Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.

Absent are the complex branched surface domains
that typically occur in misoriented grains of iron-
based material. Only occasionally regular domains
may be replaced by a smoothly varying magnetiza-
tion configuration as actually expected for zero an-
isotropy (Fig. 6(c)). In fine-grained Permalloy
material (Fig. 6(b)), the domain structure resembles
that of Permalloy thin films in which the domains
extend over the grains (see Fig. 1, and the interpre-
tation in Sect. 2.5). Uniformly magnetized grains are
also found in coarse-grained NiFe materials after
annealing in magnetic fields. Such regular magnetic
microstructures cause reversible magnetization pro-
cesses and thus low noise in sensor applications.
Similar domains as in coarse-grained Permalloy
ribbons are observed in soft magnetic spinel ferrites
with a sufficiently large grain size (Fig. 6(d)). Ferrites
are prepared by sintering and they are used in high-
frequency applications due to their insulating prop-
erties (see Ferrites). Domain wall processes are
important in the whole application range of ferrites
up to GHz frequencies.
2.4 Amorphous Ribbons
Amorphous magnetic ribbons are alloys with typi-
cally 80% iron, cobalt, or nickel, and 20% ‘‘metal-
loids’’ like silicon or boron. They are quenched from
the melt with a thickness around 20 mm (see Amor-
phous and Nanocrystalline Materials). In application,
they compete with high-permeability NiFe alloys.
While crystal anisotropy is small in NiFe materials, it
cannot exist in metallic glasses by definition. Never-
theless, ‘‘regular’’ domains do exist in such ribbons,
again caused by residual anisotropies.
The main source of anisotropy is internal stress,
frozen in by the rapid quenching process. The mag-
netization is coupled to the stress by the magneto-
striction constant, so that the domain complexity in
as-quenched ribbons depends on magnetostriction as
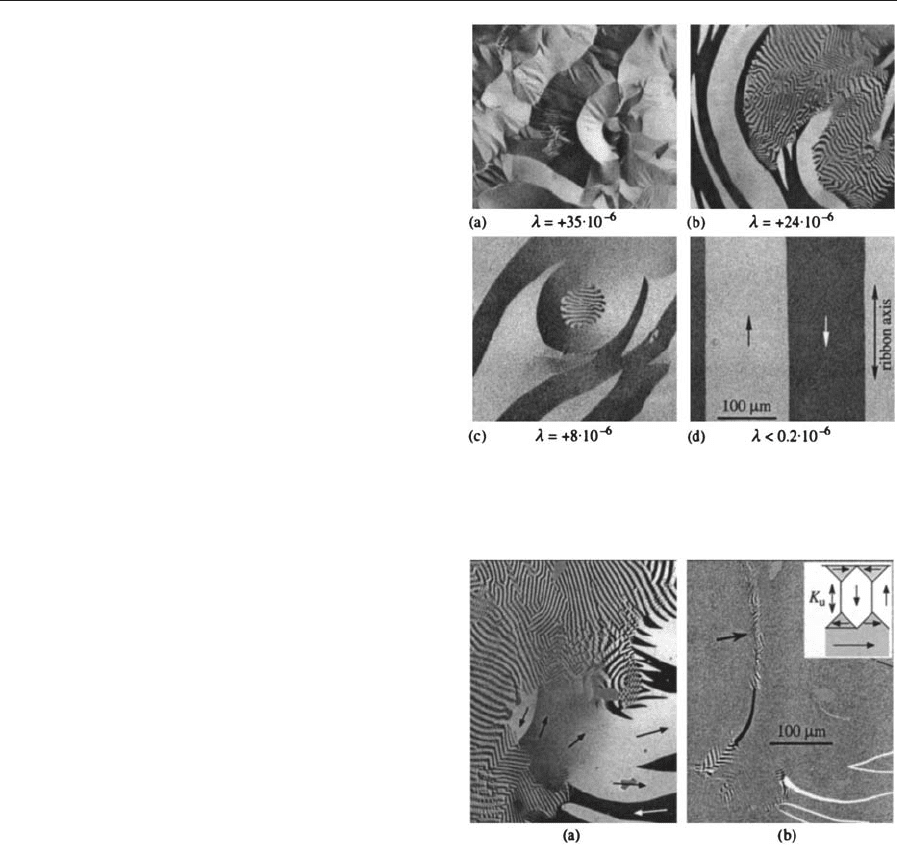
shown in Fig. 7. Two types of domains are typical:
wide curved domains that follow a varying in-plane
easy axis (caused by dominating tensile stress for
positive magnetostriction constant), and narrow fin-
gerprint domains. The fingerprints are closure do-
mains of underlying perpendicular domains, caused
by planar compressive stress which induces an easy
axis perpendicular to the surface. Internal stress can
not only vary laterally, but also in depth. This leads
to layered domain structures as demonstrated in
Fig. 8. A layered in-plane anisotropy with twisted
easy directions is visible in Fig. 7(a).
