3) 2.5–3
a
epo, evening primrose oil; bor, borage oil; bck, blackcurrant seed oil.
b
Also 20:1 4%, 22:1 2.5%, 24:1 1%.
c
Generally 9–10%.
FATTY ACIDS/Gamma-linolenic Acid 2309

series of n-9 higher monoene acids viz. 20:1, 22:1,
and 24:1 at a combined level of about 8%. Blackcur-
rant oil is intermediate between the other two sources
in its level of GLA, but it also contains the 18:3 and
18:4 n-3 acids.
0008 Molds belonging to the Mucorales family produce
GLA as the only 18:3 acid, and commercial processes
have been developed in Japan based on Mortierella
isabelina and in the UK based on Mucor javanicus.
UK production has been discontinued following a
change in ownership of the producer company.
These products differ from the seed oils in their higher
levels of saturated and monoene acids and their
lower level of linoleic acid. This makes the oil more
suitable as a source of pure GLA or of upgraded
triacylglycerols, since the necessary separations are
easier to effect.
0009 The blue–green algae Spirulina platensis (18–21%
GLA) and S. maxima (12% GLA) are also potential
sources of GLA. However, this is present in various
galactosyldiacylglycerols or phosphatidic acids rather
than in triacylglycerols.
0010 The distribution of these fatty acid chains between
the sn-1, 2, and 3 positions has been examined in a
number of ways. Results for GLA are summarized in
Table 3.
0011 Because of the high level of linoleic acid in evening
primrose oil the major triacylglycerols contain two
(or three) linoleic acyl chains: LLL 54.3 mol%, LLG
17.6%, LLO 13.7%, LLP 7.9%, and other 6.5% (L ¼
linoleic, G ¼ g-linolenic, O ¼ oleic, and P ¼ palmitic
acyl group). The triacylglycerol composition of
borage oil is more complex, with 10 molecular
species each exceeding 4 mol%: LLG 15.4, OLG
12.3, LLL 10.1, LGG 9.8, PLG 9.5, PLL/OOG 8.9,
OLL 8.2, SLG/POG 5.0, LLE 4.1, OOL 4.1, and
other 12.6 (symbols as above and E ¼ 20:1, S ¼
stearic and). No sterochemistry is implied in these
symbols, and PLG, for example, represents the sum
of six isomers.
0012 Evening primrose oil grows in many countries in
Europe and also in New Zealand, but for commercial
reasons, the crop is now grown mainly in China (75–
80%) and Eastern Europe. Seed is produced at a level
of 1000–1200 kg ha
1
. Annual production is esti-
mated to be about 10 000–12 000 tonnes of seed
yielding 1300–1500 tonnes of oil. Over 10 years to
1999, the price of the oil fell from $45 to $17.5.
0013Britain is the main source of borage (starflower),
but it is also grown in Holland, Canada, New Zea-
land, and Poland. Annual production of seed is esti-
mated at 3000–4000 tonnes, which provides about
800 tonnes of oil. Borage seed is $3000–4000 per
tonne. The price of the oil fell from $60 to $35 per
kilogram over the 10 years to 1999.
0014Blackcurrant seed oil is a byproduct of the produc-
tion of blackcurrant juice and jelly, and production is
estimated to be perhaps 50–100 tonnes per annum.
0015These oils are most often supplied in capsules with
added tocopherol and ascorbyl palmitate to serve as
antioxidants. The products have a shelf-life of about
3 years.
Sources with Higher Levels of GLA
0016It is possible to raise the level of GLA in these oils by
selective enzymic reaction, and enhanced evening
primrose and borage oils, with twice the normal
level of GLA, have been produced and offered for
sale. These materials are mainly triacylglycerols but
may contain up to 20% of diacylglycerols. Proced-
ures are based on the fact that some enzymes discrim-
inate against acids having a double bond close to the
acyl function and GLA with D6 unsaturation can be
distinguished from the more common unsaturated
acids with D9 unsaturation (oleic and linoleic). This
differentiation is apparent in hydrolysis, esterifica-
tion, acidolysis, and alcoholysis reactions promoted
by appropriate lipases. The following examples are
typical.
.
0017Borage oil (22% GLA) is hydrolyzed completely
(Pseudomonas species, 35
C, 24 h), and the acids
are selectively esterified with lauric alcohol in
the presence of Rhizopus delemar lipase at 30
for 20 h. The latter enzyme discriminates against
GLA, and this acid is concentrated in the unester-
ified acids, which finally contain 70% GLA (74%
recovery). If the esterification is repeated, 94%
GLA is obtained with 68% recovery (C. rugosa,
35
C, 15 h).
.
