Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


inactivity. (See Dietary Fiber: Physiological Effects;
Effects of Fiber on Absorption.)
0021 -Predigested, chemically defined formulas Pre-
digested, chemically defined formulas are also called
elemental formulas or diets. These preparations are
used in the nutritional management of patients with
impaired gastrointestinal function, such as severe im-
pairment of luminal nutrient hydrolysis or substan-
tially reduced functional absorptive capacity (e.g.,
short-bowel syndrome, exocrine pancreatic insuffi-
ciency). Predigested diets contain carbohydrate in the
form of glucose polymers, peptides and amino acids as
the nitrogen source, fat, usually as a mixture of
medium-chain and long-chain triglycerides, and the
full complement of vitamins and minerals.
0022 -Disease-specific formulas Specific dietary formula-
tions have been developed for patients with condi-
tions such as liver disease, renal disease, or
respiratory disease.
0023 Liver disease Patients with encephalopathy and cir-
rhosis have abnormal plasma amino acid profiles
characterized by raised levels of aromatic amino
acids (i.e., tyrosine and phenylalanine) and depressed
levels of branched-chain amino acids (i.e., leucine,
valine, and isoleucine). These changes in the pattern
of amino acid levels have been implicated in the
pathogenesis of hepatic encephalopathy. Enteral
feeding preparations containing low levels of aro-
matic amino acids and higher levels of branched-
chain amino acids are available for oral or tube
feeding: the administration of these diets may be of
benefit to patients with encephalopathy associated
with acute or chronic liver disease. (See Liver: Nutri-
tional Management of Liver and Biliary Disorders.)
0024 Renal disease Formulas containing all of the essen-
tial amino acids plus histidine, without the non-
essential amino acids, have been developed for
patients with impaired renal function. It is suggested
that these diets reduce urea production and promote
the reutilization of nonessential amino acids in the
liver by transamination. At present, there is little
evidence to support their chronic use to delay pro-
gression of chronic renal disease.
0025 Respiratory disease The level of energy from carbo-
hydrate in enteral formulas can result in further in-
creased production of carbon dioxide and respiratory
distress in some patients. In order to reduce the pro-
duction of carbon dioxide, the use of enteral formulas
which have a higher fat:carbohydrate ratio has been
suggested for patients with respiratory disease. These
formulas may facilitate more rapid weaning from
ventilatory support.
0026-Modular diets Modular diets allow specific com-
ponents of a diet to be altered according to the indi-
vidual patient requirement. These include products
that provide various types of isolated carbohydrate,
protein, and fat compounds.
Initiation and Contraindications of Enteral Feeding
0027Some care teams administer small volumes of diluted
enteral feeds during the initial stages of nutrition
support in order to minimize any gastrointestinal
side-effects. Some studies report that the use of dilute
‘starter regimens’ does not decrease the frequency of
gastrointestinal side-effects but may result in reduced
energy and nutrient intake and thus may adversely
affect the nitrogen balance. Enteral nutrition is
contraindicated in patients with an inaccessible or
nonfunctioning gastrointestinal tract (e.g., paralytic
ileus, intestinal obstruction).
Complications of Enteral Nutrition
0028Malposition of enteral feeding tubes may occur, but
this can usually be avoided by strict adherence to the
protocol for the insertion of feeding tubes, and con-
firmation of the position of the tube. Unintentional
removal of feeding tubes is commonly noted in
agitated, disoriented patients. Blockage of tubes can
occur unless they are flushed regularly.
0029Diarrhea is the most commonly reported side-
effect, and the factors implicated are high osmotic
loads, concomitant antibiotic therapy, and exces-
sively rapid infusion. Problems of lactose intolerance
are limited, since the majority of commercial enteral
formulas are low in lactose or lactose-free. Tube-
feeding-related diarrhea occurs in up to 30% of
patient, but its etiology is unclear. Nausea, vomiting,
bloating, cramps, and abdominal distension can
occur in some patients, especially following bolus
feeding or when the feed is infused at a high flow
rate. Pulmonary aspiration and regurgitation may be
reduced by elevating the head of the patient’s bed.
Duodenal (transpyloric) feeding may be beneficial in
patients at risk for aspiration.
0030Abnormalities of liver function tests are generally
minor and reversible with an enteral feeding regime.
Regular monitoring of patients enables early detec-
tion of biochemical imbalances, including electro-
lyte and fluid balance abnormalities. Drug–nutrient
interactions can occur with many medications, in-
cluding theophylline, warfarin, methyldopa, and
digoxin.
ENTERAL NUTRITION 2117

Monitoring Patients Receiving Enteral
Nutrition
0031 Monitoring enterally fed patients is an essential as-
pect of providing safe and effective nutrition support.
Fluid Balance Charts and Weight
0032 Continuous fluid-balance records enable an accurate
review of the patient’s actual intake of enteral for-
mula compared to the prescribed regimen, as well as
other fluid intake. The prescribed regimen and the
actual intake may not be the same, and without
records, patients may receive suboptimal (inadequate
or excessive) levels of nutrition support. All patients
receiving enteral nutrition support should be weighed
regularly. The frequency of the weight assessment
depends upon the patient’s age, nutritional status,
and medical condition.
Biochemical and Hematological
0033 Plasma glucose, electrolytes, calcium, phosphate, and
magnesium should be monitored regularly, and more
frequently in severely malnourished patients. Plasma
proteins are useful markers of nutritional status, trans-
ferrin and prealbumin in the short-term, and albumin
in the long-term. Hemoglobin and the white blood cell
and lymphocyte count should be monitored regularly
and often. (See Nutritional Assessment: Biochemical
Tests for Vitamins and Minerals.)
Anthropometric and Dynamometric
0034 Measurements of mid-arm circumference and triceps
and subscapular skinfolds should be taken regularly
and are commonly used for research purposes. (See
Nutritional Assessment: Anthropometry and Clinical
Examination.)
Ending Enteral Tube Feeding
0035 As the patient is able to increase oral intake of regular
food, the administration of the enteral tube feed is
decreased. Oral and enteral tube intake should be
monitored using food record charts to document the
nutritional quality of the total intake. Enteral feeding
should not be discontinued until adequate nutrition
can be taken from food and/or oral dietary supple-
ments, and normal hydration and weight maintained.
Nutrition Team
0036 Team management of enterally fed patients reduces
associated morbidity and optimizes nutrition
support. This interdisciplinary team usually includes
a dietitian/nutritionist, nurse, and physician.
See also: Carbohydrates: Requirements and Dietary
Importance; Dietary Fiber: Physiological Effects; Effects
of Fiber on Absorption; Energy: Measurement of Food
Energy; Fats: Requirements; Liver: Nutritional
Management of Liver and Biliary Disorders; Malnutrition:
The Problem of Malnutrition; Minerals – Dietary
Importance; Nutritional Assessment: Anthropometry
and Clinical Examination; Biochemical Tests for Vitamins
and Minerals; Protein: Requirements; Deficiency;
Vitamins: Overview
Further Reading
Alpers DH and Klein S (1999) Approach to the patient
requiring nutritional supplementation. In: Yamada T,
Alpers DH, Laine L, Owyang C and Powell DW (eds)
Textbook of Gastroenterology 3rd edn, pp. 1081–1107.
Philadelphia, PA: Lippincott Williams & Wilkins.
