Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


tbl0002 Table 2 Major drug–nutrient interactions of clinical relevance
Drug Class Food/nutrient Effect/mechanism
Acarbose Antidiabetic Food Delays carbohydrate breakdown: delays glucose
absorption
Iron Decreased iron absorption
Acetaminophen Analgesic Food May delay extended release; high-pectin food delays
absorption
Alcohol Increased risk of hepatotoxicity
Acetohexadmide Antidiabetic Glucose Hypoglycemia
Alcohol Flushing, headache, nausea, vomiting, sweating,
tachycardia
Sodium Hyponatremia, SIADH
Acyclovir Antiviral Food No effect; may take with meals
Aluminum hydroxide Antacid Thiamin Affects bioavailability, because of pH
Iron Decreased iron absorption
Phosphorus Inhibits phosphorus absorption
Vitamin A Inhibits vitamin A absorption
Amikacin Antibiotic Calcium, potassium,
magnesium
Causes renal wasting of these nutrients
Amoxicillin Antibiotic Food Decreased absorption by delayed gastric emptying
Amphotericin B Antifungal Potassium,
magnesium
Causes renal wasting of potassium and magnesium
Ampicillin Antibiotic Food Decreased absorption by delayed gastric emptying
Potassium High doses increase urinary potassium losses
Antipyrine Green vegetables,
beef protein
Decreased absorption
Aspirin Analgesic Food Decreased rate of absorption
Folic acid Increased excretion of folate
Amino acids Decreased intestinal absorption of amino acids,
increased urinary excretion of tryptophan
Iron Chronic high dose 3–4 g day
1
, iron deficiency possible
Alcohol Gastric irritation, leading to possible gastric bleeding
Curry powder,
licorice, teas,
raisins, paprika
Potential salicylate accumulation
Ascorbic acid,
vitamin C, fresh
fruits, high
vitamin C
Increased urinary excretion; decreased concentration in
serum and platelets
Astemizole Antihistamine Food Decreased bioavailability
Atenolol Antihypertensive Food Delayed absorption
Atovaquone Antibiotic Food Bioavailability increased, especially high-fat foods
Atropine Anticholinergic Iron Delayed absorption
Azithromycin Antibiotic Food Decreased rate and delayed absorption
Bacampicillin Antibiotic
(penicillins)
Food Decreased absorption
Barbiturates Anticonvulsant Alcohol Enhanced CNS depression
Calcium, vitamin D Increased vitamin D requirements, owing to increased
metabolism
Cyanocobalamin Increased bone resorption
Folic acid Decreased serum levels, leading to megaloblastic
anemia
Serum lipids Decreased CSF folate and erythrocyte concentration;
may increase cholesterol, HDL triacylglycerols
Benzodiazepines Anticonvulsant Nutrient Enhanced CNS depression
Clonazedam Calcium Increased vitamin D requirements, secondary to
increased metabolism
Clorazepate dipotassium Vitamin D Increased bone resorption
Lopazepam Cyanocobalamin Decreased serum levels, leading to megaloblastic
anemia
Oxazepam Folic acid Decreased CSF folate and erythrocyte concentration
Serum lipids May increase cholesterol, HDL triacylglycerols
Buprenorphone HCL Analgesic Alcohol Enhanced CNS depression
Narcotic
Agonist–antagonist
Continued
1906 DRUG–NUTRIENT INTERACTIONS

Drug Class Food/nutrient Effect/mechanism
Butorphanol tartate Analgesic Alcohol Enhanced CNS depression
Narcotic
Agonist–antagonist
Calcium carbonate Antacid Iron Decreased iron absorption
Fats May cause steatorrhea
Captopril Antihypertensive
angiotensin-
converting
enzyme inhibitor
Food Reduced absorption
Carbamazpine Anticonvulsant Sodium SIADH
Food Enhanced absorption, increased bile production
Carbenicillin iandanyl sodium Antibiotic Food Decreased rate of absorption
Cephalosporins Antibiotic General alcohol Flushing, headache, nausea, vomiting, tachycardia
Cefachlor Food Decreased rate of absorption
Cefadroxil Food No effect (may take food)
Cefamandole Vitamin K Decreased vitamin K hypoprothrombinemia
Cefixime Food Decreased rate of absorption
Cefoperazone Vitamin K Decreased vitamin K hypoprothrombinemia
Cefotetan Vitamin K Decreased vitamin K hypoprothrombinemia
Cefpodoxime proxetil Food Bioavailability increased with food
Ceftibuten Food Decreased absorption
Cefuroxim axetil Food Bioavailability increased with food
Cephalexin Food Absorption reduced for suspension; delayed for
capsules
Cephradine Food Rate of absorption delayed
Cetrizine Antihistamine Food Delays time to serum peak; no effect on overall
absorption
Chlorambucil Antineoplastic Food Reduced absorption
Chloramphenicol Antibiotic Iron Increased serum level iron; increased total iron-binding
capacity
Folic acid Antagonist to physiological action; increased
requirements of folic acid
Vitamin B
12
Increased requirements of vitamin B
12
can cause
peripheral neuropathy
Chloroquine Antimalarial Food Increased bioavailability
Chlorothiazide Diuretic Food Increased drug absorption owing to delayed gastric
emptying
Chlorpromazine Antiemetic Food Decreased absorption owing to delayed gastric
emptying
Chlorpropamide Antidiabetic Glucose Decreased blood glucose concentration
Sodium Hyponatremia, SIADH
Alcohol Flushing, headache, nausea, vomiting, tachycardia
Cholchicine Antigout Cyanocobalamin Decreased absorption of cyanocobalamin
Cimetidine Histamine 2
antagonist
Food Delays absorption