0018Selective hydrolysis of borage oil (22% GLA) with
C. rugosa lipase at 35
C for 15 h gives glycerol
esters with 46% of this acid that can hardly be
raised above this value. After removal of free
acids, a second hydrolysis raises the concentration
to 54% with 76% recovery. On a pilot-plant scale,
7 kg of borage oil gives 1.5 kg of upgraded oil (56%
tbl0003 Table 3 Distribution of GLA between the sn-1, 2, and 3
positions in evening primrose, borage, and blackcurrant seed
oils
epo
a
epo
b
bor
b
bck
b
Tag 9.6 9.3 24.8 15.9
sn-1 7.2 3.6 4.0 4.1
sn-2 10.7 10.7 40.4 17.4
sn-3 10.9 13.5 30.1 25.8
Forabbreviations,see Table 2.
a
From Laakso P and Christie WW (1990) Lipids 25: 349–353.
b
From Lawson LD and Hughes BG (1988) Lipids 23: 313–317.
2310 FATTY ACIDS/Gamma-linolenic Acid

GLA) after removal of free acid by molecular dis-
tillation. Starting with an already upgraded borage
oil (10 kg, 45% GLA), high-quality GLA (2.1 kg,
98% pure, 49% recovery) is obtained by hydrolysis
and two separate partial esterifications.
There are several reports of plants such as rapeseed/
canola genetically modified to produce GLA, but
commercial products are not yet available. One
example that has been cited has the following fatty
acid composition: 16:0 4.2%, 18:0 3.7%, 9–18:1
21.6%, 11–18:1 3.4%, 18:2 26.0%, 6,9,12–18:3
37.0%, 9,12,15–18:3 1.3%, and other 2.8%.
Nutritional and Medical Uses
0019 The major applications for the oils are in the area of
healthfood supplements, but markets have also been
developed in infant nutrition, pet food, and cosmet-
ics, and the total market for these oils is around $50
million per year.
0020 Available evidence indicates that only around
5–10% of the daily intake of linoleic acid can be
converted to GLA and beyond. For a 60-kg adult
with a dietary intake of 5–20 g day
1
of linoleic acid,
the endogenous rate of formation of GLA will be
250–1000 mg day
1
or around 4–17 mg kg
1
day
1
.
0021 Human breast milk contains 100–400 mg l
1
of
GLA þ DGLA (20:3 n-6). A 5 kg baby consuming 1 l
of milk per day receives 20–80 mg kg
1
day
1
of these
two acids combined. DGLA is converted to two main
eicosanoids: PGE
1
and 15-hydroxy DGLA. PGE
1
(prostaglandin E
1
) is antiinflammatory, antithrombo-
tic, a vasodilator, and stimulates the formation of
cyclic adenosine monophosphate, which inhibits
phospholipase A
2
, whereas 5-hydroxy DGLA is a
potent natural antiinflammatory agent inhibiting the
conversion of AA (arachidonic acid) to its 5- and 12-
lipoxygenase metabolites. GLA and DGLA are con-
sidered to be useful in the treatment of diabetes,
atopic eczema, inflammation, stress, cardiovascular
disease, and cancer.
0022 Soft gelatin capsules containing evening primrose
oil have been sold as nutritional supplements for
more than 20 years and of borage oil (also marketed
as starflower oil) for more than 10 years. Samples
may be blended with other oils (fish, linseed) or
have added vitamins, minerals, or herbal extracts.
Nutritional supplements probably account for 80%
of the total usage of GLA oils.
0023 GLA has been used to treat a wide range of condi-
tions including: atopic eczema, dermatitis and other
inflammatory skin conditions, diabetic neuropathy,
breast pain, premenstrual syndrome, high blood pres-
sure, and cancer, but not all of these claims have been
confirmed.
0024Doses have not been fully defined, but the
following levels have been recommended (levels
refer to GLA and not to GLA-containing oils): nutri-
tional purposes (25–50 mg day
1
), therapeutic use
(100–500 mg day
1
), and pharmacological effect
(500–2000 mg day
1
).
0025Two products have been licensed as pharmaceut-
icals Efamast
TM
for relief of cyclical mastalgia and
Epogam
TM
for treatment of atopic eczema. There are
also a range of preterm and term infant formulations
on the market (particularly in Europe) containing
GLA from various sources. Levels of GLA in infant
formula range from 0 to 0.9%. Many skin-care
products (creams, lotions, soaps) contain GLA oils
at levels between 0.1 and 2.0%.
See also: Essential Fatty Acids; Fatty Acids:
Metabolism; Dietary Importance; Vegetable Oils: Types
and Properties; Oil Production and Processing;
Composition and Analysis; Dietary Importance
Further Reading
Christie WW (1999) The analysis of evening primrose oil.
Industrial Crops and Products 10: 73–83.
Clough P (2001) Specialty vegetable oils containing gamma
linolenic acid and stearidonic acid. In: Gunstone FD
(ed.) Structured and Modified Lipids. New York: Marcel
Dekker.
Gunstone FD (1992) Gamma linolenic acid – occurrence
and physical and chemical properties. Progress in Lipid
Research 31: 145–161.
Horrobin DF (1992) Nutritional and medical importance of
gamma linolenic acid. I. Progress in Lipid Research 31:
163–194.