DeLegge MH and Copenhaver (1999) Enteral and paren-
teral nutrition. In: Brandt LJ and Daum F (eds) Clinical
Practice of Gastroenterology, 1st edn, vol. 2, pp. 1567–
1575. Philadelphia, PA: Churchill Livingstone.
Forchielli ML, Hendricks KM and Lo CW (1999) Home
enteral parenteral nutrition. In: Walker WA and Watkins
JB (eds) Nutrition in Pediatrics: Basic Science and
Clinical Applications, 2nd edn, pp. 727–733. Hamilton,
Ontario: B.C. Decker.
Mascarenhas MR, Tershakovec AM and Stallings VA
(1999) Parenteral and enteral nutrition. In: Wyllie R
and Hyams JS (eds) Pediatric Gastrointestinal
Disease, 2nd edn, pp. 741–757. Philadelphia, PA: W.B.
Saunders.
Mascarenhas MR, Kerner JA and Stallings VA (2000) Par-
enteral and enteral nutrition. In: Walker WA, Durie PR,
Hamilton JR, Walker-Smith JA and Watkins JB (eds)
Pediatric Gastrointestinal Disease, 3rd edn, pp.
1705–1752. Hamilton, Ontario: B.C. Decker.
Shike M (1999) Enteral feeding. In: Shils ME, Olson JA,
Shike M and Ross AC (eds) Modern Nutrition in Health
and Disease, 9th edn, pp. 1643–1656. Philadelphia, PA:
Williams & Wilkins.
Smith P, Zeitzer J and Leibowitz AB (1995) Enteral nutri-
tion. In: Haubrich WS, Schaffner F and Berk JE (eds)
Gastroenterology, 5th edn, pp. 3209–3220. Phila-
delphia, PA: W.B. Saunders.
Stoker TW and Castle JL (1999) Special diets. In: Walker
WA and Watkins JB (eds) Nutrition in Pediatrics: Basic
Science and Clinical Applications, 2nd edn, pp.
727–733. Hamilton, Ontario: B.C. Decker.
Torosian MH (ed.) (1995) Nutrition for the Hospitalized
Patient: Basic Science and Principles of Practice. New
York: Marcel Dekker.
2118 ENTERAL NUTRITION

Enterobacteriaceae See Escherichia coli: Occurrence; Detection; Food Poisoning; Occurrence and
Epidemiology of Species other than Escherichia coli; Food Poisoning by Species other than Escherichia coli
Enzymatic Browning See Browning: Nonenzymatic; Toxicology of Nonenzymatic Browning; Enzymatic
– Biochemical Aspects; Enzymatic – Technical Aspects and Assays
Enzyme Immunoassay See Immunoassays: Principles; Radioimmunoassay and Enzyme
Immunoassay
ENZYMES
Contents
Functions and Characteristics
Uses in Food Processing
Uses in Analysis
Functions and Characteristics
J R Whitaker, University of California, Davis, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Enzymes are absolutely essential for life. They occur
in all animals, plants, and microorganisms. Nearly all
chemical reactions in living organisms are a result of
enzyme activities. Enzymes cause reactions to occur
rapidly at ambient temperatures with a high degree
of specificity. As an example, it takes 24–72 h at
100–110
C and 6 N hydrochloric acid to hydrolyze
a protein. Gastrointestinal-tract enzymes accomplish
this in 2–4 h at 37
C. There are thousands of
different enzymes in living organisms.
Definition of an Enzyme
0002 An enzyme is a protein with catalytic properties due
to its power of specific activation. Some enzymes also
require cofactors for activity. The properties and
characteristics of enzymes are described below. A
few ribonucleic acids, called ribozymes, also act as
catalysts for a limited number of hydrolytic reactions,
but they do not fit the definition of an enzyme as
defined above.
History
0003Although the effects of enzymes, such as fermenta-
tion, digestion, milk clotting and meat tenderization,
were known for centuries, the first clear recognition
of an enzyme as a unique compound was in 1833,
when Payen and Persoz reported that an alcohol
precipitate of malt extract contained a thermolabile
substance ‘diastase’ (now known as amylase) because
of its ability to separate starch from insoluble envel-
opes of starch grains. They identified some character-
istics of enzymes – precipitation with alcohol,
thermolability, and specificity – and designed a
naming system (stem of substrate name plus the suffix
‘-ase’) that is used universally today. Schoenbein, in
1855, discovered peroxidase in plants that, in the
presence of hydrogen peroxide, causes a brown
solution of gum guaiac to turn blue. Later (1856),
Schoenbein also discovered polyphenol oxidase in
mushrooms, which causes them (and many other
plant tissues) to turn brown. Berthelot in 1860
ENZYMES/Functions and Characteristics 2119

discovered invertase in yeast, which causes an inver-
sion in the optical rotation of a sucrose solution.
Several thousand enzymes are now well known.
0004 The relationship between enzymes and living cells
was a controversial topic between 1875 and 1900.
Pasteur, a microbiologist and enologist, argued that
fermentation was inseparable from living cells. Lie-
big, a chemist who demonstrated the action of pepsin
on proteins, held that enzymes are chemical sub-
stances, active in the absence of cells. Bu
¨
chner, in
1897, separated broken yeast cells from liquid, show-
ing that the cell-free extract carried out fermentation,
thereby ending the controversy. In 1878, Ku
¨
hne pro-
posed the name ‘enzyme’ (Greek for ‘in yeast’) for the
substance transforming one compound to another.
(See Fermented Foods: Origins and Applications.)
Protein Nature of Enzymes
0005 Enzymes are proteins. This was generally accepted in
the late 19th and early 20th centuries. However, this
idea was challenged in the late 1920s by Willsta
¨
tter. He
purified peroxidase until no protein was detectable,
but appreciable activity remained. Therefore, he con-
cluded that enzymes could not be proteins. Sumner, in
1926, crystallized urease from Jack Bean meal and
showed it to be a protein. This set off a polemic debate
between Willsta
¨
tter and Sumner that involved many
scientists. In 1930, the famous biochemist Haldane
indicated that, with the single exception of urease,
almost nothing was known about the chemical nature
of enzymes. However, soon thereafter, scientists at the
Rockefeller Institute in New York, led by the distin-
guished biochemist, John Northrop, crystallized
pepsin, trypsin, chymotrypsin, and carboxypeptidase
A, among others. All were proteins. More than 600
enzymes, all proteins, have now been crystallized.
0006 As proteins, enzymes have specific structures. Each
has a fixed molecular size and a specific amino acid
sequence (primary sequence), as determined by the
gene for that enzyme. The primary structure is folded,
via a-helices, b-pleated sheets, b and g bends and
random-coil segments, to give secondary structure.
Further folding, giving a tertiary structure, results
from thermodynamic and kinetic requirements that
most of the hydrophobic amino acid residues must be
inside the protein, away from water, and most of
the hydrophilic amino acid residues must be on the
surface. (See Protein: Chemistry.)
0007 Many enzymes consist of single polypeptide mol-
ecules. Others have two or more subunits (identical or
different) per molecule, giving rise to a quaternary
structure. Some enzymes associate to form macro-
molecular structure systems to convert substrate to
product(s) more efficiently. Escherichia coli pyruvate
dehydrogenase is a complex of three different
enzymes and five cofactors (M
r
of 4.44 10
6
Da).
0008Early on, researchers investigated water-soluble
enzymes. Now, much emphasis is on structurally
bound enzymes.