Ciprofloxacin Antibiotic
(quinolone)
Caffeine Decreased rate of absorption
Food Cipro decreased elimination of caffeine
Calcium Calcium can bind quinolones
Mineral supplement Absorption of divalent cations and trivalent cations
decreased by binding to quinolones
Clarithromycin Antibiotic
(macrolide)
Food Decreased onset of absorption; no change in total
amount absorbed
Clonazepam Anticonvulsants
(benzodiazepine)
Nutrient Enhanced CNS depression
Clorazepate dipotassium Calcium Increased vitamin D requirements, owing to increased
metabolism
Vitamin D Increased bone resorption
Cyanocobalamin Decreased serum levels, leading to megaloblastic
anemia
Folic acid Decreased CSF folate and erythrocyte concentration
Serum lipis May increase cholesterol, HDL triacyclglycerols
Cloxacillin Antibiotic
(penicillin)
Food Decreased rate of absorption
Table 2 Continued
Continued
DRUG–NUTRIENT INTERACTIONS 1907

Drug Class Food/nutrient Effect/mechanism
Codeine Narcotic agonist,
analgesic
Alcohol Enhanced CNS effect
Glucose Can cause hyperglycemia
Corticosteroids Steroid Calcium,
phosphorus,
vitamin D
Decreased absorption of Ca, P; increased urinary
excretion; chronic high dose can cause osteomalacia
Dexamethazone
Hydrocortisone
Methylprednisolone
(Prednisone, Prednisolone)
Corticosteroids Steroid Nitrogen Increased urinary nitrogen losses
Zinc Increased urinary excretion and decreased serum levels
Glucose Impairs glucose tolerance; increases plasma levels
Triacylglycerols,
cholesterol
Increased serum levels
Co-trimaxazole Antibiotic Potassium Decreased excretion hyperkalemia
Sodium Increased excretion hyponatremia
Folic acid Potential for folate deficiency
Cyclosporine Antirejection Milk, fat, pineapple
juice
Increased absorption
Demeclocycline Antibiotic Food, calcium, iron Decreased absorption of dairy products and divalent and
trivalent cations
Diazepam Anticonvulsant Food Increased absorption with high-fat meals and delayed
gastric emptying
Clonazedam
Clorazepate dipotassium
Lopazepam
Oxazepam
Dicumarol Anticoagulant Food Increased absorption with high-fat meals and delayed
gastric emptying
Didanosine Tab Antiviral Food Decreased rate and extent of absorption
Oral suspension Fruit juice or acid
liquid
Didanosine unstable in acid pH
Digoxin Cardiac Food Delay absorption; adsorbent to high-fiber, high-pectin
foods
Dirithromycin Antibiotic
(macrocide)
Food Slightly increased absorption
Divalproex Anticonvulsant Food Decreased rate of absorption; extent of absorption not
affected
Doxycycline Antibiotic Food Decreased absorption of food and milk
Erythromycin Antibiotic
(macrocide)
Food Increased absorption by delayed gastric emptying
Erythromycin stearate Food Reduced absorption by delayed gastric emptying
Ethionamide Antituberculosis Pyridoxins Reports of peripheral neuritis and paresthesia
Etodolac Nonsteroidal
antiinflammatory
drug (NSAID)
Food (milk) Decreased total bioavailability of tolmetin; decreased
absorption of ibuprofen
Sodium Hyponatremia (indomethacin/ketorolac)
Potassium Hyperkalemia (indomethacin/ketorolac)
Food Increased rate of absorption
Felbamate Anticonvulsant Glucose Hypoglycemia
Magnesium Hypomagnesemia
Phosphorus Hypophosphatemia
Potassium Hypokalemia
Sodium Hyponatremia
Fenoprofen NSAID Food (milk) Decreased total bioavailability of tolmetin
Fenoprofen calcium Sodium Hyponatremia (indomethacin/ketorolac)
Potassium Hyperkalemia (indomethacin/ketorolac)
Food (milk) Decreased bioavailability of tolmetin; decreased
absorption of ibuprofen
Food Increased rate of absorption
Fluconazole Antifungal Potassium Hypokalemia
Flucytosine Antifungal Food Decreased rate of absorption; no change in extent of
absorption
Table 2 Continued
Continued
1908 DRUG–NUTRIENT INTERACTIONS

Drug Class Food/nutrient Effect/mechanism
Foscarnet Antiviral Calcium Hypocalcemia; drug chelates; divalent metal ions
Magnesium Hypomagnesemia
Phosphorus Hypo- and hyperphosphatemia
Potassium Hypokalemia
Furazolidone Antiinfective Tyramine-rich foods
(avocados,
canned figs, aged
cheese, cola
beverages,
coffee, chocolate,
wines, soy
sauces,
fermented meats,
yeast
preparation,
yogurts)
Prolonged large doses result in increased risk for
hypertensive crisis
Alcohol Flushing, headache, nausea, vomiting, sweating,
tachycardia
Furosemide Diuretic Food Delayed absorption
Ganciclovir Antiviral Food Increased area under curve plasma concentration
Glipizide Antidiabetic Food Delayed absorption
Alcohol Flushing, headache, nausea, vomiting, sweating,
tachycardia
Sodium Hyponatremia, SIADH
Griseofluvin Antifungal Alcohol Can increase alcohol effect, flushing, tachycardia
High-fat food Increased drug absorption rate
Hydralazine Diuretic Food Increased absorption
Hydrochlorothiazide Diuretic Food Increased absorption by delayed gastric emptying
Ibuprofen NSAID Food (milk) Decreased total bioavailability of tolmetin; decreased
absorption of ibuprofen
Sodium Hyponatremia (indomethacin/ketorolac)
Potassium Hyperkalemia (indomethacin/ketorolac)
Food Increased rate of absorption
Indinavir Antiviral Food Decreased absorption of high-calorie, high-fat, and
protein
Grapefruit juice Decreased area under curve concentration
