Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Table 1 Typical drying conditions and properties of some spray-dried foods
Product Total solid
content of
feed (%)
a
Air inlet tem-
perature (
C)
Type of
atomizer
Type of cham-
ber
Air outlet tem-
perature (
C)
Product
moisture
content
(%)
a
Particle
size of
powder
(mm)
Bulk dens-
ity of
powder
(kgm
3
)
Notes
Skim milk (ordinary) 40–50 180–230 Various 85–100 3.5–4.0 30–50 600–700
Skim milk
(agglomerated
b
)
40–50 180–230 Various 85–90 3.5–4.0 150–200 450–600 Bulk density may be as low as 250 kg m
3
if rewetted
Whole milk (ordinary) 40–50 180–200 Various 75–95 2.5–3.0 30–50 550–650
Whole milk
(agglomerated)
b
45–50 175–200 Various 90–100 2.5–3.0 150–200 450–550
Whey (ordinary) 45–50 180–200 Centrifugal
9
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
=
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
>
;
Concurrent 90–100 3.0–4.0 30–40 600–700
Whey (precrystallized
and agglomerated)
40–60 180–190 Centrifugal 80–90 2.0–3.0 100–150 550–650 Particle size may be >1000 mm and bulk density
<500 kg m
3
if a second crystallization stage
follows spray drying
Buttermilk 45–50 175–190 Various 75–90 3.5–4.5 30–50 750–830
Eggs (yolk and whole) 25–48 150–200 Centrifugal 50–80 2.0–3.0 450–550
Egg (white) 10–12 145–200 Centrifugal 50–80 2.0–3.0
Instant coffee 35–40 250–300 Nozzle Concurrent 110–115 2.0–3.0 150–300 180–300
Instant tea 35–40 200–250 Nozzle Tall chamber 90–100 2.0–2.5
Tomatoes 25–40 140–150 Centrifugal Concurrent
with cooled
wall
70–80 3.0–3.5
a
Wet weight basis.
b
Agglomeration in fluidized bed.
tbl0001

lecithin to improve their wettability when reconsti-
tuted in water or aqueous solutions.
0024 Whey is also spray-dried. A number of drier
designs, usually featuring nozzle atomizers, may be
used. To reduce the hygroscopicity and improve the
handling characteristics of whey powder, the lactose
is crystallized into the stable a-monohydrate form,
prior to drying.
0025 Buttermilk is also spray-dried. Driers similar to
those used for skim milk are used.
0026 Icecream mixes have high sugar contents, up to
30%, which cause problems during drying. One solu-
tion is to keep back some of the sugar and add it to the
dried mix. Two-stage drying yields a good noncaking
powder. (See Ice Cream: Methods of Manufacture.)
0027 Cheese is ground and mixed with water prior to
drying. Some deposition on chamber walls may occur.
This can be overcome using devices to assist in
powder removal (see above) and/or two-stage drying.
Strong odors can escape to the atmosphere during
cheese drying. The exhaust air may need to be
deodorized or a semiclosed drying system used (see
above).
0028 Butter and high-fat powder (60–80% fat) may be
spray-dried. Precautions must be taken to avoid
breakdown of the emulsion structure and the product
must be cooled after drying.
0029 Sodium caseinate after manufacture is relatively
viscous and is fed to the drier at a lower than usual
solids content (20–25%). Centrifugal atomizers and
two-stage drying are usually used.
Eggs
0030 Whole egg, egg yolk and egg white may be spray-
dried. The feeds are pasteurized prior to drying. Glu-
cose, if present in the dry powder, can give rise to the
development of discoloration and off-odor during
storage. Glucose may be removed, prior to drying,
by fermentation using bacteria, yeasts, or enzymes.
This process is mainly applied to egg white. Whole
egg and egg yolk are fed to the drier at 25–27% and
45–48% solids contents, respectively. Centrifugal
atomizers are usually used. Egg white feed usually
contains 10–12% solids, but this may be increased
by reverse osmosis.
Beverages
0031 Instant coffee is another product spray-dried on a
large scale. Ground, roasted coffee beans are ex-
tracted with hot water in semicontinuous or continu-
ous extractors. The extract contains 20–25% solids
which may be fed directly to the spray drier or pre-
concentrated up to 60% solids. Driers featuring tall
cylindrical bodies (Figure 4a) are used, and nozzle
atomizers. Air and spray follow a streamlined path
down the tower. The conical base of the drier may be
jacketed and cool air passed through this to keep the
wall temperature low. Fines may be recycled to the
drying chamber. Coffee powder may be agglomerated
by rewetting. Instant coffee may also be freeze-dried.
Coffee substitute made from mixtures of chicory,
barley, rye, wheat, and coffee may be dried in a simi-
lar manner to the pure coffee. (See Coffee: Instant.)
0032Instant tea may also be produced by spray drying.
Dried leaves are extracted with hot water to give an
extract containing 3–5% solids. This extract is con-
centrated by vacuum evaporator. The volatile aroma
compounds which flash off in the early stages of evap-
oration are condensed and added back to the concen-
trate prior to drying. Driers similar to those used for
coffee extract are used but at lower inlet air tempera-
tures. (See Tea: Types, Production, and Trade.)
Fruits and Vegetables
0033Whole fruits and fruit juices may be spray-dried.
However, with the exception of tomatoes, additives
must be used to facilitate drying.
0034Tomatoes are prepared for drying by the hot-break
or cold-break processes. The former method is
favored as the reconstituted powder made in this
way has less of a tendency to settle than that made
by the cold-break process. The pulped tomatoes are
comminuted and sometimes homogenized and then
concentrated to 26–48% solids by vacuum evapor-
ation. The drier most commonly used has a relatively
short cylindrical body and conical base. The whole of
the drying chamber may be jacketed and cool air
drawn through the jacket to cool the chamber wall.