Solvation of Enzymes
0009Water is an important factor in determining native
enzyme structure, as well as activity. Hydrophobic
amino acid residues are largely inside the enzyme
molecule, away from water, and most of the hydro-
philic amino acid residues are on the outside. Enzymes
contain 30–40% (w/v) of bound surface water
(170–220 mol of water per 10 000 gram of protein).
The relatively high surface hydrophobicity of a folded
polypeptide chain, and its lack of hydration by water
molecules, determines whether an enzyme has a qua-
ternary structure or not. In less polar solvents than
water, but miscible with water, an enzyme probably
would not fold in the same way, and is most likely
inactive. Enzymes can be more stable suspended in
immiscible organic solvents than in water. (See Water
Activity: Effect on Food Stability.)
Size
0010Enzymes are large molecules. Ribonuclease (M
r
¼
13 683) and lysozyme (14 100) are small enzymes,
alkaline phosphatase (80 000) and mushroom poly-
phenol oxidase (128 000) are of intermediate size,
and b-galactosidase (520 000) and glutamate dehy-
drogenase (2 000 000) are large enzymes.
Catalytic Nature of Enzymes
0011The most unique feature of enzymes is their ability to
bind compounds (called substrates) stereospecifically
and to convert them to other compounds (called prod-
ucts) very efficiently. Two measures of enzyme effi-
ciency are turnover number and rate enhancement.
Example turnover numbers are 10
2
–10
7
mol of sub-
strate converted to product per second per mole of
enzyme active site for chymotrypsin and catalase,
respectively. Some examples of rate enhancement
(based on the same temperature and concentration)
are: hydrogen peroxide is converted to water and
oxygen by catalase 3.5 10
8
times faster than non-
catalytically; invertase hydrolyzes sucrose to glucose
and fructose 5.6 10
10
times faster than does 1 M
hydrochloric acid; urease hydrolyzes urea to carbon
dioxide and ammonia 4.2 10
11
times faster than
does 1 M hydrochloric acid. This remarkable rate
enhancement makes life possible at ambient tem-
peratures.
2120 ENZYMES/Functions and Characteristics
001 2 Enzymes increase rates of conversion of substrate
to product by lowering the activation energy, E
a
. E
a
is
the minimum energy that a substrate must acquire
above the ground-state energy to go to product. In
two of the examples above, catalase lowers E
a
from
18.0 to 6.4 kcal mol
1
(3.5 10
8
rate enhancement)
for the oxidoreduction of hydrogen peroxide to prod-
ucts and urease lowers E
a
from 24.2 to 8.7kcalmol
1
(4.2 10
11
rate enhancement) in the hydrolysis of
urea.
001 3 Enzymes convert substrates to products by well-
known chemical mechanisms. Because of their effi-
ciency, it was once considered that they must have
magical qualities. We now know that several well-
known factors can account for this efficiency. These
are: (1) conversion from inter- to intramolecular
reaction; (2) proximity and specific orientation of
substrate in the active site of the enzyme; (3) catalysis
by distortion of bonds; (4) general acid–general base
catalysis; and (5) nucleophilic–electrophilic catalysis.
These will be explained below.
Active Sites of Enzymes
0014 Reactions occur at active sites of enzymes. The
active site is a small area, a cavity or hole on the
surface of the enzyme. The active site consists of 10–
15 amino acid residues brought together by folding
from different parts of the primary structure of the
protein. One part of the active site is responsible for
stereospecific binding of the substrate in proximity
to the (second) transforming part of the active site. In
a-chymotrypsin, residues Ser189, Gly216, and
Gly226 are responsible for binding the peptide sub-
strate, and residues His57, Ser195, and Asp102 are
responsible for catalyzing hydrolysis of the scissile
peptide bonds. The amino group of Ile16 and the
carboxyl group of Asp194 assist in maintaining
the shape of the active site. The binding site recog-
nizes l-Tyr, l-Phe or l-Trp residues of the substrate.
Peptide bonds involving other l-amino acids or d-
amino acids are not hydrolyzed. There must be very
close complementarity, following any induced fit, be-
tween the shape of the substrate and the active site for
binding to occur. (See Amino Acids: Properties and
Occurrence.)
Proximity and Orientation Effects
0015 Proximity and orientation effects include (1) intra- vs
intermolecular catalysis, (2) reaction entropy, and (3)
effective concentration of reactive groups. An absolute
requirement for catalysis is that the substrate must
bind stereospecifically in a correct orientation at the
active site to form the enzyme–substrate complex, as
shown in eqn (1), where E is the enzyme, S is the
substrate, ES is the enzyme–substrate complex, EP
is the enzyme–product complex, and P is the product.
The obligatory ES complex converts an intermolecu-
lar reaction to an intramolecular reaction. This in-
creases the rate of an enzyme-catalyzed reaction
compared with a noncatalyzed reaction by 10
3
,
10
15
,and10
22
times for bimolecular, trimolecular,
and termolecular reactions, respectively.
E + S E
.
S E
.
P E + P.
k
1
k
−1
k
2
k
−2
k
3
k
−3
(1)
0016Binding of the substrate at the active site involves
multiple (a minimum of three and up to 12) contacts
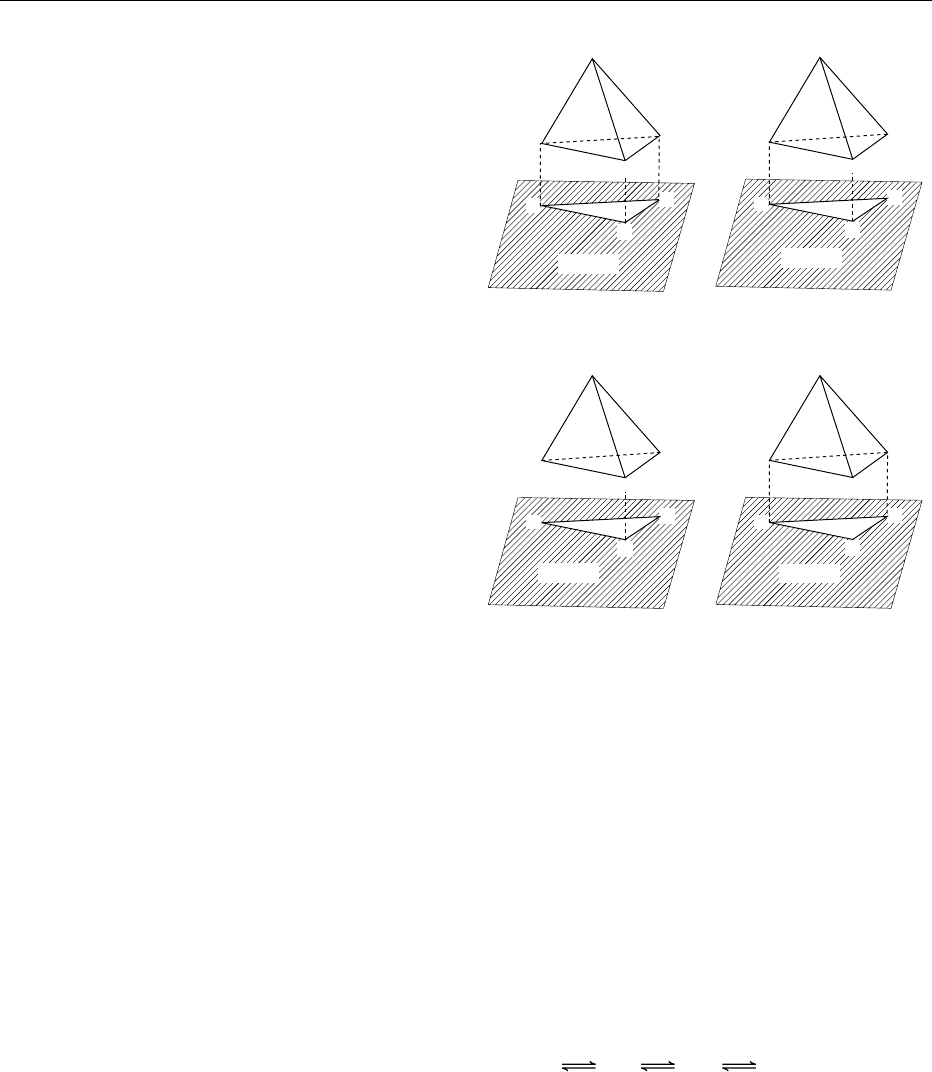
between the substrate and the enzyme (Figure 1).