Even magnetostriction-free metallic glasses show
regular (but wide) domains in the as-quenched state
(Fig. 7(d)). They are often aligned along the ribbon
axis, caused by magnetization-induced anisotropy
(see Sect. 1). The easy axis follows the magnetization
direction present during quenching, which is frozen in
while cooling through the Curie point and which is
along the ribbon axis for magnetostatic reasons. Such
induced anisotropies are also present in magnetos-
trictive materials where it is difficult to separate them
Figure 7
Typical zero-field domain patterns in 20 mm-thick
amorphous ribbons of different magnetostriction
constant l.
Figure 8
Demagnetized state (a) of a magnetostrictive amorphous
ribbon, and the image difference (b) between image
(a) and a state where a small magnetic field was applied,
showing domain changes. The 1801-wall displacement
continues in the area of the fingerprint structure (see
arrow in (b)), proving that the fingerprint pattern must
be restricted to the surface zone as sketched. Obviously
there is a layered stress distribution: compressive stress
near the surface and dominating tensile stress
underneath.
850
Magnets, Soft and Hard: Magnetic Domains
from stress-induced contributions. Residual anisotro-
pies may also be caused by the flow pattern during
quenching, by the surface structure of the ribbons
(due, for example, to ‘‘air bubbles’’ on the wheel
side), and by mechanical creep due to different
quenching speed at different ribbon locations.
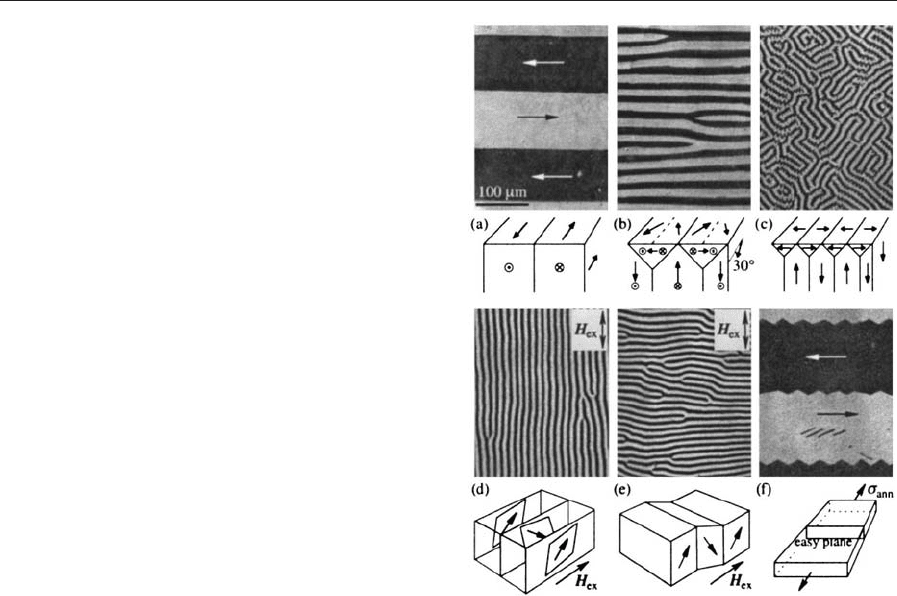
Controlling anisotropy in metallic glasses is possi-
ble. Frozen-in stress can be relaxed by more than
95% by carefully annealing below the crystallization
temperature. In addition, annealing can be performed
in uniform magnetic fields below the Curie temper-
ature or under external mechanical load. This leads to
well-defined anisotropies by the same mechanisms as
discussed before. A variety of ordered domain-states
is possible in this way (Fig. 9), allowing tailoring of
magnetization curves for specific applications. For
instance, transverse and perpendicular domains
shown in (a) and (e), respectively, are essentially
magnetized by rotation in fields along the (vertical)
ribbon axis, causing a linear hysteresis curve with
only minor domain rearrangement processes. Losses
are correspondingly low.