A centrifugal atomizer is used. The powder leaving
the chamber is cooled on a vibrating fluidized bed.
0035Citrus and other fruits such as bananas, apples,
pears, strawberries, raspberries, peaches, and apricots
need to have fillers added to permit spray drying.
Without such additives the wall of the drying cham-
ber will become coated with sticky powder and the
product will be tacky and hygroscopic, with a strong
tendency to cake. The most common additive used is
liquid glucose with a dextrose equivalent in the range
15–20. This may be added in amounts up to 75% of
the fruit, on a solids basis. Skim milk powder has also
been used as an additive. To insure good atomization
the fruit must be comminuted thoroughly. Both
centrifugal and nozzle atomizers and various designs
of drying chamber, some featuring cooled walls and
aftercoolers, have been used.
0036Whole vegetables and vegetable juices may also be
spray-dried. These include carrots, potatoes, cab-
bage, peas, and asparagus. These are easier to dry
than fruits. Again, efficient comminution is necessary
and additives may be required.
DRYING/Spray Drying 1937

Other Spray-dried Products
0037 Beverage whiteners These are oil-in-water emul-
sions whose ingredients include vegetable fat, sodium
caseinate, corn syrup, and emulsifying agents. They
are spray-dried, usually using pressure nozzles, down
to a moisture content of 2–5%. Often two-stage
drying is used.
0038 Vegetable proteins These may be extracted from
soya beans, peanuts, potatoes, and other sources.
They are spray-dried using concurrent chambers and
pressure jet atomizers. Some dried products may be
tacky and hygroscopic and require cooling during
handling and packaging.
0039 Single-cell protein (SCP) SCP is produced from
methane, methanol, ethanol, and Z-paraffins by
fermentation, concentrated by centrifugation and
spray-dried. The fine powder is often agglomerated.
Relatively high inlet and outlet temperatures are used.
There is a danger of dust explosion and semiclosed,
self-inertizing spray-drying systems may be used.
0040 Meat Pure
´
es of beef and chicken may be spray-dried
for use in soups and sauces. Concurrent drying cham-
bers and centrifugal atomizers are usually used. Blood
from slaughterhouses is spray-dried to produce a
range of blood, albumin, and plasma powders. Rela-
tively low drying temperatures are used.
0041 Fish byproducts A range of products, including sol-
uble fish powder, fish meal, fish flour, and fish pro-
tein, produced by hydrolysis of fish flesh, is produced
by spray drying. The odor released during drying can
be a problem.
0042 Enzymes A number of enzyme powders are pro-
duced by spray drying. To minimize inactivation,
inorganic salts may be added and relatively low
drying temperatures used. In some cases aseptic
spray drying may be carried out.
0043 Vitamins Vitamins from various sources may be
spray-dried. Vitamins B and C are spray-dried dir-
ectly. Vitamins A and D are usually microencapsu-
lated (See Vitamins: Determination.)
See also: Agglomeration; Ascorbic Acid: Properties and
Determination; Physiology; Cholecalciferol: Properties
and Determination; Physiology; Coffee: Instant; Drying:
Theory of Air-drying; Fluidized-bed Drying; Physical and
Structural Changes; Chemical Changes; Eggs: Use in the
Food Industry; Fish: Miscellaneous Fish Products; Fish
Meal; Ice Cream: Methods of Manufacture;
Instrumentation and Process Control; Powdered Milk:
Milk Powders in the Marketplace; Characteristics of Milk
Powders; Retinol: Properties and Determination;
Physiology; Single-cell Protein: Algae; Yeasts and
Bacteria; Soy (Soya) Beans: Processing for the Food
Industry; Tea: Types, Production, and Trade; Processing;
Vitamins: Determination; Whey and Whey Powders:
Production and Uses
Further Reading
Barbosa-Canovas GV and Vega-Mercado H (1996) Dehy-
dration of Foods. New York: Chapman and Hall.
Brennan JG (1994) Food Dehydration – A Dictionary and
Guide. Oxford: Butterworth-Heinemann.
Brennan JG, Butters JR, Cowell ND and Lilly AEV (1990)
Food Engineering Operations, 3rd edn. London:
Elsevier Applied Science.
Masters K (1991) Spray Drying Handbook, 5th edn. New
York: Longmans.
Mujumdar AS (ed.) (1995) Handbook of Industrial Drying,
2nd edn. New York: Marcel Dekker.
Van Arsdel WB, Copley MJ and Morgan AI Jr. (1973) Food
Dehydration, Drying Methods and Phenomena, vol. 1
Principles, vol. 2 Practices and Applications. Westport,
CT: AVI.
Dielectric and Osmotic Drying
J G Brennan, The University of Reading, Reading, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Application of Dielectric Energy to Drying
Principles
0001Here the term dielectric heating is used to represent
the radiofrequency (RF) and microwave (MW) bands
of the electromagnetic spectrum. RF heating is in
the frequency range 1–200 MHz and MW from
300 MHz to 300 GHz. By international agreement,
certain frequencies have been allocated for industrial
use, in order to avoid interference with telecommuni-
cations. These are RF 13.56 and 27.12 MHz and MW
2450 and a band within the range 896–915 MHz.