0017As a result of ES, catalytic groups of the enzyme
are 0.1–0.2 nm from the substrate bond to be
transformed, usually by general acid–general base or
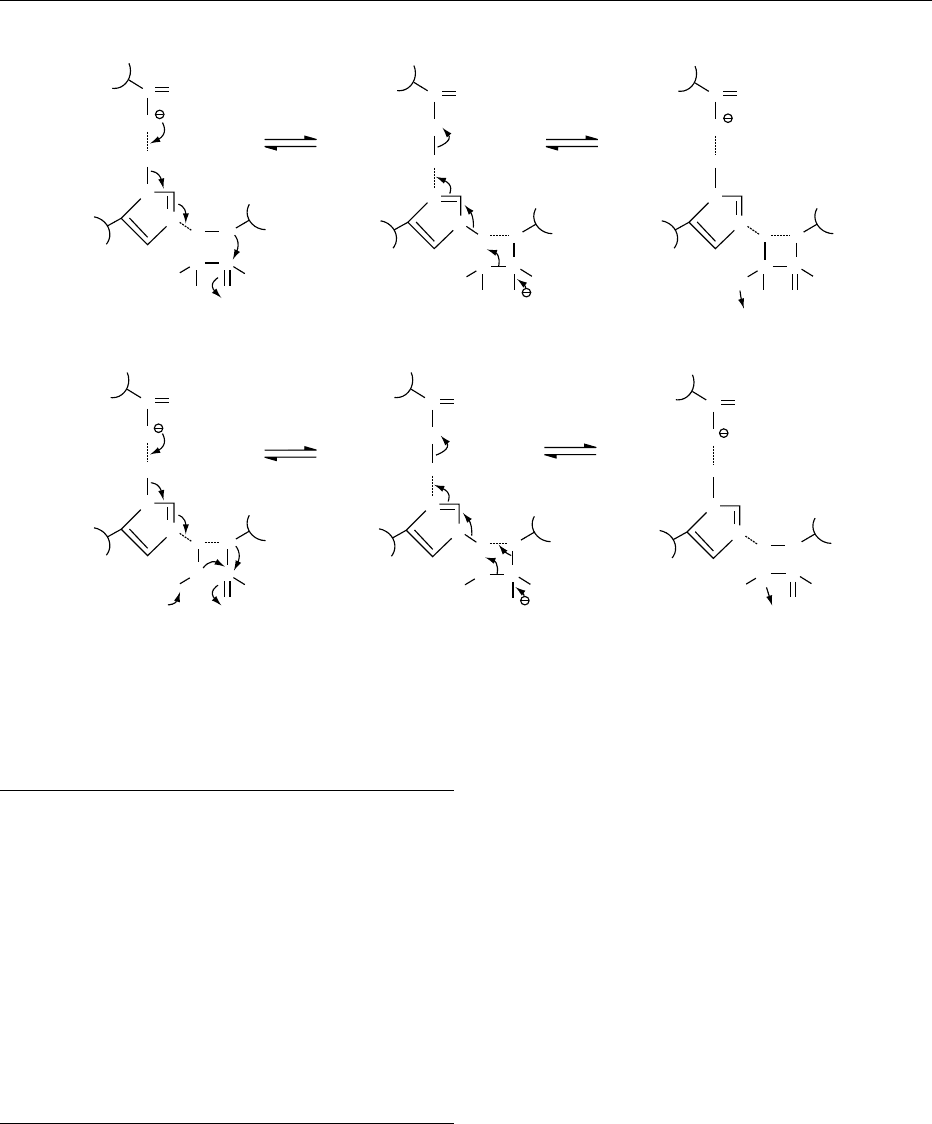
nucleophilic–electrophilic catalysis (Figure 2). This
proximity effect is equivalent to about a 10 M
concentration of the corresponding intermolecular
catalyst (His57 in Figure 2).
H
H
OH
CH
3
Enzyme
B
A
C
H
H
OH
CH
3
Enzyme
B
A
C
H
H
HO
CH
3
Enzyme
B
A
C
H
H
OH
CH
3
Enzyme
B
A
C
(a) (b)
(c) (d)
fig0001Figure 1 Schematic representation of several possible modes
of orientation of ethanol on the surface of alcohol dehydrogen-
ase. A, B, and C are binding points for two Hs, OH, and CH
3
of
ethanol on the surface of the enzyme. Only (a) shows the ethanol
bound properly with the enzyme surface.
ENZYMES/Functions and Characteristics 2121
Catalysis by Distortion
0018 There is a close complementarity between the struc-
ture and shape of a substrate and its binding position
in the active site. This led Emil Fischer to the ‘lock
and key’ analogy for the fit between a substrate and
an enzyme. Later data established that enzymes
permit some (small) latitude in the structure and
shape of a substrate, leading to the ‘rack mechanism’
(Lumry) or the ‘induced-fit’ concept (Koshland). This
results in a decrease in the activation energy needed,
with a rate enhancement of 10
2
–10
3
.
0019Based on the factors above, an enzyme increases
rates of reactions by 10
8
–10
28
, depending on how
many substrates are involved in the reaction. Bender
et al. in 1964 experimentally and theoretically
determined rates of hydrolysis of N-acetyl-l-trypto-
phanamide by the three catalysts hydroxide ion, imi-
dazole, and a-chymotrypsin (Table 1). There is
remarkable agreement between the rate calculated
by theory (Table 1, 4d) and determined experimen-
tally (Table 1, 3).
Effect of Environmental Factors on Rates
of Enzyme-catalyzed Reactions
0020Time, enzyme concentration, substrate concentra-
tion, nature of the substrate (including physical
state), pH, temperature, solvent, activators, and in-
hibitors all affect rates of enzyme-catalyzed reactions.