Huang Y-S and Mills DE (1996) g-Linolenic Acid Metabol-
ism and its Roles in Nutrition and Medicine. Cham-
paign, IL: AOCS Press.
Huang Y-S and Ziboh A (2001) Recent Advances in Bio-
technology and Clinical Applications of Gamma Lino-
lenic Acid. Champaign, IL: AOCS Press.
Ucciani E (1995) Sources potentielles d’acide gamma-
linolenique: une revue. Oleagineux Corps gras Lipides
2: 319–322.
Analysis
E W Hammond, Greenisle-Consulting, Warkton,
Kettering, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Fatty acids occur in food lipids mainly as esters
of glycerol. Fatty acids are represented in food
lipids (typical ranges shown in brackets) by
FATTY ACIDS/Analysis 2311
triacylglycerides (> 90%), diacylglycerides and mono-
acyglycerides (in the range 0.5–4%), other polar
lipids such as lecithin (0.5–5%), phytosterol esters
(up to 1%), and free fatty acids (up to 1%).
Extraction of Lipids from Foods
0002 The extraction of lipids from foods in order to ana-
lyze their fatty acids is often difficult and specialized.
For example, foods may be essentially anhydrous
(e.g., biscuits, wheat flour) or wet (e.g., fresh meats,
fish). Each food type may require very different
handling to obtain the total lipids present. The fat
in biscuits can be obtained just by suspending the
crushed samples in light petroleum. However, to
obtain the total lipids from flour requires rigorous
extraction with solvents such as n-butanol. Samples
containing water in any quantity require dehydrating
first, which process might change the lipid materials,
or extraction with alcohol based solvent mixtures.
The references included in the bibliography cover a
range of techniques for specific materials.
Determination of Total Free Fatty Acids
0003 Free fatty acids (FFA) occur in refined edible oils at
less than 0.1% (w/w) and in crude oils typically from
1 to 15%. FFA at such levels are present because of
lipid hydrolysis and not as a normal natural com-
ponent of vegetable or animal lipids. The high levels
sometimes found in crude oils are as a consequence of
tissue damage leading to the release of natural lipases.
Tropical fruits such as palm are particularly prone to
high FFA levels in crude oils. (See Phospholipids:
Determination; Triglycerides: Characterization and
Determination; Vegetable Oils: Composition and
Analysis.)
0004 The normal method of determination is titration
(AOAC28:029–28:034) with a solution of potassium
hydroxide [approximately 0.1 M in 95% (v/v) etha-
nol]. This procedure is applicable to all oils and fats
that are soluble in the solvent mixture 1/1 (v/v) of
ethanol 95% (v/v) and diethyl ether. The titrimetric
procedure uses an indicator solution which is phenol-
phthalein [10 g l
1
in ethanol 95% (v/v)], for deter-
mining the end point. In the case of fats, which
give colored solutions, a potentiometric technique
should be used to determine the end point. The result
of the titration is the average of two duplicate
determinations and is expressed as one of the
following:
1.
0005 Acid value (AV): the number of milligrams of
potassium hydroxide required to neutralize 1 g
of the fat. This is given by the formula:
A:V: ¼ð56:1PVÞ=m,
where V ¼ number of milliliters of potassium
hydroxide; P ¼ exact molarity of potassium hy-
droxide; and m ¼ mass (g) of the test portion.
2.
0006FFA%: the acidity in per cent given by the
formula:
Acidity ¼ðPVM
r
Þ=ð10mÞ,
where V ¼ number of milliliters of potassium
hydroxide; P ¼ exact molarity of potassium
hydroxide; M
r
¼ relative molecular mass (see
Table 1); and m ¼ mass (g) of the test portion.
0007When determining FFA%, it is conventional to
express the value as ‘oleic acid.’ However, where the
type of fat is known, the relative molecular mass
should be used for improved accuracy. This can be
calculated from the average molecular weight of fatty
acids, determined from the analysis of fatty acids by
gas chromatography. Otherwise, the values for M
r
,as
shown in Table 1, should be used.
0008To maintain precision, the size of sample to be used
in the determination will depend on the expected level
of FFA and should follow the data in Table 2.(See
Chromatography: Gas Chromatography.)
Method: Total FFA by Titration
1. 0009A quantity of the solvent mixture (1/1 ethanol/
diethyl ether) should be neutralized, just prior to
use, by dropwise addition of 0.1 M potassium hy-
droxide solution after adding the phenolphthalein
indicator at the rate of 0.5 ml per liter of solvent.
2.
0010The quantity of sample required is determined
by reference to Table 2. Duplicate samples are
weighed out accordingly, paying attention to
accuracy. The weights are recorded. The samples
are dissolved in 50–150 ml of the solvent, each.
tbl0001Table 1 Total free fatty acids
Type of fat Expressedas M
r
Coconut, palm kernel Lauric acid 200
Palm oil Palmitic acid 256
All other oils Oleic acid 282
tbl0002Table 2 Sample mass required for determination of FFA
Expected
acid value
PercentageFFA Mass of test
portion (g)
Accuracy of
weighing (g)
<1 (<1) 20 0.05
1–4 (<2) 10 0.02
4–70 (2–10) 2.0 0.01
20–70 (10–40) 0.5 0.001
>70 (>40) 0.1 0.0002
2312 FATTY ACIDS/Analysis

3.0011 While the solution is stirred continuously, titration
with 0.1 M potassium hydroxide should proceed
to the end point. The end point is a pink color,
which should persist for at least 10 s. The volume
of titrant is recorded.