The advantages of dielectric heat for drying, cooking,
thawing, and melting of foods are that heat gener-
ation is rapid and occurs throughout the body of the
food material. An additional advantage for drying is
that water is heated more quickly than the other
components of the food. In conventional methods of
drying, heat is transferred to the surface of the food
by convection, conduction, or radiation. It has then to
transfer throughout the body of the food by conduc-
tion. Some penetration does occur in the case of
radiant heat but this is limited and often difficult to
1938 DRYING/Dielectric and Osmotic Drying
control. The penetration of electromagnetic waves in
the dielectric range into materials such as foods
depends on frequency and the characteristics of the
material. The lower the frequency, hence the longer
the wavelength, the greater the penetration. Penetra-
tion may be quantified by half-power depth, i.e., the
thickness of the material, which reduces the wave
energy to 50% of the incident level. The half-power
depth for water is about 12 mm at a frequency of
2450 MHz and 75 mm at 100 MHz. Penetration into
foods is less than into water.
0002 In the case of dielectric heating, the heat is trans-
ferred into the body of the material rather than just
to the surface. When a wet material is exposed to
high-frequency electromagnetic radiation molecules,
which are dipolar, such as water, are stressed by the
alternating magnetic field and this results in the gen-
eration of heat. This is the main mechanism which
causes heating on the application of MW energy. At
the lower RF frequencies, ionic conduction plays a
part. Ions present in the material are accelerated by
the electric fields, and this leads to the generation of
heat. Since most foods contain ions in the aqueous
phase, RF heating can be effectively applied to them.
The mechanism of drying using dielectric heating is
different from that which operates when convected or
conducted heat is used. The rapid generation of heat
within the material causes rapid evaporation of water.
This creates a total pressure gradient, which results in
the rapid movement of liquid water and water vapor
to the surface. Thus, shorter drying times and lower
product temperatures are attainable compared to
more conventional methods of heating.
0003 Overheating of the surface can be avoided. Drying
is more uniform as thermal and concentration gradi-
ents are less. There is also less movement of solutes
within the material. Since the water absorbs most of
the heat there is more efficient use of energy com-
pared to drying by the application of convected or
conducted heat. However, too high a heating rate can
lead to scorching or burning of the material. If the
water vapor becomes entrapped within the material,
high pressures can develop, which can lead to the
rupture of solid pieces.
Equipment
0004 A basic RF (platen) applicator consists of two metal
plates between which the food is located or conveyed.
Often the conveyor belt may represent one of the
electrodes. The plates are at different electrical volt-
ages. The whole system is housed in a metal chamber
to prevent leakage of radiation. In a stray field appli-
cator a thin layer of material passes over electrodes
of alternating polarity. In the staggered applicator
rods extend beyond the plates to form staggered
throughfield arrays between which the layer of ma-
terial passes. This last type can cope with thicker
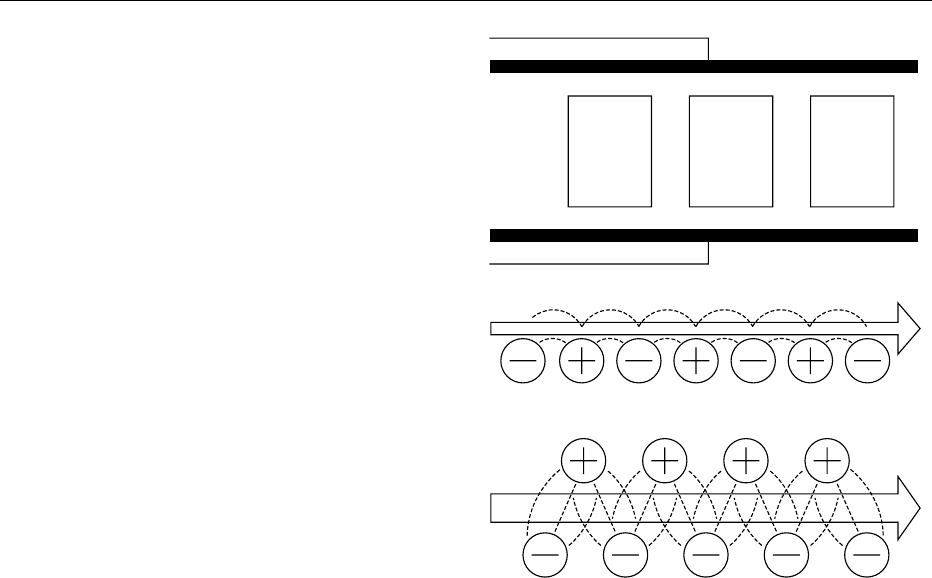
layers Figure 1.
0005In a basic batch MW applicator, MWs, generated
by a magnetron or klystron, are directed into a metal
chamber via a waveguide or coaxial cable. The food is
placed in the chamber. To improve the uniformity of
heating the beam of MWs may be disturbed by a
mode stirrer, resembling a slowly turning fan, which
causes reflective scattering of the waves. Alterna-
tively, the food may be rotated in the chamber. In
continuous systems, the food is conveyed through a
chamber, which has a number of wave injection ports
in its sides, top, and bottom.
Applications
0006In drying of foods, dielectric heating is usually com-
bined with conventional heating. It may be used to
preheat the feed to a hot-air drier. By raising the tem-
perature of the feed quickly and causing moisture to
move to the surface, it can decrease the overall drying
time. Dielectric heating may be applied part-way
through the drying cycle, when the food enters the
falling rate period. (See Drying: Theory of Air-drying.)
(a)
−
+
(b)
(c)
fig0001Figure 1 Electrode configurations for dielectric heating sysU
`
-
tems. (a) Platen type for bulky objects. (b) Stray field type for thin
layers. (c) Staggered type for thicker layers. Adapted from Schiff-
mann RF (1995) Microwave and dielectric drying. In: Mujumdar
AS (ed.) Handbook of Industrial Drying, 2nd edn, vol. 1, pp. 345–372.
New York: Marcel Dekker.