Asp-102
C
C
O
O
O
O
H
H
H
N
N
N
R
Ser-195
His-57
R
Asp-102
C
C
O
O
O
O
H
H
H
N
N
N
R
Ser-195
His-57
R
Asp-102
C
C
O
O
O
O
H
H
H
N
N
N
R
Ser-195
His-57
R
(A)
Asp-102
C
C
O
O
O
O
H
H
N
N
O
R
Ser-195
His-57
H
(D)
(B) (C)
Asp-102
C
C
O
O
O
O
H
H
N
N
O
R
Ser-195
His-57
H
(F)
Asp-102
C
C
O
O
O
O
H
H
N
N
O
R
Ser-195
His-57
H
(E)
(a) Acylation
(b) Deacylation
fig0002 Figure 2 Proposed mechanism for a-chymotrypsin-catalyzed reactions. (a) Acylation. (b) Deacylation.
tbl0001 Table 1 Factors responsible for the rate enhancement of
chymotrypsin-catalyzed hydrolysis of N-acetyl-
L-tryptophan-
amide relative to the hydroxide ion and imidazole
a
(1) Rate constant for hydroxide ion
catalysis
3 10
4
m
1
s
1
(2) Rate constant for imidazole catalysis 4.8 10
10
m
1
s
1
(3) Experimental rate constant for
a-chymotrypsin catalysis
4.4 10
2
s
1
(4) Calculated rate constant for
a-chymotrypsin catalysis
(cumulative):
(a) Conversion of imidazole catalysis
to intramolecular reaction (10
1
)
4.8 10
9
s
1
(b) Nucleophilic catalysis by
serine-OH(10
2
)(see Figure 2)
0.8 10
7
s
1
(c) Proximity and orientation factors
(10
3
)
4.8 10
4
s
1
(d) General acid catalysis by
imidazole(10
2
)(see Figure 2)
4.8 10
2
s
1
a
At 25
C; data from Bender ML, Kezdy FJ and Gunter CR (1964) The
mechanism of action of proteolytic enzymes. XXXIII. The anatomy of an
enzymatic catalysis. a-Chymotrypsin. Journal of the American Chemical
Society 86: 3714–3721.
2122 ENZYMES/Functions and Characteristics
Time
0021 Enzyme-catalyzed reactions are time-dependent. Ini-
tially, the concentration of product, [P], formed is
linearly related to reaction time. How long the linear
relation holds depends on the initial substrate concen-
tration, [S]
0
, in relation to the Michaelis constant
K
m
([S]
0
at which v
0
¼ 0.5V
max
; see eqn (2)), enzyme
stability, pH, temperature and activators and revers-
ibility of reaction. A tangent drawn to the initial part
of the curve gives the initial velocity, v
0
( ¼ dP/dt), an
invaluable experimental parameter in enzymology.
Enzyme Concentration
0022 For most enzyme-catalyzed reactions, v
0
is directly
proportional to the enzyme concentration, [E]
0
.
Doubling [E]
0
doubles v
0
. This is analytically very
convenient since enzyme concentrations in biological
systems are based on determination of v
0
under
standard conditions. However, there are several
cases where direct relations do not hold, as described
by Whitaker in 1994.
Substrate Concentration
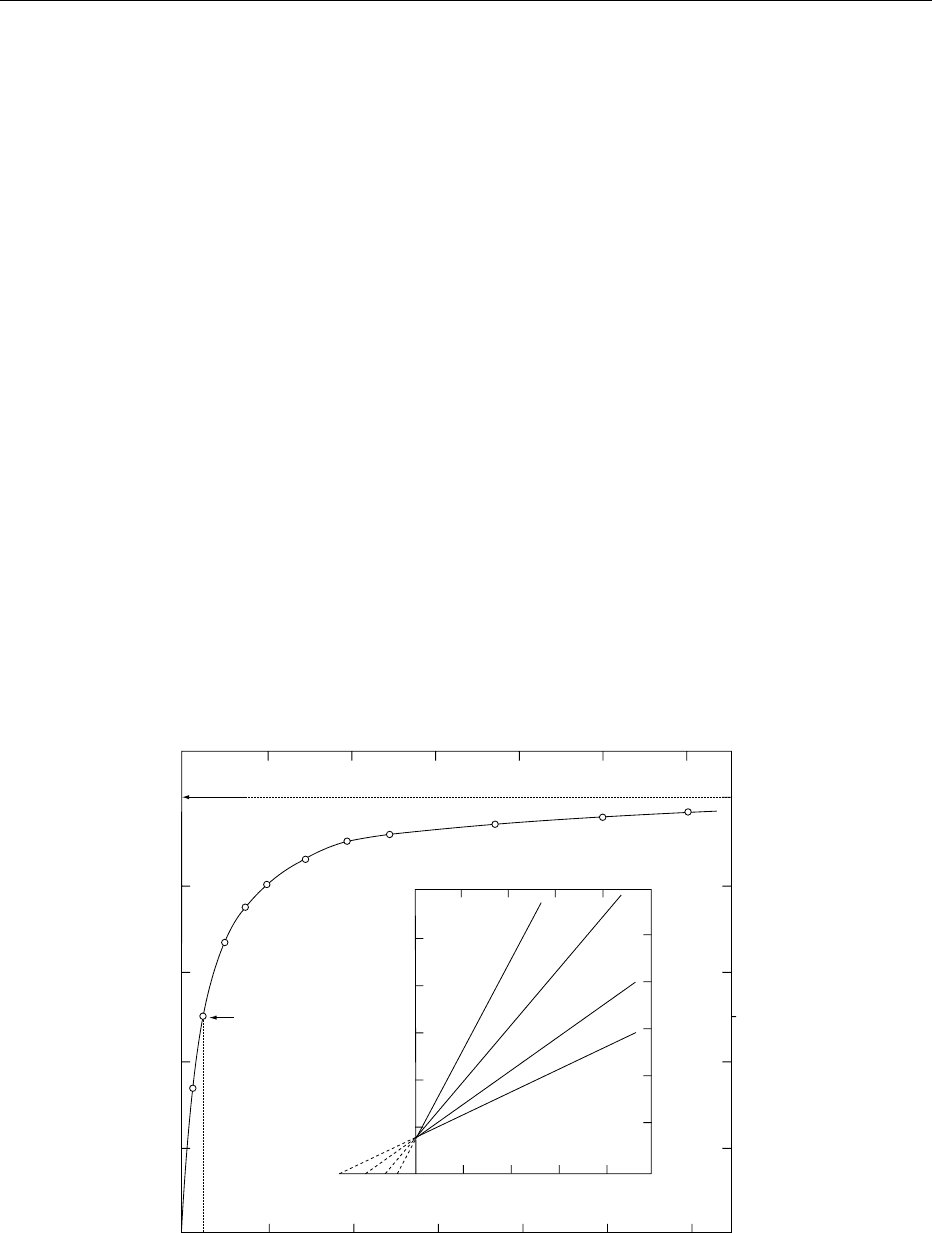
0023Because of the obligatory E S (eqn (1)), the relation
between [S]
0
and v
0
gives a right-hyperbolic plot
(Figure 3). The extent of saturation of an enzyme
with a substrate, to form ES, is dependent on the
relation of [S]
0
to K
m
.At[S]
0
<< K
m
, the reaction
rate is first order with respect to [S]
0
(dP/dt ¼k[E]
0
[S]
0
/K
m
). At [S]
0
>> K
m
, the reaction rate is zero order
with respect to [S]
0
(dp/dt ¼k[E]
0
), thereby giving the
maximum velocity, V
max
, under the conditions used.
At [S]
0
¼K
m
, v
0
¼0.5V
max
.When[S]
0
0.01K
m
100K
m
, the reaction rate is a mixture of first and
zero order.
0024Enzyme-catalyzed systems that follow Figure 3
obey Michaelis–Menten kinetics
v
0
¼ k½E
0
½S
0
=ðK
m
Þþ½S
0
Þ: ð2Þ
0025Substrate activation and inhibition and allosteric
behavior cause deviations from this relation.
0026A plot of 1/v
0
vs 1/[S]
0
gives a linear relationship
(Lineweaver–Burk equation), permitting V
max
and
K
m
to be determined readily (Figure 3 insert).