Indomethicin NSAID Food (milk) Decreased total bioavailability of tolmetin; decreased
absorption of ibuprofen
Sodium Hyponatremia (indomethacin/ketorolac)
Potassium Hyperkalemia (indomethacin/ketorolac)
Food Increased rate of absorption
Iron Mineral Ascorbic acid Increased absorption
Amino acids Increased absorption
Calcium phosphate Decreased absorption
Zinc Inhibits absorption
Vitamin A Vitamin A deficiency inhibits iron utilization and
accelerates development of anemia
Tea/coffee Decreased absorption owing to formation of iron tannate
Vegetable
polyphenols
Binds and insolubilizes iron
Isoniazid Antituberculosis Food Decreased intestinal absorption
Pyridoxine Decreased metabolism, antagonism
Food and histamine,
tuna, liver,
aubergine,
parmesan
cheese, tomato,
spinach,
tyramine-
containing foods
Headache, redness, itching of eyes and face, chills,
diarrhea, palpitation
Potential hypertensive crisis owing to monoamine
oxidase inhibitor activity
Table 2 Continued
Continued
DRUG–NUTRIENT INTERACTIONS 1909

Drug Class Food/nutrient Effect/mechanism
Itraconazole Antifungal Food Increased absorption, increased triacylglycerols
Potassium Hypokalemia
Ketoconazole Antifungal Alcohol Flushing, headache, nausea, vomiting, sweating,
tachycardia
Labetalol Antihypertensive Food Increased absorption
Lamivudone (3TC) Antiviral Food Decreased rate of absorption
Levodopa Anti-Parkinson Food Decreased absorption; with high-protein meals amino
acids compete for absorption
Lithium Antimanic Low-sodium diet Increased lithium concentrations
High-sodium diet Increased lithium clearance
Food Increased absorption
Lomefloxacin Antibiotic
(quinolone)
Food Decreased rate and extent of absorption
Loracarbef Antibiotic Food Increased rate of absorption
Lovastatin Antihyperlipidemia Food Increased absorption
Mebendazole Anthelmintic Food Increased absorption
Meclofenamate NSAID Alcohol Additive CNS effects; increased prothrombin time
Food Decreased bioavailability
Melphalan Antineoplastic Food Reduced absorption
Mercaptopurine Antineoplastic Food Reduced absorption
Methacycline Antibiotic Food, calcium, iron Decreased absorption of dairy products, cereals,
divalent and trivalent cations
Methenamine mandelate Urinary antiinfective Milk products, citrus
fruits
Excessive amounts inhibits drug conversion
Methosuximide Anticonvulsant Alcohol, calcium Additive CNS effects; hypocalcemia
Methotreate Antineoplastic Food Increased absorption
Methyldopa Antihypertensive Vitamin B
12
, folate In high doses methyldopa can increase vitamin B
12
and
folate losses
Food High-protein meals compete for absorption
Metoprolol Antihypertensive Food Increased absorption
Metronidazole Antibiotic Alcohol Flushing, headache, nausea, vomiting, sweating,
tachycardia
Food Decreased peak serum concentration but total amount of
drug absorbed not affected
Minocycline Antibiotic Food, calcium Decreased absorption
Nafcillin Antibiotic Food Decreased absorption; decreased serum levels owing to
altered gastric pH
Potassium High doses can cause hypokalemia owing to increased
urinary losses
Nifedepine Antihypertensive
calcium channel
blocker
Grapefruit juice Increased serum level of nifedepine flavonoids inhibits
cytochrome P-450
Food Decreased bioavailability, formulation dependent
Nitrofurantoin Antibiotic Food Increased absorption by delayed gastric emptying
NSAIDs NSAID Food (milk) Decreased bioavailability of tolmetin
Diclofenal Sodium Hyponatremia (indomethacin/ketorolac)
Etodolac
Fenoprofen Ca Potassium Hyperkalemia (indomethacin/ketorolac)
Ibuprofen
Ketoprofen Food Decreased absorption of ibuprofen
Ketorolac
Naproxen
Oxaproxin
Piroxican
Sulindac
Tolmetin NA Food Increased rate of absorption
Norfloxacin Antibiotic
(quinolone)
Food, dairy products Decreased rate of absorption
Multivitamin and
mineral
supplements
Decreased absorption owing to formation of divalent/
trivalent cation complexes with quinolones
Oflaxacine Antibiotic
(quinolone)
Dairy products and
mineral
supplements
Decreased absorption by polyvalent cations
Table 2 Continued
Continued
1910 DRUG–NUTRIENT INTERACTIONS

Drug Class Food/nutrient Effect/mechanism
Ondansetron Antiemetic Food Increased extent of absorption
Potassium Hypokalemia
Oral contraceptives Ascorbic acid Decreased ascorbic acid concentration in plasma,
platelets, leucocytes
Vitamin C, folic acid Decrease in their serum levels
Vitamin B
12
Impairs tryptophan metabolism
Amino acids,
vitamin A, vitamin
E, copper
Increase in their serum levels
Oxacillin Antibiotic Food Decreased absorption and decreased serum
concentration
Fats Oxacillin can cause steatorrhea
Paromomycin Amoebicide Food Increased absorption by delayed gastric emptying
Vitamins A, D, E, K Malabsorption of fat-soluble vitamins owing to
hypocholesterolemia
Penicillamine Antidote (chelating
agent)
Food Decreased absorption
Iron/zinc Decreased absorption 30–70% of increased zinc
absorption; decreased penicillamine absorption
Penicillin G & VK Antibiotic Food Decreased absorption by delayed gastric emptying
Glucose Hyperglycemia
Pentamidine Antibiotic Calcium,
magnesium
Hypomagmanesemia, hypocalemia
Potassium Hyperkalemia due to nephrotoxicity