DRYING/Dielectric and Osmotic Drying 1939

This can boost the rate of drying. If dielectric heating is
applied near the end of hot-air drying it can also
shorten the drying time significantly and hence increase
the throughout of the drier. It is more usual to use
dielectric heating in the later stages of drying.
0008 One of the major applications of RF heating is in
the postbaking of biscuits. The objectives in baking
biscuits are to produce a product of the right size,
shape, color, and moisture content. In a conventional
oven, reducing the moisture content to the desired
level can take up a large part of the total baking
time. The application of RF heating can shorten the
baking time. The oven is set to produce biscuits of the
right size, shape, and color, but the RF heating is used
to remove the remaining moisture, without any
further change in these properties of the biscuit. The
capacity of an oven can be increased by more than
50% by the use of RF heating. Postbaking by RF
heating has also been applied to breakfast cereals
and cereal-based baby foods.
0009 MW heating is used, in combination with heated
air of high humidity, to dry pasta products. The humid
air prevents cracking of the product. The drying time
is reduced from 8 h, using heated air only, to 1 h. MW
heating is used in drying onions. The moisture con-
tent is reduced from 80% to 10% by means of heated
air and then down to 5% using MWs. This combined
process results in more uniform drying and a saving in
energy of up to 30%, compared to hot-air drying.
Potato chips (French fries) and crisps are finish-dried
after frying to prevent darkening, if the sugar content
is high.
0010 MW heating has been used in vacuum drying. (See
Drying: Equipment Used in Drying Foods.) Pasta is
dried under vacuum using MW heating. This results
in much shorter drying times, compared to the use of
conducted or radiant heat. There are reports of other
dried products being produced in this way, including
fruit juice, tea and enzyme powders, mushrooms,
asparagus, and soya beans.
0011 MW heating in freeze drying (See Freeze-drying:
The Basic Process; Structural and Flavor (Flavour)
Changes), has been widely studied. The loss factors
of ice and liquid water are much higher than that of
dry tissue. When a frozen food is exposed to MWs,
the ice will absorb energy much faster than the solid.
This should be an advantage in freeze drying. How-
ever, ionization of the rarefied gases can occur be-
cause of the very low pressures used. This can result
in plasma discharge and overheating of the food. The
use of higher-frequency MWs, 2450 MHz, can reduce
this problem. The loss factor of liquid water is much
higher than that of ice. If some melting of the ice
occurs, the water formed will absorb energy quickly
and vaporize. This could cause solid food particles to
explode because of the build-up of pressure internally.
Good control of the freeze-drying process should pre-
vent this happening. The use of MW heating in freeze
drying is technically feasible. However, it has not
been used on a large scale as yet.
Osmotic Drying
Principles
0012This term is applied to the removal of water from fresh
foods, mostly fruits and vegetables, by immersing
pieces of the food in a solution with a higher osmotic
pressure, and hence a lower water activity, than the
food. This solution is sometimes referred to in the
literature as the hypertonic solution. Water will pass
from the food into the solution under the influence of
the osmotic pressure gradient. In this process, the walls
of the cells in the food act as semipermeable mem-
branes. However, the membranes are not completely
selective. Some soluble natural substances, such as or-
ganic acids, sugars, salts, and vitamins, may be lost
with the water, while solutes from the solution may
penetrate into the food. This gain of solutes by the
food can contribute to the reduction in its water
activity, but may affect the taste of the product.
Osmotic Agents
0013The solutes used to make up the hypertonic solution
must be highly osmotically active, nontoxic, edible,
with an acceptable taste and flavor. In the case of
fruits, sugars are the solutes used to make up the
hypertonic solution. Sucrose is the most widely used
sugar, but glucose, fructose, glucose/fructose and glu-
cose/polysaccharide mixtures, high-fructose syrups,
and lactose have been used experimentally, with vary-
ing degrees of success. The addition of small amounts
of sodium chloride (0.5–2.0%) to the sugar solution
can enhance the rate of water loss. Other low-
molecular-weight substances, such as malic acid,
lactic acid, and even hydrochloric acid, can have a
similar effect. There is some evidence that the mole-
cular size of the osmotic solute can affect its penetra-
tion into the food. The smaller the size of the
molecule, the greater the depth and extent of
the penetration. For vegetables, sodium chloride is
the solute most widely used. Glycerol and starch
syrup have also been investigated.
Processing Conditions
0014In osmotic drying, the rate of water loss is high
initially but reduces significantly after 60–120 min.
However, it can take days for the process to reach
equilibrium. A typical processing time to reduce the
weight of the food by 50% is 4–6 h. Some researchers
1940 DRYING/Dielectric and Osmotic Drying

have reported that, while water loss falls off rapidly in
the first 2 h, the gain of solutes from the hypertonic
solution continues for longer. The initial concentra-
tion of solute in the solution can affect the rate of
water loss. Sugar concentrations in the range 40–70%
are used. In general, the higher the initial concentra-
tion of the sugar, the greater the rate and extent of
drying. Sometimes, the upper limit in concentration
may be dictated by the gain of solids, which can affect
the taste of the product, i.e., making it too sweet. In
the case of vegetables, sodium chloride concentra-
tions in the range 5–20% have been used. Again, at
the high concentrations, the taste of the product may
be adversely affected.
0015 The temperature at which the osmosis takes place
can affect the rate of water loss. Generally, increasing
the temperature will increase this rate. However,
there will be an upper limit on temperature above
which the food may be adversely affected. Cell walls
may be damaged resulting in excess loss of soluble
materials, such as vitamins, from the cells. Discolor-
ation of food may also occur. This upper limit on
temperature will be different for different foods. Tem-
peratures above 70
C are seldom used, and for some
heat-sensitive fruits, osmosis may be carried out at
room temperature, i.e., c. 20
C.