4 8 12 16 20 24
Initial velocity
0.5 V
max
0
0
K
m
Substrate concentration
0
0.5
1.0
v
0
/V
max
(A
0
)/K
m
1/v
0
1/(A
0
)
(I
0
)
3
(I
0
)
2
(I
0
)
1
(I
0
)
0
=0
0
0
V
max
fig0003 Figure 3 Relation between initial velocity, v
0
, and initial substrate concentration, [S]
0
, for an enzyme-catalyzed reaction. Insert:
effect of three different concentrations of a competitive inhibitor on 1/v
0
versus 1/[S]
0
plot. [I
0
]
0
,[I
0
]
1
,[I
0
]
2
, and [I
0
]
3
are 0, 0.5, 1.5, and 3.0
times K
i
, respectively.
ENZYMES/Functions and Characteristics 2123
pH
0027 Enzyme-catalyzed reactions are sensitive to the pH of
the reaction, resulting in a pH optimum (maximum
v
0
). Examples of pH optima are: porcine pepsin, pH 2;
horse-radish peroxidase, pH 6; bovine trypsin, pH 8;
bovine milk alkaline phosphatase, pH 10; catalase,
pH 4–9. The relation between pH and v
0
, bell-shaped
or sigmoidal, results from the effect of pH on the
ionization of essential catalytic or binding groups in
the active site of the enzyme and the effect of pH on
stability of the enzyme as a protein. (See pH – Prin-
ciples and Measurement.)
Temperature
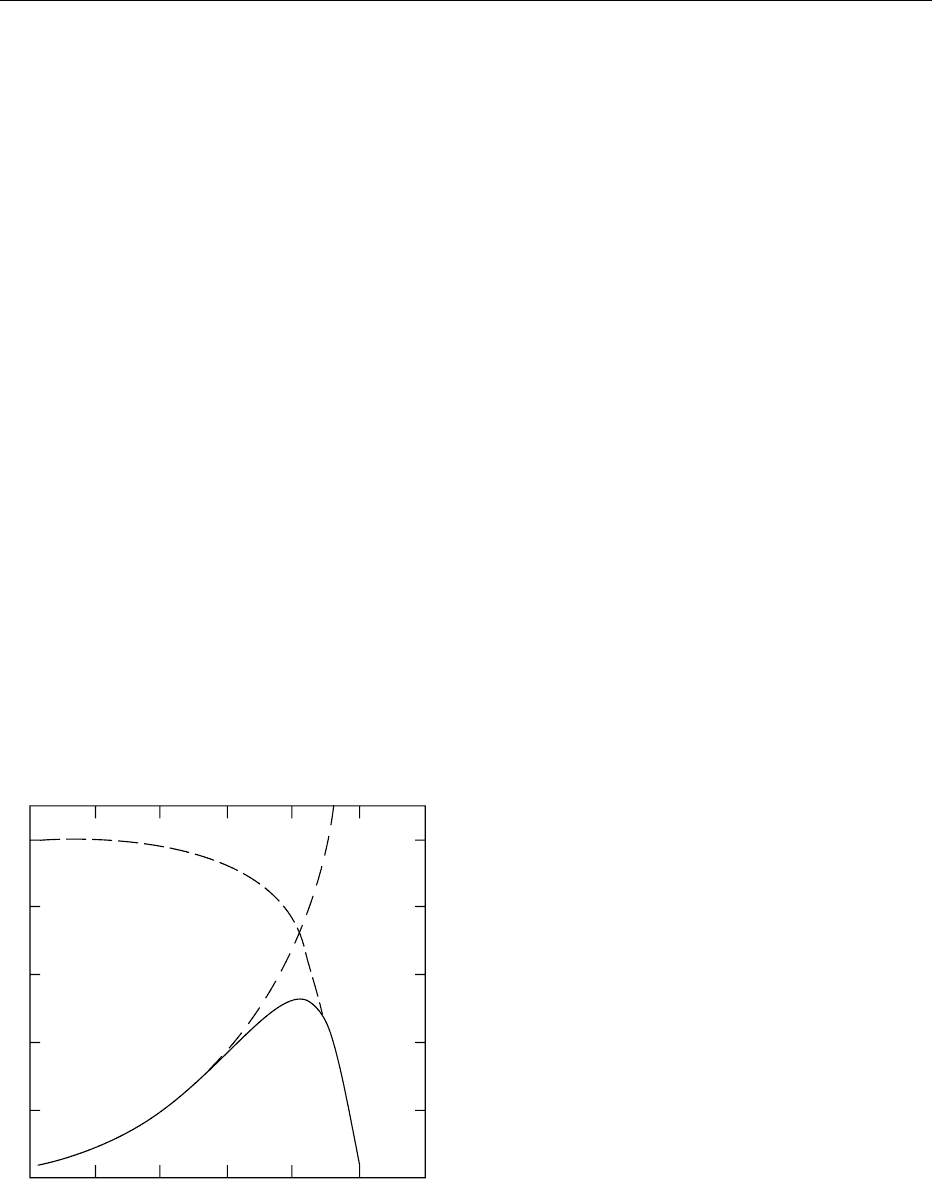
0028 Temperature affects enzyme-catalyzed reactions by
influencing v
0
, the stability of the enzyme as a protein
and various equilibria, including K
m
and pK
a
of cata-
lytic groups. There is a logarithmic effect of tempera-
ture on the rate of reaction (Figure 4, curve A), as
predicted by the Arrhenius relation:
k ¼ A
E
a
=RT
e
, ð3Þ
where k is a rate constant, A is a proportionality
constant, T is temperature (degrees Kelvin), R is the
universal gas constant, and E
a
is the Arrhenius acti-
vation energy. At higher temperatures, depending on
the specific enzyme, enzymes lose activity because of
instability (see curve B, Figure 4), resulting in a
decrease in activity. The temperature at which
the maximum v
0
is obtained is the temperature
optimum (apex of curve C, Figure 4) under the con-
ditions used.
Enzyme Activation
0029Any compound that increases v
0
when added to
an enzyme–substrate system is an activator. These
compounds can be cofactors (coenzymes or pros-
thetic groups), allosteric effectors, conformational
effectors, or converters of proenzymes to enzymes
(by proteolysis, usually).
0030Cofactors are small organic or inorganic molecules
essential for enzyme activity. Organic cofactors
are often built around the B vitamins (pyridoxine,
thiamin, riboflavin, niacin, and pantothenic acid),
whereas inorganic cofactors include Zn
2þ
,Mn
2þ
,
Mo
5þ
,Mo
6þ
,Cu
2þ
,Mg
2þ
,Co
2þ
,Ca
2þ
,Fe
2þ
,Fe
3þ
and Cl
. For example, more than 150 enzymes
require Zn
2þ
as a cofactor. Coenzymes are bound
via noncovalent bonds, whereas prosthetic groups
generally are covalently bound to the protein. (See
Coenzymes.)
Enzyme Inhibition
0031Any compound that decreases v
o
when added to
an enzyme–substrate system is an inhibitor. Inacti-
vation caused by adverse pH, temperature or solv-
ents is not included. Inhibitors occur naturally in
many biological materials, may be produced syn-
thetically, or may be metal ions (particularly Hg
2þ
,
Pb
2þ
,Ag
þ
,Cu
2þ
). Enzyme inhibitors are important
in pathogenic microorganism control, food storage,
and food-quality preservation. Many pharmaceut-
icals are designed to inhibit specific microbial
enzyme systems. Fungicides, herbicides, and insecti-
cides are targeted to specific enzymes. Products of
enzyme reactions are often inhibitors, since they
resemble substrates. (See Fungicides; Pesticides and
Herbicides: Types, Uses, and Determination of
Herbicides; Storage Stability: Mechanisms of Deg-
radation.)