Phenacemide Anticonvulsant Fresh fruits and
vitamin C
Increased urinary excretion of phenacemide
Phenobarbital Anticonvulsant (see
Barbiturates)
Food Decreased absorption owing to protein binding
Protein Low-protein diet increases duration of action of
phenobarbitol
Vitamin D, calcium Decreased serum vitamin D by cytochrome P-450
hypocalcemia
Fresh fruits and
vitamin C
Increased urinary excretion of phenobarbitol
Phensuximide Anticonvulsant
(succinimides)
Calcium, vitamin D Decreased serum vitamin D by P-450 cytochrome
hypocalcemia
Vitamin B
12
, folic
acid
Decreased absorption and serum levels of folates;
inhibits vitamin B
12
transport
Copper Increased serum levels
Phenytoin Anticonvulsant
(hydantoins)
Fresh fruits and
vitamin C
Increased urinary excretion
Vitamin D, calcium Decreased serum vitamin D by cytochrome P-450
hypocalcemia
Enteral feeds Decreased absorption
Food Increased absorption by delayed gastric emptying
Piroxicam Food Delayed absorption
Praziquantel Anthelmintic Food Decreased rate and extent of absorption
Primidone Anticonvulsant Fresh fruits and
vitamin C
Increased urinary excretion of primidone
Protein Low-protein diet increases duration of action of
primidone
Propranolol Antihypertensive High-protein foods Increased absorption
Propantheline Food Decreased absorption
Proxyphene Analgesic Food Increased absorption by delayed gastric emptying
Pyrimethamine Antimalarial Folic acid Decreased serum folate concentrations
Quinidine Antiarrhythmic Food Delayed absorption owing to protein binding
Riboflavin Vitamin Food Increased absorption by delayed gastric emptying
Rifampin Antibiotic Food Decreased absorption
Ritonavir Antiviral Vitamins Can cause vitamin deficiency
Potassium Hyper- and hypokalemia
Cholesterol Hypercholesterolemia
Triacylglycerols Hypertriacylglycerolemia
Food Delayed absorption
Oral solution capsules Food Increased extent of absorption
Table 2 Continued
Continued
DRUG–NUTRIENT INTERACTIONS 1911

into the brain by the same transport system as the
large neutral amino acids, and thus must compete
with them for use of the carrier binding sites. Diet
composition, by affecting the postprandial amino
acid profile, may change significantly the clinical effi-
cacy of drugs such as l-dopa, used in the treatment of
Parkinson’s disease.
0018 Body composition is also a relevant determinant of
drug disposition and action. Although most drug
dosages are calculated by total body weight, most
drugs only act in the fat-free body mass. Thus, at a
given body weight, individuals with more body fat
will tend to receive a higher effective dose than those
with lower body fat. The size of the body fat mass is
also important for drugs that are stored in adipose
tissue.
Major Drug–Nutrient Interactions of
Clinical Relevance
0019Table 2 provides information on the major drug–
nutrient interactions of clinical relevance. The list
reflects well-known interactions of drugs that have
been in the market for some time. The US Food and
Drug Administration (FDA) maintains an on-line
database of recently reported interactions and
those of new drugs. The database can be assessed at
www.fda.gov.
Drug Class Food/nutrient Effect/mechanism
Salicylates Analgesic Iron Long-term chronic use decreases serum iron
Magnesium salicylate
Choline salicylate Vitamin C Decreases concentration in serum and platelets
Sodium salicylate Amino acids Decreases their intestinal absorption and increases
urinary secretion
Saquinavir mesylate Antiviral Food Increased absorption of high-calorie, high-fat foods
Calcium Hypercalemia
Glucose Hyper- and hypoglycemia
Phosphorus Changes in serum phosphorus
Potassium Hyper- and hypokalemia
Spironolactone Diuretic Food Increased absorption by delayed gastric emptying
Sulfonamides Antibiotic Food Delayed with no effect on extent of absorption
Sulfdiazine
Sulfisoxazole Folic acid Decreased intestinal synthesis, absorption and serum
levels
Sulfamethoxazole
Te t r a c y c lin e Antibiotic Food Decreased absorption
Minerals Inhibits absorption of iron, calcium, zinc and
magnesium; chelation by polyvalent cations
Fats Decreases their absorption
Vitamin K Decreases bioavailability
Vitamin C Increase urinary losses; decreases plasma and
leucocytes and levels
Theophylline Broncodilator Charbroiled beef Increased metabolism of theophylline
High-fat meals Increased absorption dependent on formulation
Tola z a mid e Antidiabetic Sodium Hyponatremia and SIADH
Tolb u t amid e
Trimethoprim Antibiotic Folic acid Decreased serum folate levels
Valproic acid Anticonvulsant Milk, food,
carbonated drinks
Delayed absorption but no effect on extent of absorption
Divalproex sodium
Valproate sodium
Oral solution
Warfarin Anticoagulant Alcohol Inhibits warfarin metabolism
Vitamin K Beef liver, pork liver, green tea, leafy green vegetables
high in vitamin K inhibit anticoagulant effect
Vitamin E Can increase warfarin response
Zalcitabine Antiviral Food Decreased rate and extent of absorption
Zidovudine Antiviral Food Decreased rate of absorption
CSF, cerebrospinal fluid; NSAID, nonsteroidal antiinflammatory drug; SIADH, Syndrome of Inappropriate Antidiuretic Hormone excretion.