Notes:
1.
0012 If the FFA is very low (< 0.2%), atmospheric
carbon dioxide may interfere significantly. It is
useful to replace the air in the titration flask with
nitrogen.
2.
0013 If the solution becomes cloudy during titration,
the volume of neutralized solvent may be in-
creased. Warming should be avoided.
3.
0014 If the quantity of hydroxide required for titration
exceeds 10 ml, a solution of 0.5 M may be used.
Determination of Individual Free Fatty
Acids
0015 Certain situations arise where it may be advantageous
to determine a single fatty acid or the distribution of
fatty acids in the FFA fraction of a fat or oil. In this
case, titration is of little use, since it does not discrim-
inate between the different fatty acids. The quickest
method is to use gas chromatography with an added
internal standard. In this method, quantitation of
both individual and total FFA is obtained and can be
measured down to 0.001% with confidence.
0016 It is possible to analyze free fatty acids directly by
capillary gas–liquid chromatography (GLC) using
special deactivated acidic phases, such as Restek
Stabilwax-DA (Stabilwax-DA crossbond fused silica
capillary column; Thames Restek UK Ltd., Berkshire,
UK). These reduce the tailing effects and nonpropor-
tional losses that are a consequence of hydrogen
bonding of FFA on the column. Analysis of deriva-
tives is advised to reduce quantitative errors that can
result.
0017 Where FFA is to be analyzed directly, it should first
be isolated by thin-layer chromatography (TLC) or
clean-up column procedures (see below). Prior to this,
an internal standard of heptadecanoic acid (C17:0)
should be added to the fat at a level consistent with
the expected level of FFA present in the fat. (See
Chromatography: Thin-layer Chromatography.)
0018 The FFA, dissolved in toluene (up to 5 mg ml
1
,
total FFA), is injected (0.5 ml) using the direct on-
column technique. The column can be a 30-m length
of 0.53-mm ID fused silica, with a 0.25-mm, cross-
bonded film suitable for the analysis of underivatized
fatty acids (see Figure 1). A 1.0-m length of fully
deactivated, blank fused silica should be fitted as
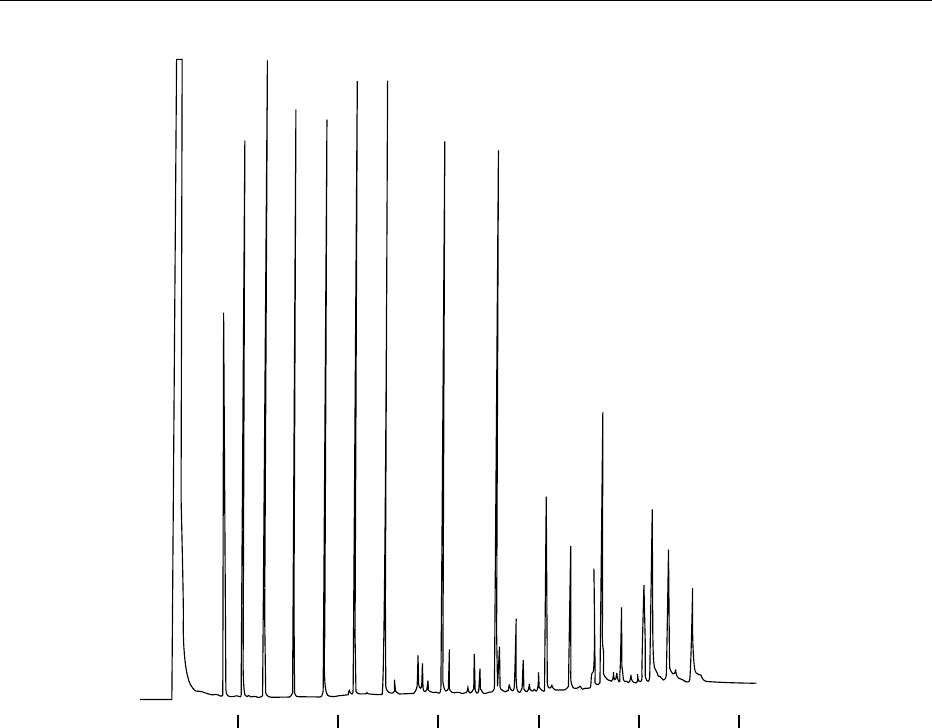
a ‘retention gap.’ Typical conditions for analysis
are shown in Figure 1. A full profile, including
unsaturated fatty acids, from butyric acid (C4:0) to
arachidic (C20:0) acid can be achieved within 30 min.