0016 The size and shape of the food pieces can also affect
the osmotic process. The smaller the pieces, the
greater the surface area through which water loss
can occur and hence the higher the rate of water
loss. However, the smaller the pieces, the more cell
damage will occur during slicing. This can result in
excessive loss of soluble matter. The shape and size of
the pieces may be dictated by the form of the end
product, e.g., some fruits such as plums may be pro-
cessed whole or in halves. Apples and peaches may be
in slices or segments. Vegetables may be in the form of
slices or cubes. The literature suggests that the upper
limit on thickness is 10 mm.
0017 Movement of the osmotic solution over the surface
of the food pieces would be expected to increase the
rate of water loss. However, vigorous mixing of the
pieces is seldom desirable because it causes damage to
them. The food pieces may be immersed motionless in
the solution. To increase the rate, the solution may be
recirculated through the tank by means of a pump.
The pieces may be suspended in a vibrating basket in
the solution. Another option is to pump the solution
through a bed of the pieces contained within a tall
vessel.
0018 The weight ratio of food to solution can affect the
process, in terms of rate of water loss and solute gain.
In general, the lower this ratio, the higher the water
loss and the solute gain. Ratios of 1:4 to 1:5 are
commonly used.
0019Blanching, either in water or osmotic solution, will
prevent browning. In the case of large fruit pieces,
blanching can sometimes shorten the osmotic pro-
cess, due to relaxation of the structural bonds in the
fruit. For some small fruit pieces and for vegetable
pieces, blanching can adversely affect the osmotic
process, reducing water loss and increasing solids
gain. However, this effect is different for different
foods.
Recycling of Hypertonic Solution
0020Following the osmotic process, the isotonic solution
will be diluted with the water removed from the food.
In order to reuse this solution, its concentration has to
be increased to that of the original solution. This can
be done by adding more solute to the diluted solution.
However, this will result in a surplus of solution
which has to be used for some other purpose. An
alternative approach is to reconcentrate the solution
by vacuum evaporation. There will be a limit to the
number of times the solution can be recycled as some
darkening can occur. It is usually necessary to filter
the solution free of insoluble solids before evapor-
ation. It is necessary to monitor the microbiological
quality of the solution which is being recycled. There
can be a build-up of microorganisms, mainly yeasts,
in sugar solutions, if the processing temperature is
low. Pasteurization of the solution can overcome
this problem.
0021Since there is no change of phase during osmotic
drying, the energy requirements should be less that
in conventional drying methods. Heat is required
to dissolve the solute in preparing the hypertonic
solution, to maintain the temperature of the solution
and to evaporate water from the solution, if it is being
recycled. Additional energy is necessary to mix and
recirculate the solution. It has been reported that the
energy consumption during osmotic drying of fruits is
100–2400 kJ kg
1
of water removed, depending on
the processing temperature and how the surplus
solution is treated, compared to 5000 kJ kg
1
when
drying in heated air.
Further Processing of Osmotically Dried Foods
0022The amount of water removed in osmotic drying,
typically about 50% of the fresh weight of the food,
does not result in a shelf-stable product. The water
activity attainable in fruits and vegetables is usually in
the range 0.90–0.95. Consequently, osmosed prod-
ucts have to be processed further to obtain a product
with an extended shelf-life.
0023Air drying Osmotically dried fruits and vegetables
may be stabilized by further drying in heated air. The
DRYING/Dielectric and Osmotic Drying 1941
rate of drying of such products is usually lower than
that of fresh material and relatively low air tempera-
tures may have to be used to avoid caramelization
and case hardening. The sensory properties of such
products are different to those produced by air-drying
alone. They have a good color and flavor, with re-
duced acidity, and a soft chewy texture. Shrinkage is
usually less than in air-dried products.
0024 Vacuum drying Osmosed products may be stabil-
ized by vacuum drying. The quality of such products
can be superior to those completed by air drying but
the process, which is known as osmovac, is more
expensive.
0025 Freeze drying Predrying by osmosis can shorten the
drying time, and hence reduce, the cost of freeze
drying. The end products are less brittle and less
prone to browning than those freeze-dried without
the osmotic step.
0026 Freezing Freezing of fruits and vegetables is an
energy-intensive operation. By partly drying food
before it is frozen, freezing costs can be reduced.
This process is known as dehydrofreezing. The partial
drying prior to freezing can be achieved by osmosis.
When thawed, such products can have a better
texture, with less drip, than those frozen from fresh.
Unlike conventionally frozen foods, they require
rehydration after thawing.
0027 Solar drying It has been reported that osmotic
drying before solar drying can reduce the load on
the solar driers and enhance product quality. Solar
energy may be used to reconcentrate the solution
before it is recycled.
0028 The range of osmotically dried products available
commercially is limited to date. Some good-quality
fruit products are on the market. These are osmotic-
ally dried, followed by air or vacuum drying. How-
ever, there is considerable interest in this process
worldwide.
See also: Drying: Theory of Air-drying; Freeze-drying:
The Basic Process; Structural and Flavor (Flavour)
Changes; Drying: Equipment Used in Drying Foods
Further Reading
Brennan JG (1994) Food Dehydration – A Dictionary and
Guide. London: Butterworth-Heinemann.
Jones PL (1993) Alternative methods of industrial drying.
In: Encyclopaedia of Food Science, Food Technology
and Nutrition, vol. 3, pp. 1476–1480. London: Aca-
demic Press.
Lewicki PP and Das Gupta DK (1995) Osmotic dehydra-
tion of fruits and vegetables. In: Mujumdar AS (ed.)