Naming of Enzymes
0032Enzymes are named by adding the suffix ‘-ase’ to the
stem of the substrate name, e.g. amylum (Latin for
‘starch’)/amylase, maltose/maltase, carbohydrate/car-
bohydrase, lipid/lipase. However, there are several
enzymes that act on a carbohydrate, for example, or
a single enzyme may act on several substrates. Other
trivial names are derived from the source of the
B
C
A
Temperature
Velocity (or k) for lines A and C
1/velocity (for line B)
fig0004 Figure 4 Effect of temperature on v
0
of an enzyme-catalyzed
reaction. Curve A shows the effect of temperature on the velocity
of substrate conversion to product. Curve B shows the effect of
temperature on the stability of the enzyme. Curve C shows the
actual effect of temperature on the velocity of substrate convers-
tion to product.
2124 ENZYMES/Functions and Characteristics
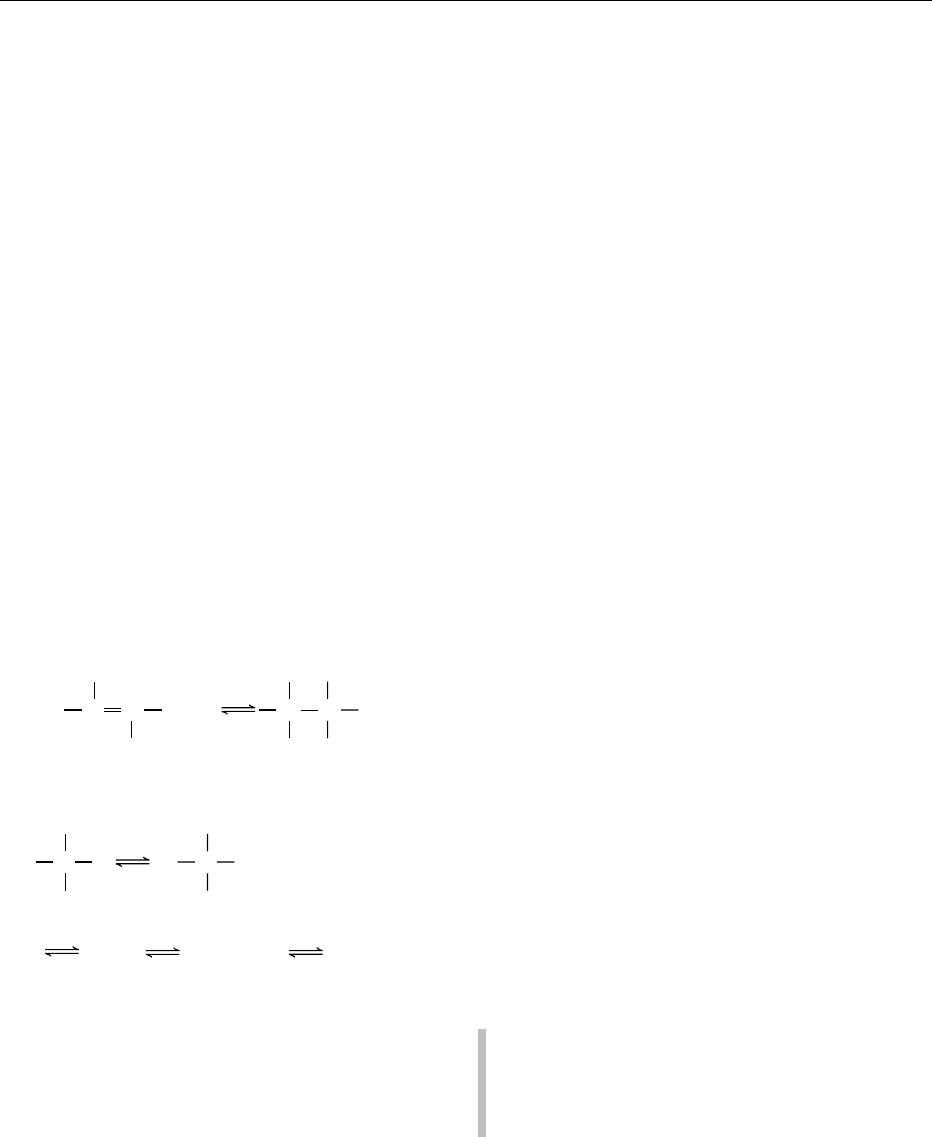
enzyme or the color of the purified enzyme. In 1955,
the International Union of Biochemistry appointed
members from many countries to a commission on
enzymes, with the charge to develop a systematic
method of naming enzymes. In 1961, a classification
was adopted, based on dividing ‘the enzymes into
groups on the basis of the type of reaction catalysed
and this, together with the name(s) of the substrate(s),
provided a basis of naming individual enzymes’.
Retention of the trivial name (already in general use)
and addition of a four-digit numbering system were
also adopted.
003 3 All enzymes fit one of six types of chemical reac-
tions. These are (with the first digit of the number and
a generalized reaction):
EC1. Oxidoreductases
AX þ B Ð A þ BX, where X is H or e
;Bis
oxygen etc.
EC2. Transferases
AY þ B Ð A þ BY, where Y is methyl, acyl,
glycosyl, etc.
EC3. Hydrolases
AB þ H
2
O Ð AH þ BOH, where water is a
substrate.
EC4. Lyases
+
RX
H
CC
H
H
C
R
C
H
X
EC5. Isomerases
H
CR′R
X
X
CR′
R, i.e
H
D− L−, cis
trans, keto enol, etc.
EC6. Ligases (synthetases)
A þ B þ AJP Ð AB þ ADP þ P
i
(or AMP þ PP),
where P
i
is inorganic phosphate.
0040 The first digit of the EC number indicates the class to
which the enzyme belongs, the second digit the sub-
class, the third digit the subsubclass, and the fourth
digit serial number of the enzyme in its subsubclass.
For example, the trivial name of the enzyme catalyz-
ing the reaction shown in eqn (4) is alcohol dehydro-
genase, the systematic name is alcohol:NAD
þ
oxidoreductase, and the number is EC 1.1.1.1. The
first digit indicates that it is an oxidoreductase, the
second that the donor is a primary alcohol, the third
that the acceptor is NAD
þ
or NADP
þ
, and the fourth
that the enzyme is alcohol dehydrogenase.
CH
3
CH
2
OH þ NAD
þ
ÐCH
3
CHO þ NADH þ H
þ
:
ð4Þ
0041The naming of an enzyme is complete when the
organism, organ, and isozyme (if present) are indi-
cated. For example, one of the two pig pancreas
a-amylases is porcine pancreatic a-amylase-1, and
the other is porcine pancreatic a-amylase-2. The iso-
zyme number is assigned consecutively, on the basis
of the fastest moving isozyme towards the anode by
electrophoresis, as ‘1’, and so on.
See also: Amino Acids: Properties and Occurrence;
Coenzymes; Fermented Foods: Origins and
Applications; Fungicides; Pesticides and Herbicides:
Types, Uses, and Determination of Herbicides; pH –
Principles and Measurement; Protein: Chemistry;
Storage Stability: Mechanisms of Degradation; Water
Activity: Effect on Food Stability
Further Reading
Anonymous (1992) Enzyme Nomenclature, Recommenda-
tions of the International Union of Pure and Applied
Chemistry and the International Union of Biochemistry.