Table 2 Continued
1912 DRUG–NUTRIENT INTERACTIONS
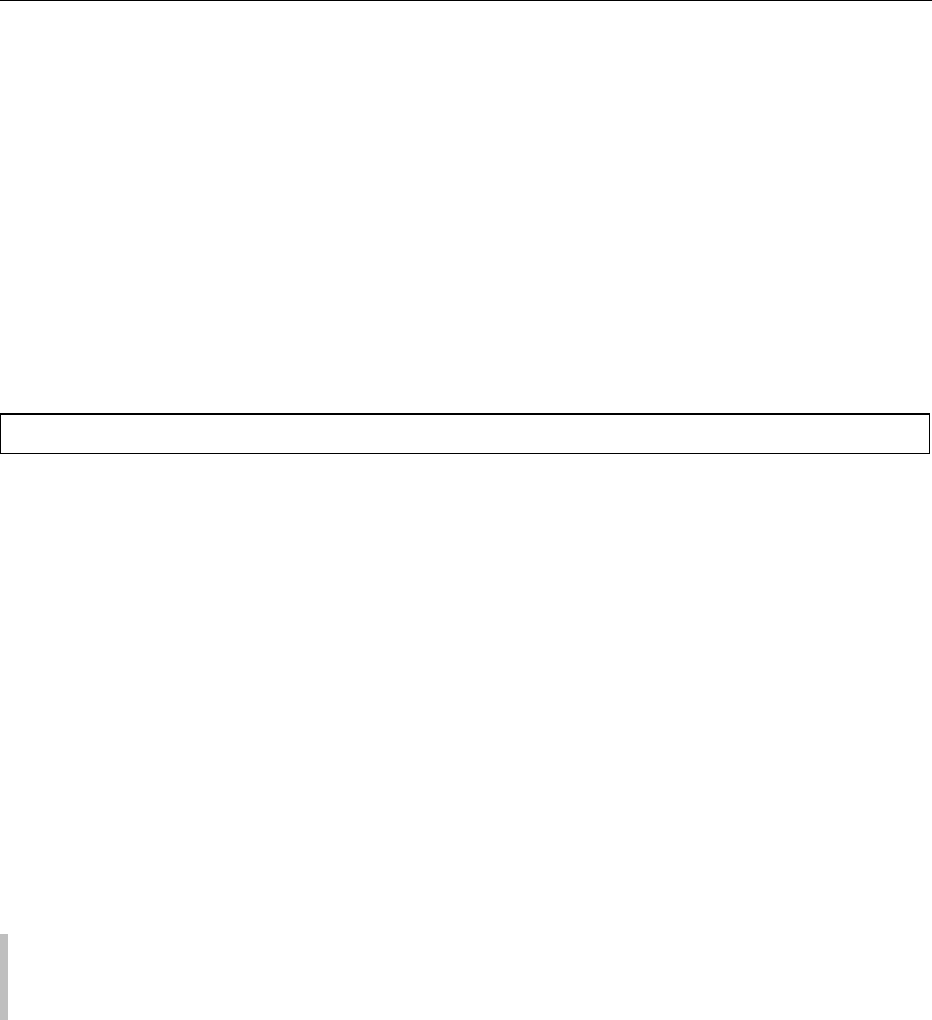
See also: Amino Acids: Metabolism; Malnutrition: The
Problem of Malnutrition; Malnutrition in Developed
Countries
Further Reading
Caballero B (1988) Nutritional implications of dietary
interactions: a review. Food Nutrition Bulletin 10: 9–20.
Knapp HR (1996) Nutrient–drug interactions. In: Ziegler
FF and Filer LJ (eds) Present Knowledge in Nutrition,
pp. 540–546. Washington, DC: ILSI Press.
Neuvonen P (1989) Clinical significance of food–drug inter-
actions. Medical Journal of Australia 150: 36–40.
Roberts J (1988) Age and diet effects on drug action.
Pharmacology and Therapeutics 37: 111–149.
Roe DA (1986) Drug food and drug nutrient interactions.
Journal of Environmental Pathology, Toxicology and
Oncology 5: 115–135.
Roe DA (1994) Diet, nutrition and drug reactions. In: Shils
ME, Olson JA and Shike M (eds) Modern Nutrition in
Health and Disease, 8th edn, pp. 1399–1416. Phila-
delphia, PA: Lea & Febiger.
Wurtman RJ, Caballero B and Salzman E (1988) Facili-
tation of DOPA-induced dyskinesias by dietary carbo-
hydrates. New England Journal of Medicine 390:
1288–1289.