0019Where the chain-length distribution of the FFA is
wider than C14 to C20, it may be necessary to apply
correction factors to the detector response. The flame
ionization detector responds to nonoxidized carbon
in a linear relationship. However, the carboxyl carbon
is oxidized and does not respond; therefore, formic
acid gives no response, while acetic shows the re-
sponse of one carbon, and so on through the series.
Since an internal standard is used in this method, it is
a simple job to determine the response factors for the
FFA of interest relative to this standard.
Analysis of Derivatized FFA
0020Methods There are often problems in the measure-
ment of FFA by GLC. It is advisable to derivatize for
improved accuracy. Again, it will be necessary to use
an internal standard of C17:0 and relative response
factors. Two approaches may be used: methyl esters
of FFA or silyl ethers of FFA.
0021In the case of derivatives of FFA, one must consider
whether they are to be analyzed free (isolated) or in
the presence of other lipid classes such as triacylgly-
ceride (TAG) and partial glycerides. If short-chain,
volatile FFA is present (e.g., in butterfat), it is best
not to have any concentration step. By far the most
specific reagent is diazomethane, but care must be
exercised in its use, as the reagent is highly toxic and
potentially explosive.
0022The preparation of methyl esters in this way will
provide good quantitative data on the FFA portion of
the fat only. If silyl ethers are prepared, and the whole
fat mixture is analyzed by GLC, total fat information
is obtained.
0023Methyl Esters via Diazomethane An appropriate
amount (up to 50 mg) of the isolated FFA is dissolved
in diethyl ether (2 ml) containing a few drops of
methanol. The diazomethane in diethyl ether solution
is prepared, with all manipulations carried out in a
fume cupboard. Sufficient diazomethane solution is
added to the sample to leave a slight excess of yellow
color remaining. The mixture is left for no longer
than 5 min; otherwise, artificially high results may
occur. Formic acid in methanol (10%) solution is
added dropwise to remove the excess reagent. This
solution is now ready for analysis without concen-
tration. The type of GLC column used is typically a
30 m 0.32 mm i.d. fused silica, with a 1.0-m reten-
tion gap of fully deactivated fused silica. A polar
phase of bonded FFAP at 1.0-mm film thickness is
suitable. The sample is injected using the on-column
technique and not split injection. The carrier gas may
be hydrogen (linear velocity 40 cm s
1
) or helium
FATTY ACIDS/Analysis 2313
(linear velocity 20 cm s
1
). The column initial tem-
perature will depend upon the type of sample but
typically is 100
C, held for 5 min. The temperature
is then programmed to 200
Cat5
Cmin
1
. The
elution order and profile will appear very similar to
that for FFA above (Figure 1), with the advantage that
peak width and retention time will be improved.
0024 O-Trimethylsilyl Ethers (OTMSi) The most useful
reagents for the derivatization of fats are bis-
trimethylsilyl acetamide (BSA) and trimethylsilyl imi-
dazole (TSIM) (Pierce Chemical Co.), with the latter
being the stronger reagent, particularly when diacyl-
glycerides (DAG) and monoacylglycerides (MAG)
are present. Derivatization is achieved in the neat
reagent or in a solution of the lipid in chloroform or
tetrahydrofuran (10 mg ml
1
). During the procedure,
the anhydrous solution is warmed at 50
C for 5 min
in a closed vial. In this reaction, all free hydroxyls and
carboxyls are derivatized. Normally, 100% volume
excess of the reagent over the fat is sufficient, where
the level of FFA and partial glycerides is not abnor-
mally high. The minimum level of reagent will be 50–
100-fold molar excess over total free hydroxyls and
carboxyls. When using chloroform, allowance must
be made for the 2% of ethanol stabilizer. The
resulting solution is stable for up to 5 h if maintained
anhydrous in a closed vial. After this time, it should
be discarded. The solution is injected directly ‘on
column’ and not via a split technique. For GLC, use
a column of fused silica, 0.53 mm i.d. by 10 m in
length with a bonded phase of OV1, OV101 (or
min. 4 8 12 16 20 24
2
1
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
c-gram#162
fig0001 Figure 1 Organic acids and free fatty acids [Stabilwax DA, direct injection; 30 m, 0.53 mm i.d., 0.25 mm Stabilwax-DA (cat. #11025),
0.5 ml direct injection of a 5 mg ml
1
standard]. Oven temperature: 100
C (hold for 2 min) to 250
Cat8
C min
1
. Injection and detection
temperature: 280
C. Carrier gas: helium. Linear velocity: 40 cm s
1
(flow rate: 5.2 cm
3
min
1
). FID sensitivity: 8 10
11
AFS. 1: C2:0,
acetic acid; 2: C3:0, propionic acid; 3: C4:0, butyric acid; 4: C5:0, valeric acid; 5: C6:0, caproic acid; 6: C7:0, enanthic acid; 7: C8:0,
caprylic acid; 8: C9:0, capric acid; 9: C10:0, lauric acid; 10: C11:0, myristic acid; 11: C12:0, pentadecanoic acid; 12: C13:0, palmitic acid;
13: C14:0, palmitoleic acid; 14: C15:0, stearic acid; 15: C16:0, oleic acid; 16: C17:0, linoleic acid; 17: C18:1, linolenic acid. Reproduced by
permission of Restek Corporation.