Handbook of Industrial Drying, 2nd edn, vol. 1, pp.
691–713. New York: Marcel Dekker.
Schiffmann RF (1995) Microwave and dielectric drying. In:
Mujumdar AS (ed.) Handbook of Industrial Drying, 2nd
edn, vol. 1, pp. 345–372. New York: Marcel Dekker.
Physical and Structural
Changes
W F A Horner, University of Hull, Hull, UK
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001The concentration of solutes as drying proceeds has
consequences on the nature of foodstuffs: some of
these changes are reversible and some are irreversible.
The manner of drying, its conditions, and extent de-
termine this reversibility and, therefore, how closely
the characteristics of the rehydrated food resemble
the material from which it originated.
Physical Changes
0002Changes in color and texture, accompanied, in solid
structured materials, by shrinkage and deformation,
are expected outcomes of drying. As solutes concen-
trate in the milieu, osmotic gradients cause water
molecules to move out of their close association
with hydrophilic macromolecules. The latter tend to
aggregate: some, like starch and cellulose, to such an
extent that they became almost crystalline. Such close
aggregations of molecules are not easily dissociated
on rehydration. This helps to explain the frequent
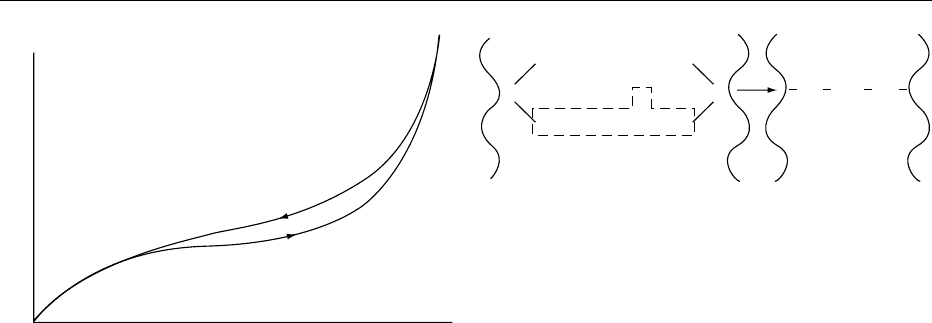
observation, illustrated in Figure 1, that many foods
with the same water content have a higher water
activity-(a
w
) (which usually means greater suscepti-
bility to microbiological spoilage) when rehydrating
than when dehydrating. Although dehydration may
also cause changes in the structure of macromol-
ecules, e.g., the denaturation of proteins, experimen-
tal evidence has shown that many such denatured
structures hold more hydration water than undena-
tured structures. This is because the denatured form
often exposes hydrophilic groups not exposed in
the native form. (See Water Activity: Effect on Food
Stability.)
1942 DRYING/Physical and Structural Changes
000 3 It seems likely, therefore, that the major textural
changes in food, characterized by collapse of cell
walls and membranes and the change from the gel-
like to the fibrous, are due more to the purely physical
moving together of macromolecules than to denatur-
ation. Such aggregation may subsequently favor the
cross-linking between macromolecules via hydrogen
bonds and sulfur bridges, further restricting the
extent to which rehydration can occur. If meat, for
example, is overdried, down to the a
w
0.15–0.40
region, it reaches maximum toughness and elasticity.
Moreover, upon rehydration, muscle tissue dried in
air to this extent has lost its natural succulence. To
chew such material is to chew inert wetted fibers
which very quickly yield their absorbed water to
become an unpleasantly dry ball of fibers in the
mouth.
Texture Changes and Rehydratability
0004 The modulus of elasticity in vegetable tissue decreases
rapidly as, in drying, cells lose their turgor and the
plant wilts. In this way, the trend in texture for vege-
table tissue appears opposite to that in flesh foods as
a
w
decreases. It seems, therefore, that the textural
properties of foods, such as crispness, hardness, and
toughness, on drying, depend on their cellular nature.
Pretreatment with a glycerol solution, however, helps
to protect vegetable cell walls from disruption and
fracture during freeze and air-drying and allows the
tissue to return to its original shape on rehydration.
Untreated tissue remains partly shriveled on rehydra-
tion.
000 5 In dehydrated animal tissues, aggregation of the
actomyosin fraction causes the texture to become
tough and woody so that the flesh rehydrates slowly
andincompletely.Theformationofmethylene(-CH
2
-)
linkages between myosin molecules was thought to be
responsible for the gradual toughening of fish muscle
texture during frozen storage. The plausible initiator
of such cross-linking was formaldehyde released
when trimethylamine oxide, naturally present in
marine and some fresh-water fish species, is enzyma-
tically broken down to dimethylamine (eqn (1)). For-
maldehyde from this reaction could react with certain
protein end-of-side-chain groups like the amino
group and possibly form methylene bridges between
protein molecules (Figure 2). Analytical evidence sug-
gests, however, that such methylene bridges between
protein chains occur rarely, if ever. Spoilage product
molecules, however, being much smaller than the
structural protein molecules, are much more mobile
and tend to react together, excluding water and caus-
ing irreversible texture changes. The muscle protein
myosin, if very fresh, can dry like a gel and can be
easily rehydrated. Myosin which is less fresh or is
dried under harsh conditions tends to dry as diffi-
cult-to-rehydrate fibers.
NðCH
3
Þ
3
O ! HNðCH
3
Þ
2
þ HCHO ð1Þ
0006Freezing is a form of dehydration and as such pro-
motes the macromolecule aggregation which hinders
the rehydration process. Nevertheless, it is used to
improve rehydratability in dried vegetables. Ice crys-
tal growth during freezing forces tissues apart and
leaves pores in the material upon drying. Thus,
being more porous, the dried food imbibes water
more quickly upon rehydration. Water-holding
capacity, however, is not improved. The use of cryo-
protectants in freezing reduces the tendency of
macromolecules to aggregate by holding more water
molecules around them. (See Freezing: Structural and
Flavor (Flavour) Changes.)