Drugs See Antibiotics and Drugs: Uses in Food Production; Residue Determination
DRYING
Contents
Theory of Air-drying
Drying Using Natural Radiation
Fluidized-bed Drying
Spray Drying
Dielectric and Osmotic Drying
Physical and Structural Changes
Chemical Changes
Hygiene
Equipment Used in Drying Foods
Theory of Air-drying
J G Brennan, The University of Reading, Reading, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Drying, or dehydration, is the oldest method of food
preservation practiced by man, and today it is a major
contributor to the convenience food market. The main
objective of drying food is to prolong its shelf-life
beyond that of the fresh material, while at the same
time minimizing undesirable changes in appearance,
texture, flavor, and nutritional value during the drying
process. In addition to the preservation of the product,
drying can bring about savings in storage and
transport of foods owing to the reduction in weight
and bulk that occurs during most methods of drying.
Water in Foods
0002Water is an important contributor to the sensory qual-
ity of foods and a loss or gain of moisture from a
product can adversely affect its acceptability. However,
a certain amount of moisture in foods enables undesir-
able changes to occur as a result of the activity of
microorganisms and enzymes or through nonenzymic
chemical reactions. (See Drying: Chemical Changes.)
Thus, removing moisture from a food, or making it less
available, is one way of extending its shelf-life.
0003The moisture content of a wet material may be
expressed on a wet-weight basis (wwb), i.e., the mass
of water per unit mass of the wet material, or on a
DRYING/Theory of Air-drying 1913
dry-weight basis (dwb), i.e., the mass of water per unit
mass of dry solids. The latter way of expressing mois-
ture content is usually used in drying formulae and
calculations; see section Theory of Air Drying.
0004 However, when the influence of moisture on the
stability of foods is being studied, it is not only
the total moisture content that is of importance but
also how much of that moisture is available to sup-
port the activity of microorganisms and enzymes and
enable chemical changes to occur. It is now widely
accepted that a fraction of the total moisture in food
is strongly bound to individual sites on the solid
components and is not available to support these
activities. An additional amount of moisture is less
firmly bound but is still not readily available as a
solvent for soluble components of the food. Such
‘bound’ moisture exerts a vapor pressure less than
the vapor pressure of pure water at the prevailing
temperature. A property known as water activity
(a
w
) is used to represent the availability of moisture
in foods thus:
a
w
¼
p
v
p
w
ð1Þ
where p
v
is the water vapor pressure exerted by a
solution or wet solid, and p
w
is the vapor pressure
of pure water at the same temperature. This
expression also represents the relative humidity of
an air–water vapor mixture ( 100 to give the per-
centage relative humidity). A graph of moisture con-
tent as a function of water activity, with which it is in
equilibrium, is known as a sorption isotherm.Such
plots may be constructed by adsorption or desorp-
tion, which, in the case of many foods, results in two
different curves, exhibiting hysterisis (Figure 1). Food
isotherms may be considered to be made up of three
regions. In region A, water is strongly bound to spe-
cific sites on the solid components, is not available as
a solvent, and so does not support, microbiological,
enzymic or chemical activity. The amount of moisture
held in this way is known as the monolayer or mono-
molecular moisture content. This type of moisture
usually exits below a
w
0.35, corresponding to a mois-
ture content range 0.05–0.11 (dwb). Above region A,
water is less strongly bound to the solids but still does
not exert its full vapor pressure. In some publications,
region B (Figure 1), is said to contain moisture that is
bound in multilayers to the solid, and in region C,
structural and solution effects lower the water vapor
pressure. However, it is more likely that all these
effects have an influence over the whole isotherm.
At moisture contents of 1.0 (dwb) and above, most
foods exert vapor pressures equal to that of pure
water. Temperature affects the sorption behavior of
foods according to the expression:
d ðlna
w
Þ
d
1
T
¼
Q
s
R
, ð2Þ
where T is the absolute temperature, Q
s
is the heat of
adsorption, and R is the gas constant. The amount
of adsorbed water at any given value of a
w
decreases
as the temperature increases.
0005Numerous mathematical models have been proposed
to represent sorption isotherms of foods. One of the
earliest was the Brunauer–Emmet–Teller isotherm:
a
w
ð1a
w
ÞW
¼
1
W
m
C
þ
a
w
ðC 1Þ
W
m
C
, ð3Þ
where W is the total moisture content (dwb), W
m
is
the monolayer moisture content (dwb), and C is con-
stant. This model is said to apply over the a
w
range
0.05–0.45. An extended version of eqn (3), which
takes into account multilayer adsorption and is said
to be applicable up to a
w
0.90, is the Guggenheim–
Anderson–DeBoer model:
W
W
m
¼
Cka
w
ð1 ka
w
Þð1 ka
w
þ Cka
w
Þ
, ð4Þ
where C is known as the ‘Guggenheim’ constant, and
k is a factor correcting properties of the multilayer
molecules with respect to the bulk liquid.
0006A knowledge of the sorption characteristics of
foods is useful in the prediction of drying times and
energy requirements for drying processes. When
0 0.2 0.4
A
B
Desorption
Adsorption
Moisture content
0.6
C
Water activity
0.8 1.0
fig0001Figure 1 Adsorption and desorption isotherms showing hys-
teresis. From Brennan JG, Butters JR, Cowell ND and Lilly AEV
(1990) Food Engineering Operations, 3rd edn. London: Elsevier
Applied Science, with permission.