2314 FATTY ACIDS/Analysis

equivalent) of film thickness 0.1–0.2 mm. A 1.0-m
length of fully deactivated, blank fused silica is fitted
as a ‘retention gap.’ The helium carrier gas is set high
at about 30 ml min
1
and a start temperature of 50
C
held for 5 min. The temperature is then programed at
5
C min
1
up to 320
C in a single ramp. The elution
order is fatty acids, MAG, DAG, and TAG in order of
molecular weight. The method is suitable for neutral
fat extracts. In some fats, the level of free sterols is
significant, and OTMSi ethers of sterols will appear
on the chromatogram.
Fatty Acid Methyl Esters from
Triacylglycerides
0025 There are a number of methods used to prepare fatty
acid methyl esters (FAME) from TAG to enable their
analysis by GC. The boron trifluoride/methanol re-
agent is widely used (AOAC -28:057). The reagent is
toxic and unstable during storage, and has been
found to be prone to form artefacts from some oxy-
genated, cyclic, and some polyunsaturated fatty acids.
0026 Transmethylation techniques are very rapid, but
they produce FAME only from glyceride esters and
not from any FFA component. It is important, also,
that the reagent and fat solutions are anhydrous;
otherwise, significant (and even large) amounts of
unesterified FFA can result. Of this group, 0.5 M
sodium methoxide/methanol is widely used. This
reagent is hazardous to prepare and dispose of.
Anhydrous potassium hydroxide/methanol (0.2 M)
is perhaps a more preferable reagent, being easy to
prepare as required, although storage is acceptable
provided it is kept dry. It should be noted that if
conjugated unsaturated fatty acids are known to be
present, they might be unstable in alkaline reagents.
These reagents should be stored in bottles with poly-
carbonate stoppers; glass stoppers will fuse to glass
bottles.
0027 For up to 50 mg of sample in 3–5 ml of reagent
(with 1.0 ml of toluene added as solubilizer), heated
to 50
C, the reaction time is normally 15 min. At the
end of reaction, the sample is allowed to cool, and
5 ml of aqueous acetic acid (5%) is added carefully,
followed by up to 10 ml of hexane (dependent upon
sample size) such that the ester concentration is about
5mgml
1
(suitable for direct sampling on to GLC).
This mixture needs to be shaken thoroughly and
allowed to separate. The lower layer is removed by
aspiration and discarded. A 5-ml aliquot of water is
added, and the mixture shaken and separated again.
Most of the top layer is then transferred to a vial
containing about 2 g of anhydrous sodium sulfate,
which dehydrates the solution ready for GLC. No
concentration step should normally be necessary.
0028Acid methanolysis reagents methylate most lipid
classes including FFA. They require relatively long
reaction times but very short processing times,
which means that technicians are not tied to one
technique for long periods. Since the reagents are
acidic, they are not suitable for epoxy fatty acids or
other acid-labile fatty acids. Anhydrous hydrochloric
acid/methanol (typically 5% or saturated) is suitable
and popular. This produces methyl chloride during
storage. In addition, while it is easy to use and pre-
pare by bubbling hydrogen chloride gas from a cylin-
der into anhydrous methanol, this can be hazardous.
Alternatively, acetyl chloride (50 ml) can be added to
cold (5
C) anhydrous methanol (450 ml); methyl
acetate is formed as a byproduct. However, several
workers, when using this latter reagent, have reported
yield problems.
0029Sulfuric acid/methanol with toluene as solubilizer
(1:10:20 by volume of sulfuric acid–toluene–metha-
nol) is a convenient reagent. It is easy and safe to
prepare, is stable at ambient for long periods, and is
easy to use. Sulfuric acid is added to cooled and
stirred methanol, followed by the toluene. The solu-
tion is stored in a brown stoppered bottle and must be
kept dry. Acid-resistant gloves and a face shield
should be worn while mixing in the acid. For use,
5 ml of reagent is added to up to 50 mg of sample,
which is refluxed for 60 min in a tube. The sample is
then diluted with 5 ml of water. A volume of hexane is
added (to give an approximate sample concentration
of 5 mg ml
1
), and the mixture is shaken thoroughly.
On separation, the bottom layer is aspirated to waste.
A further 5 ml of water is added and the mixture
shaken. The top layer is transferred to a vial contain-
ing 2 g of anhydrous sodium sulfate. This solution is
then ready for analysis by GLC. If short-chain (< C10)
fatty acids are present, the toluene may interfere with
the GLC analysis. In this case, a reagent made up
without the toluene solubilizer should be used, but
note that an extended reflux (90 min) may be neces-
sary, unless the fat dissolves rapidly.