0007With the exception of freeze-drying, dehydration
processes cause shrinkage which is greatest during
the initial stages. If the rate is too fast, shrinking
N
N
NH
+H
2
O
CH
2
HN
H
HH
H
O
HCH
fig0002Figure 2 Formation of a methylene bridge between two protein
molecules. Reproduced from Drying: Physical and Structural
Changes, Encyclopaedia of Food Science, Food Technology and Nu-
trition, Macrae R, Robinson RK and Sadler MJ (eds), 1993, Aca-
demic Press.
0
H
2
O content (%)
1.0
a
w
fig0001 Figure 1 Typical food sorption isotherm showing hysteresis.
Reproduced from Drying: Physical and Structural Changes, En-
cyclopaedia of Food Science, Food Technology and Nutrition,
Macrae R, Robinson RK and Sadler MJ (eds), 1993, Academic
Press.
DRYING/Physical and Structural Changes 1943

capillaries bearing water to the food surface soon
become blocked by solids carried in the flow. The
surface becomes dry and impermeable – a condition
known as ‘case hardening’–halting the drying pro-
cess whilst the interior is still wet. Flesh foods dried
thus decompose from the inside but, with some vege-
table foods, a high initial drying rate is advantageous
because the final volume is fixed early in the drying
process. As drying proceeds further, the tissues split
and rupture internally, forming an open microstruc-
ture. The product, in this case, has a low bulk density
and good rehydration characteristics. Such materials
subjected to low initial drying rates shrink inwards
progressively with process time, yielding a high bulk
density product which is difficult to rehydrate. (See
Freeze-drying: The Basic Process.)
0008 Liquids like milk and hydrocolloid solutions are
frequently spray- or roller-dried. The extremely
short contact time (microseconds for droplets of
10 mm diameter) required to dry milk to a powder in
a spray drier causes very little damage to components,
loss of nutrients, or destruction of microorganisms.
However, the powder produced, composed as it
would be of particles which are mainly very small,
does not easily or quickly reconstitute because surface
tension effects on such tiny particles prevent efficient
dispersal. To improve reconstitutibility, the fine
powder is often rehumidified, causing particles to
stick together, and redried as larger more easily
dispersible agglomerates. This process is referred to
as ‘instantization’.
0009 Roller-dried liquids and slurries are exposed to
high temperatures for a longer period than spray-
dried ones; indeed, the extra contact time may con-
tribute to ‘cooking’ the paste as well as drying it.
Instant or pregelatinized starch powder, which is
used as the main functional component of instant
pudding mixes, is manufactured in this way.
0010 Unlike the corresponding spray-dried version, the
roller-dried product tends to be coarse and more
dispersible. Thermal damage to the nutrients and
sensory characteristics of such products may be
minimized by carrying out the roller-drying process
in a vacuum chamber.
Flavor and Color Retention
0011 In spray drying, where there is a huge temperature
differential between feed material and drying
medium, the consequent large loss of volatiles close
to the point of liquid introduction to the chamber
may be reduced by the addition of an emulsified oil
phase to the feed which alters the droplet size distri-
bution and the pattern of flavor loss. Flavor oils
themselves, moreover, can be emulsified with suitable
hydrocolloids dispersed in water (continuous phase)
so that, on spray drying, the oil droplets are protected
from evaporation by the hydrocolloid ‘skin,’ which
instantly encloses them. This microencapsulation
process yields almost odorless flavor powders which
release their volatiles slowly – an advantage when
baking a cake, for example – or quickly when the
microcapsules are ruptured, as in ‘scratch-and-sniff’
perfume samplers.
0012The retention of the flavor components of a food
material during the drying process in unaltered form
is a major positive quality attribute for some dried
products. Consumers are prepared to pay a premium,
for example, on instant coffee expensively produced
by freeze-drying which, in spite of losses due to the
high vacuum, retains a greater proportion of the
highly volatile characteristic flavors than does spray
drying. (See Coffee: Instant.)
0013The loss of volatiles can be very serious in products
which depend significantly upon their aroma for ac-
ceptability. Even for milk there is evidence that the
loss, during drying, of volatiles such as methyl sul-
fides and the lower fatty acids may be responsible
for the lack of ‘fresh milk’ flavor in the dried product.
(See Sensory Evaluation: Aroma.)
0014Heat-induced reactions such as enzymatic and
nonenzymatic browning may lead to significant
changes in flavor as well as color. Such changes are
not always undesirable; indeed, they may be an essen-
tial product attribute as in the case of black tea com-
pared to green tea production. Enzymatic changes in
dried foods are dealt with in greater detail in the
following section, but control can be exercised by
selection of the variety and stage of maturity of the
material to be processed. In this way it may be
arranged that the starting material is as deficient as
possible in the substrate or enzyme involved in
browning. Blanching vegetables before dehydration
and excluding oxygen from the product, respectively,
destroy the enzyme and prevent the oxidation stage of
the sequence leading to browning. Sulfur dioxide acts
both as an enzyme poison and reducing agent and is
therefore frequently used to preserve the color of dried
fruits and vegetables. Because it is volatile, it is easily
driven off as the food is reconstituted and cooked.
Normally, sulfur dioxide is applied to vegetable matter
prior to drying through dipping them in a solution of
sodium metabisulfite for a period which yields a prod-
uct conforming to the maximum permitted limits for
that chemical in that specific material. (See Browning:
Nonenzymatic; Preservation of Food.)