1914 DRYING/Theory of Air-drying
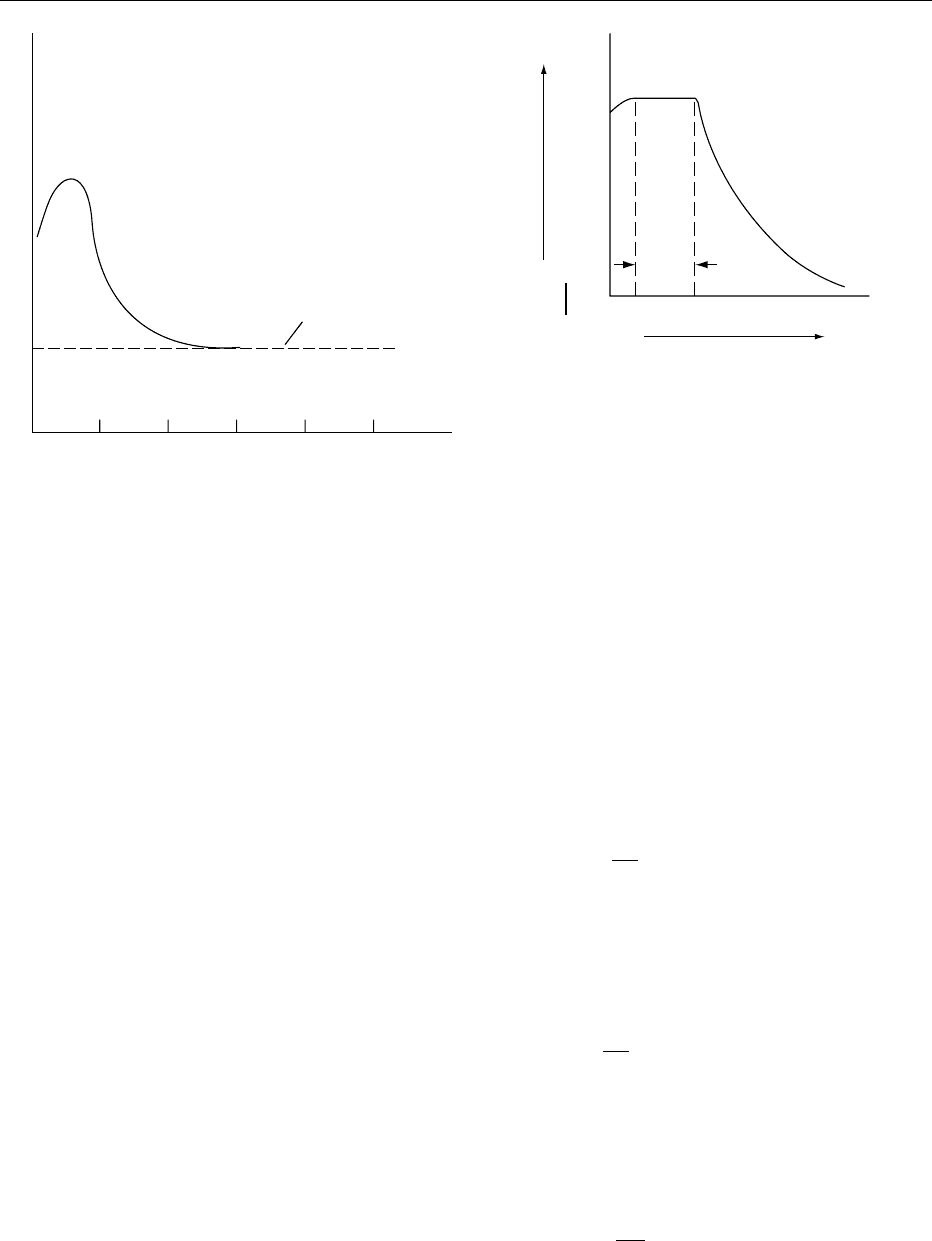
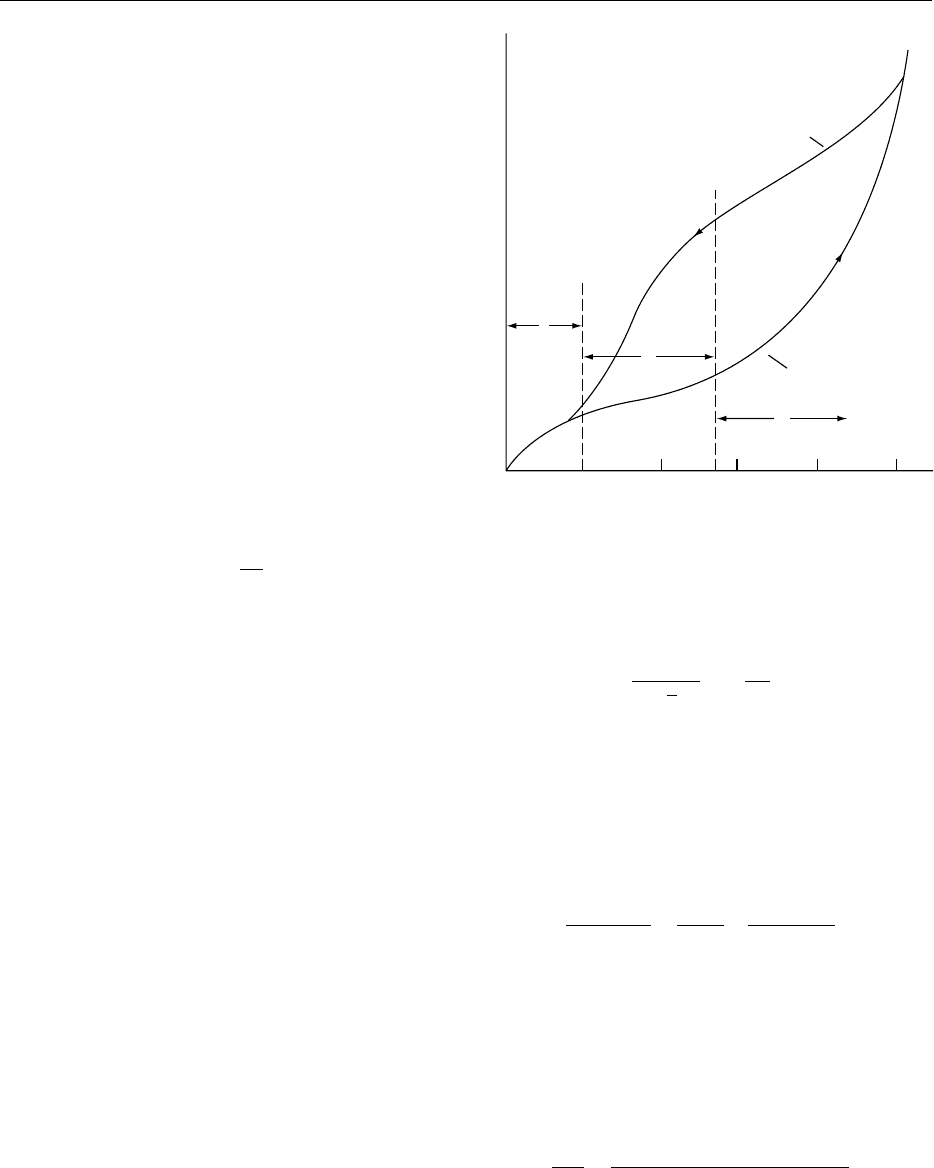
drying to very low moisture contents, extra energy is
required to overcome the bonding of the water to the
solids (Figure 2). The influence of a
w
on the stability
and shelf-life of foods is covered in Drying: Chemical
Changes. The most common method of drying foods
is air drying.
Theory of Air Drying
0007 When a wet material is placed in a current of heated
air, the air performs two main functions: it supplies
the sensible and latent heat to bring about the evap-
oration of the moisture, and it acts as a carrier to
move the water vapor formed away from the drying
surface, thus enabling further evaporation to take
place. Consider a wet solid composed of pure water
and inert solids, in the form of a thin slab, which is
placed in a current of heated air. If it is assumed that
drying takes place from one large face only, that the
temperature, humidity, and velocity of the air remain
constant, and that all the heat is transferred from the
air by convection, the drying curve should have the
familiar shape shown in Figure 3. It comprises a
number of stages or periods.
000 8 Period A–Bisanequilibration period during which
equilibrium is attained between the wet solid and the
drying air. This period usually takes up a relatively
small part of the drying curve.
000 9 Period B–C is known as the constant rate period,as
the rate of drying remains constant during the period.
Throughout this period, the surface of the solid is
fully saturated with water. The movement of water
from within the solid is sufficient to maintain satur-
ated conditions at the surface. A state of equilibrium
exists at the surface as the heat transfer to the surface
from the air balances the rate of evaporation from
the surface. The temperature at the surface of the