Chromatographic Methods
0030Some of the specific points have been covered above
and should be considered in combination with this
information.
Thin-layer Chromatography (TLC)
0031FFA is easily separated from other lipid classes on
silicic acid TLC plates. This technique is excellent
for preparative work. Plates coated with Kieselgel
with (‘G’) or without (‘H’) calcium sulfate binder can
be used. The plates should be activated before use.
For speed, individual plates may be placed flat on the
FATTY ACIDS/Analysis 2315

turntable in a microwave oven for 2 min at full power
and then cooled. Multiple samples may be spotted in
lanes or, for preparative work, the sample may be
applied as a continuous streak 1 cm above the base
of the plate. The sample area is focused before the
main chromatography by developing the plate to just
above the sample application line twice in a solvent of
diethyl ether. The plate is air-dried between each
focus development, and then developed in the main
solvent, which, for FFA, is a mixture of diethyl ether/
light petroleum 40–60
with added formic acid (pro-
portions 18/82/1 by volume). After development to
within 1 cm of the top of the plate, the plate is air-
dried and sprayed lightly with a methanolic solution
of dichlorofluorescein (0.1%). After drying, the plate
is viewed under ultraviolet (UV) light (254–320 nm),
which causes most lipid components to fluoresce.
Lipids that absorb in the UV (e.g., conjugated unsa-
turates) appear as violet spots. FFA lies between TAG
and DAG with an Rf of approximately 0.6. If there is
some confusion over the position of the FFA, a stand-
ard can be run in 1-cm lanes at the sides of the plate.
Samples that contain FFA with a wide range of chain
lengths (C4 to C20, for instance) often give a broad
band or even double bands. The longer-chain FFA is
less polar than the short-chain FFA and therefore runs
slightly higher up the plate. The FFA band should be
marked, scraped off into a sinter glass disc filter stick,
and eluted off with diethyl ether. Careful concentra-
tion yields the dry FFA fraction. If small or volatile
fractions are suspected (this is particularly useful for
radioactive labeled fractions), they may be stabilized
prior to concentration by the addition of a known,
small amount of 10% potassium hydroxide in metha-
nol (enough to form the salts). After concentration,
the salts are dissolved in a small volume of 1:1 diethyl
ether:methanol containing sufficient formic acid to
re-form the acids prior to methylation.
High-performance Liquid Chromatography (HPLC)
0032 It is recommended that FFA be analyzed by GLC, but
for various reasons, the use of HPLC may be dictated.
To overcome the lack of a chromaphore, most workers
use UV-absorbing derivatives of FFA. The most popu-
lar and successful has been the phenacyl ester. The
dansyl piperazide derivative has been applied success-
fully for fluorescence. (See Chromatography: High-
performance Liquid Chromatography.)
0033 Typical chromatography conditions for phenacyl
esters include a 25-cm 4-mm i.d. column packed
with C18 reversed phase material of 5-mm particle
size. A solvent of acetonitrile–water (80:20, v/v) is
run isocratically at 2.0 ml min
1
for the first 30 min.
The solvent is then linearly programed to 85:15 (v/v)
for another 15 min. There is a considerable overlap of
peak compositions, and it is essential that a full set of
standard materials is chromatographed to determine
retention behavior and times. For instance, myristic
acid (C14:0) has an elution time of about 16 min,
whereas arachidonic acid (C20:4) elutes around
18 min. Stearic acid (C18:0) has an elution time of
about 50 min.
Gas–liquid Chromatography (GLC)
0034This item has been covered earlier. However, the ap-
plications described are designed for capillary GLC.
Modern instruments together with capillary or wide
bore columns are relatively easy to use and produce
high-quality results. However, the methods do not
preclude the use of packed columns, although the
chromatography of unesterified FFA is less successful.
For esterified FFA, packed columns of either 2 or
4 mm i.d. by about 2 m in length should be used,
with a carrier gas of nitrogen at 30 ml min
1
for a
2-mm column and 60 ml/min for a 4-mm column. For
the column packing, a phase of 10% SP2330 on 100/
120 mesh Supelcoport should be used, and this should
be operated isothermally at 180
C or programed
from 50 to 200
Cat5
Cmin
1
after holding for
5 min. The sample solution is then injected directly
on-column into a heated injection area set isother-
mally at 200
C.
Determination of
Trans
Fatty Acids
0035Isolated, methylene-interrupted trans double bonds
show an infrared (IR) band absorbing at about
967 cm
1
. This absorption may be used to estimate
the trans content in edible fats. The AOAC publishes
a typical method AOAC 28.052–28.067 for trans
values (TV). There are some drawbacks with the IR
method, which can lead to some inaccuracies. Essen-
tially, when TV is measured on triacylglyceride, the
value obtained is some 2 units higher than when the
same sample is measured as methyl esters. This prob-
lem is worse for samples below 15% trans. Also,
isomerized or oxidized fats (mainly) exhibit conju-
gated species that show absorption close to the isol-
ated trans bond. This interferes with the correct
allocation of baseline and makes low values less easy
to define accurately. The following method takes into
account these problems and introduces the use of
standards and references, although it must be remem-
bered that problems still exist with the determination
of absolute TVs.