0015Selection of the raw material can also play a sig-
nificant role in maintaining the desired color quality
of dried foods subject to nonenzymatic browning.
Potato crisps, if made from stored potatoes, where
1944 DRYING/Physical and Structural Changes
the starch has begun to break down to sugars prior to
germination, become too dark before water content
has been reduced by the frying process sufficiently to
give the required characteristic crisp texture.
0016 The Maillard nonenzymatic browning reaction
sequence is initiated when the carbonyl group of
reducing sugar reacts with the amine group of a pro-
tein peptide or amino acid. The dire effects of this on
the visual quality of spray-dried egg have required
that one of the reactants, glucose, be removed by
treatment with glucose oxidase, before the drying
process.
0017 Fish which has spoiled considerably before drying
contains substantially more of the mobile Maillard
reactants than fresh fish and so becomes an unsightly
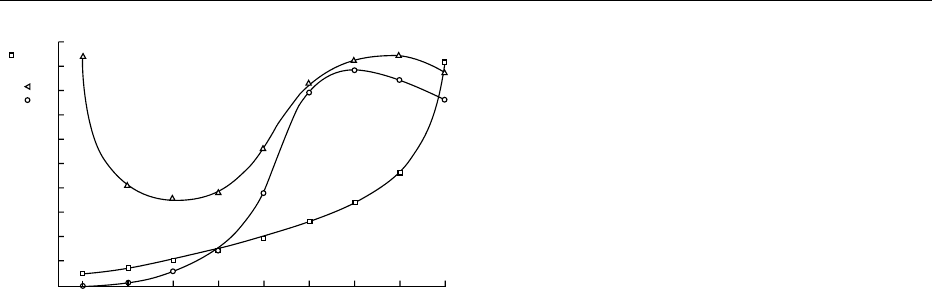
brown color upon drying. Figure 3 shows how the
rate of browning is affected by the water activity of
the product. (See Oxidation of Food Components.)
Microstructure of Dried Products
0018 Dried foods may be particulate (e.g., potato powder),
glass/rubber-like (e.g., many sugar confections and
pasta), fibrous, or a combination of these.
0019 Particulate dry foods may range from simple crys-
talline chemicals, like salt and sugar, to amorphous
conglomerates of highly complex substances like milk
powder.
0020 In many instances, dried foods are eaten without
rehydration (e.g., dried fruits and sugar confections)
and their chewy, tough, or brittle texture contributes
to the pleasure of eating them. The size range of
simple crystalline substances like sugars is critical in
determining the smoothness of a fondant and the
brittleness/crunchiness of a biscuit.
0021The manner in which lipid material is distributed in
relation to the structural matrix (carbohydrate or
protein) and interstitial components affects what is
referred to as the ‘shortness’ in texture of the product.
The lipid interrupts the potential continuity of such
matrices which would otherwise lead to toughness
and elasticity. This ‘shortening’ facility may be used
to isolate relatively small units of glutenacious mater-
ial so that no continuum is formed, in the baking of
shortbread biscuits, for example, or to isolate layers
of glutenacious matrices from one another, as in
cream crackers and puff pastry.
0022Removal of water from foods inevitably leads to
the precipitation of solutes and the aggregation of
insoluble structural components – with consequent
effects on texture and rehydratability. Some of the
soluble and colloidal components of the food starting
material, gelatinized starch, for example, form a
glassy continuum within the food material as it
dries. This phenomenon is a highly significant feature
in the cook–extrusion of starchy materials. Indeed,
the viscous glassy continuum formed in the extruder
barrel momentarily holds the water, as it suddenly
turns to steam the instant the material emerges from
the exit die, and allows the extrudate to expand and
quickly solidify as the steam escapes. The hygrosco-
picity and continuity of such glassy components favor
very rapid rehydration – an unwelcome property with
respect to long-term storage in humid environments
for many cook-extruded products destined for the
snack-food market. The ‘melt-in-the-mouth’ charac-
teristic imparted by the products’ aeration and hygro-
scopicity is, however, its strongest sensory advantage
– although with some such products rehydration in
the mouth is so rapid that the product tends to cling
to the palate and the teeth. Packaging for these hygro-
scopic, aerated extruded products must provide an
efficient barrier against the ingress of water vapor
and – if, as is usual, the product contains a significant
proportion of fat – oxygen. The porosity of dried
foods greatly assists the process of oxidative rancidity
which is also promoted (Figure 3) by the removal of
the coating of water molecules around reactive sites
at very low a
w
. In many dehydration processes, an
attempt is made to maximize dried product porosity
(as in puff, foam-mat, and freeze-drying) to improve
dehydratability. Less controlled drying processes
often yield shrunken or case-hardened products
which, due to the capillary constriction, are very dif-
ficult to rehydrate. Porosity and rapid hydratability
are not always desirable properties in dried foods. In
Indonesia, fish may be dried, smoked, and pressed
alternately until a dry horny product (ikan kaju) is
produced. This product, which is grated over soups
as a condiment, resists rehydration from the humid
0.1
0
10
20
30
40
50
Water content (% dry-weight basis) ;
relative reaction rate ,
60
70
80
90
100
0.2 0.3 0.4 0.5
a
w
0.6 0.7 0.8 0.9
fig0003 Figure 3 Dried product storage stability (susceptibility to lipid
oxidation and Maillard browning) related to its water activity.
Squares, sorption isotherm; triangles, lipid oxygenation; circles,
Maillard browning. Modified from Labuza TP, Tannenbaum SR
and Karel M (1970) Water content and stability of low moisture
and intermediate moisture foods. Food Technology 24: 543–550.
DRYING/Physical and Structural Changes 1945