Orlando, FL: Academic Press.
Bender ML, Kezdy FJ and Gunter CR (1964) The mechan-
ism of action of proteolytic enzymes. XXXIII. The anat-
omy of an enzymatic catalysis. a-Chymotrypsin. Journal
of the American Chemical Society 86: 3714 – 3721.
Boyer PD (ed.) (1970-1978) The Enzymes, 3rd edn, vols.
1–18. San Diego, CA: Academic Press.
Colowick SP and Kaplan NO (editors-in-chief) (1955–
1999) Methods in Enzymology, vols 1–300. San Diego,
CA: Academic Press.
Schwimmer S (1981) Source Book of Food Enzymology.
Westport, CA, USA: AVI.
Whitaker JR (1994) Principles of Enzymology for the Food
Sciences. New York: Marcel Dekker.
Uses in Food Processing
G B Quaglia and L Gennaro, Istituto Nazionale di
Ricerca per gli Alimenti e la Nutrizione, Rome, Italy
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Enzymes play important roles in various aspects of
food processing. In some cases they have made pos-
sible the development of new products and, in other
cases, the improvement of traditional products. At
least 75% of all industrial enzymes are hydrolytic in
ENZYMES/Uses in Food Processing 2125
action, and they are used for the depolymerization of
natural substances. Proteinases remain the dominant
type because of their wide use in dairy products
processing (as coagulants); carbohydrases, used in
baking, brewing, distilling, and especially in starch
processing, represent the second largest group. The
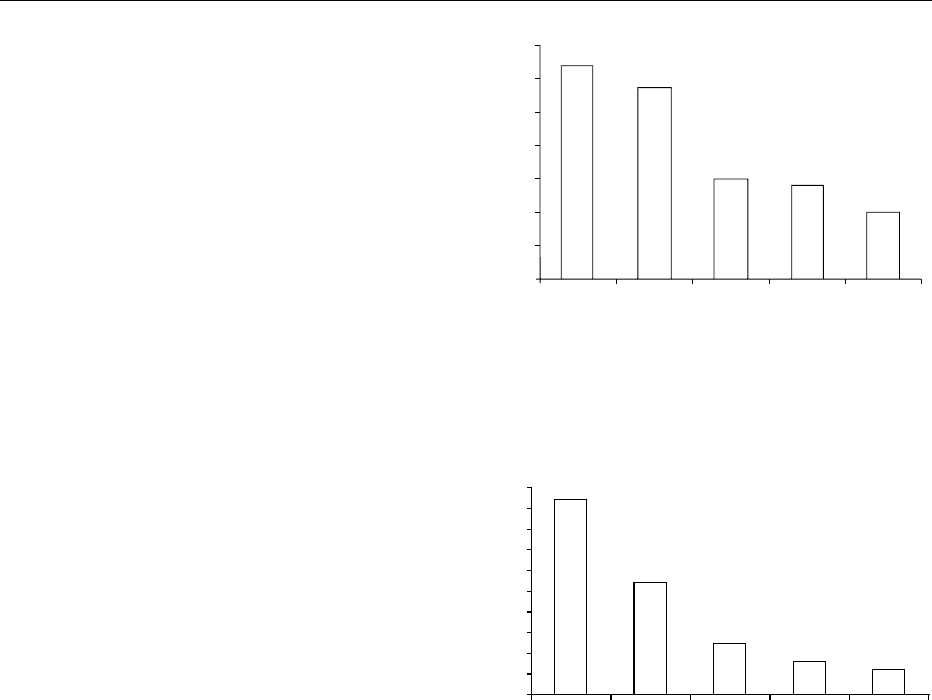
conventional distribution of the current world sales of
enzymes is to assess them by their application sectors
(dairy, detergent, starch, textiles, and other; Figure 1)
This has been the practice for many years but it is
important to consider the composition of section
listed as other in Figure 1, in order to develop a view
of future markets. The section covered by ‘others’
includes the following: alcohol, baking, fats and oils,
flavor, fruit, and wine. The distribution of sales for
2005 is expected to be very different, because ‘other’
sectors will become the single largest part of enzymes
sales, up to approximately 47% (Figure 2).
0002 The advantage of using enzymes in food technol-
ogy is primarily derived from two intrinsic properties:
catalytic rate enhancement and specificity. The ability
of enzymes to enhance reaction rates by many orders
of magnitude may seem obvious, but it is particularly
significant when these rates are achieved under very
mild conditions. For most enzymes, this means an
aqueous environment at atmospheric pressure, a pH
within the range 3–9, and a temperature between 15
and 50
C. In this way, the formation of chemically
induced byproducts is vastly reduced. For example,
in starch processing, chemical degradation yielded a
product containing up to 6% of residual polysacchar-
ide, as well as colored degradation products, the re-
moval of which required a further purification step;
the most recent enzymatic process produced a clean
product containing at most 0.1% oligosaccharide.
Moreover, the mild processing conditions facilitate
the handling of sensitive substrates (such as food-
stuffs) where extremes of temperature, pH, or pres-
sure would be undesirable. Enzyme specificity is the
other great advantage. Specificity, in fact, not only
reduces interference by undesirable substrates but
also minimizes the problem of unwanted byproducts,
which are always a costly inconvenience in industrial
processing. Finally, enzymes can be easily inactivated
by thermal or chemical means once the desired degree
of conversion has been achieved.
0003 The versatility of enzymes in food processing will
be evident by the numerous examples cited in this
work. There are a number of ways in which an
enzyme preparation can be presented to the market:
as an amorphous or microcrystalline powder, in solu-
tion, or in some immobilized form. The nature of the
preparation will be determined largely by the require-
ment of every industry. Liquid preparations are often
preferred, because volume measurements are simple,
but preparing the enzyme in an insoluble form would
not only facilitate recovery of enzymes, but also
permit reuse, which has obvious economic advan-
tages. For this reason immobilized or bound enzymes
have been developed by physically or chemically
binding the enzyme to an insoluble support. The
main cases of food processing in which immobilized
enzymes are used are the conversion of glucose to
fructose by glucose isomerase in the production of
high-fructose syrups, and the clarification of fruit
juices by means of pectic enzymes.
0004Any organism, whether plant, animal, or micro-
organism, is a potential source of enzymes. However,
the nature of the source dictates the availability,
the cost of source material, the ease of recovery,
and many other factors (Table 1). The majority of
enzymes used on an industrial scale are from microbial
sources (Table 2). One exception was that of rennin,
the protease derived from calf stomach, which is used
in cheese-making. Even here a microbial substitute,
35
30
%
25
20
15
10
5
0
Detergent Other Starch Dairy Textile
fig0001Figure 1 Distribution of enzymes sales. Data from Godfrey T
and West SI (1996) Introduction to industrial enzymology. In:
Godfrey T and West SI (eds) Enzymology, pp. 1–8. London:
MacMillan Press.
Other
Detergent Starch
Dairy Textile
50
45
40
35
30
25
20
15
10
5
0
%
fig0002Figure 2 Forecast distribution of enzyme sales. Data from
Godfrey T and West SI (1996) Introduction to industrial enzym-
ology. In: Godfrey T and West SI (eds) Enzymology, pp. 1–8.
London: MacMillan Press.
2126 ENZYMES/Uses in Food Processing