0036The method measures the TV on triacylglyceride,
but we recommend conversion to methyl esters for
values of less than 5% trans. The values are expressed
as the percentage of trielaidin compared with a stand-
ard curve of trielaidin in tristearin. Confusions in
2316 FATTY ACIDS/Analysis

interpretation exist where there are compounds pre-
sent that show absorption in the region 970 cm
1
.
Pure trielaidin and tristearin (99%) are required as a
standard and reference, together with a recording
double-beam IR spectrometer suitable for quantita-
tion between 1100 and 900 cm
1
and a matched pair
of cuvettes of path length 1 mm (deviation < 1%) with
windows of sodium chloride or potassium bromide.
The solvent is carbon disulfide and solutions and
measurements are all at 20
C. The solvent is toxic,
and therefore, all manipulations of open solutions
should be done in a fume cupboard and appropriate
solvent-resistant gloves must be worn. Alternatively,
the use of Fourier transform infrared (FTIR) greatly
improves the methodology and accuracy.
Method: Determination of
Trans
Value by IR
Spectroscopy
1.0037 The spectrometer is set to record in the range
1050–900 cm
1
, with a narrow slit and slow
recording speed. (See Spectroscopy: Infrared and
Raman.)
2.
0038 Solutions are prepared according to Table 3 and
made up to exactly 10 ml in volumetric flasks.
3.
0039 A cuvette is filled with solution 1 and placed in
the reference beam. The absorbances of the other
solutions are recorded against this.
4.
0040 For each spectral record, a straight baseline is
drawn joining the minima at 1000 and 925 cm
1
.
The absorbance of the peak is calculated. If
transmission is recorded, the absorbance can be
determined by the formula:
Absorbance A ¼ log
10
ðBD=BCÞ,
where B is the zero/? chart point, D is the center
point of the baseline, and C is the peak apex.
5.
0041 The calibration line is constructed (this should be
a straight line).
6.
0042 If necessary, all samples are liquefied and hom-
ogenized. Solutions in carbon disulfide of 200 mg
(to the nearest 0.1 mg) in 10-ml volumetric flasks
are made up to volume.
7.
0043 All sample solutions are read against the same
reference solution 1 (tristearin). The baselines are
constructed, and the peaks are measured as above.
8.
0044The values (equivalent to trielaidin) for samples
are read off the standard line, and the percentage
trans from the sample weight is calculated.
9.
0045If there is a significant degree of interference to the
construction of the baseline, the samples should be
converted to methyl esters. This should also be
done where the TV is below 5%. In this method,
the standard is methyl elaidate, and the reference
becomes methyl stearate. All other manipulations
are the same.
See also: Chromatography: Thin-layer Chromatography;
High-performance Liquid Chromatography; Fats:
Classification; Phospholipids: Determination;
Spectroscopy: Infrared and Raman; Triglycerides:
Characterization and Determination; Vegetable Oils:
Composition and Analysis
Further Reading
Borch RF (1975) Analytical Chemistry 47: 2437–2439.
Fales HM, Jaouni TM, Babashak JF et al. (1973) Analytical
Chemistry 45: 2302–2303.
Hamilton RJ and Hamilton S (eds) (1992) Lipid Analysis –
A Practical Approach. Oxford: IRL Press, Oxford
University Press.
Hammond EW (1993) Chromatography for the Analysis of
Lipids. Boca Raton, FL: CRC Press.
Horwitz W (ed.) (2000) Official Methods of Analysis of the
Association of Official Analytical Chemists, 17th edn.
Washington, DC: AOAC.
Madison BI, Depalma RA and D’Alonzo RP (1982) Journal
of American Oil Chemists’ Society 59: 178–181.
Sheppard AJ and Iverson JL (1975) Journal of Chromato-
graphic Science 13: 448.
Torres AG, Trugo NMF and Trugo LC (2002) A mathemat-
ical method for the prediction of retention times of fatty
acid methyl esters in temperature-programmed capillary
gas-chromatography. Journal of Agricultural and Food
Chemistry 50: 4156–4163.
Wood R and Lee T (1983) Journal of Chromatography 254:
237–246.
Yanagisawa I and Yamane M (1985) Journal of Chroma-
tography 345: 229–240.
Dietary Importance
C J Field, University of Alberta, Edmonton, Alberta,
Canada
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001In Western countries, an adult eats on average 75–
150 g of fat each day, representing 30–45% of the
energy in the diet. Triacylglycerols constitute more
tbl0003 Table 3 Calibration solutions for IR spectroscopy
Material (mg)
a
Solution
12345
Tristearin 200 175 150 100 50
Trielaidin 0 25 50 100 150
a
Weighed to the nearest 0.1 mg.
FATTY ACIDS/Dietary Importance 2317