solid remains constant at a value that corresponds to
the wet-bulb temperature of the air. The driving
force that causes water to evaporate into the air is
the difference in water vapor pressure at the surface
from that in the main air stream. The rate of drying,
i.e., rate of change of weight (dw/dt), may be repre-
sented by the expression:
dw
dt
¼K
g
Aðp
s
p
a
Þ, ð5Þ
where K
g
is the mass transfer coefficient, A is
the drying area, p
s
is the water vapor pressure at the
surface of the solid, and p
a
is the water vapor pressure
in the main stream of the air. Eqn (5) may also
be written as:
dw
dt
¼K
0
g
AðH
s
H
a
Þ, ð6Þ
where K
0
g
is the mass transfer coefficient, H
s
is the
absolute humidity at the surface of the solid, and H
a
is the absolute humidity in the main air stream.
0010The rate of heat transfer from the air to the drying
surface (dQ/dt) may be represented by the expression:
dQ
dt
¼ h
c
Að#
a
#
s
Þ, ð7Þ
where h
c
is the convection heat transfer coefficient, #
a
is the dry-bulb temperature of the air, and #
s
is the
wet-bulb temperature of the air (temperature at
0 0.2 0.4
Water activity
Latent heat of vaporization
for water
Total heat adsorption (Arbitrary units)
0.6 0.8 1.0
fig0002 Figure 2 Dependence of the total heat of adsorption (latent
heat plus heat of adsorption) on water activity. Adapted from
Karel M (1974) Fundamentals of drying processes. In: Spicer A
(ed.) Advances in Pre-concentration and Dehydration of Foods,
pp. 45–94. London: Applied Science.
t
c
t−(h)
B
A
dW
dt
C
D
− kg/(kg dry solids)(h)
fig0003Figure 3 Model drying curve for a slab-shaped wet solid in air
at constant temperature, humidity, and velocity. Adapted from
Brennan JG, Butters JR, Cowell ND and Lilly AEV (1990) Food
Engineering Operations, 3rd edn. London: Elsevier Applied Sci-
ence, with permission.
DRYING/Theory of Air-drying 1915