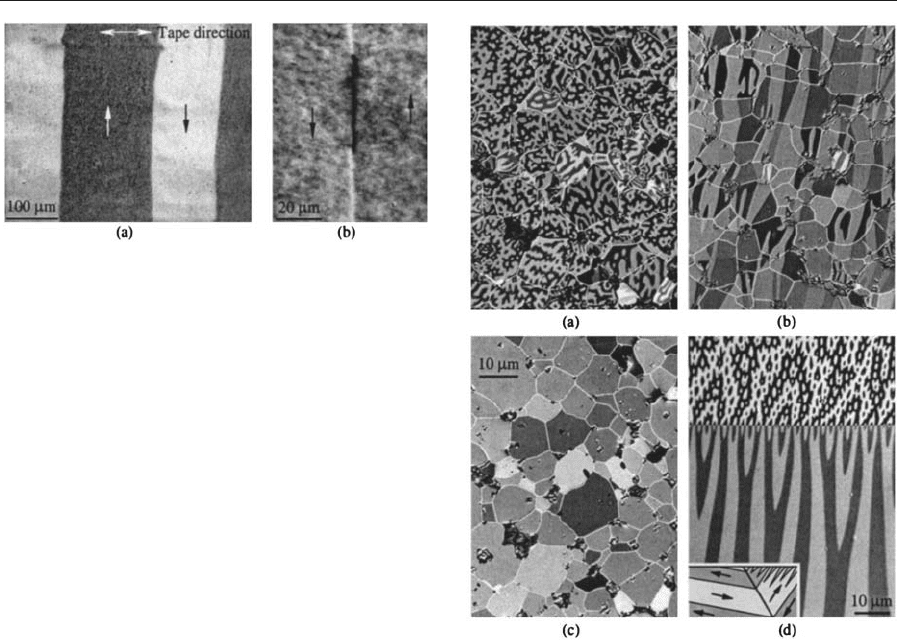
2.5 Nanocrystalline Ribbons
Modern nanocrystalline ribbons with grain sizes in
the 10 nm range compete in high-permeability appli-
cations with classical Permalloy and amorphous rib-
bons (see Amorphous and Nanocrystalline Materials).
The best-known alloy is the FeSiBNbCu system,
which consists of nanocrystalline SiFe grains embed-
ded in an amorphous magnetic matrix. Because of the
small grain size, the exchange interaction averages
over anisotropic properties of the individual grains,
so that anisotropy is smoothed out and the material is
effectively anisotropy-free (the same mechanism ap-
plies to the Permalloy films discussed in Sect. 1 and
the fine-grained Permalloy sheets of Sect. 2.3).
Together with a very small magnetostriction, excel-
lent soft magnetic properties can be achieved.
Well-defined domains and walls are found in these
materials (Fig. 10), which cannot be distinguished at
optical resolution from those in metallic glasses. An-
other class of nanocrystalline materials contains some
zirconium and has a higher iron content to obtain a
higher saturation magnetization. The patchy modula-
tion of magnetization (Fig. 10(b)) seems to be caused
by the effects of the irregular polycrystalline nature of
this material. Hard and immobile patch domains are
observed when the crystallites are getting too big or if
the coupling between the crystal grains is too weak.
3. Domains in Hard M agnetic Materials
The magnetic microstructure of hard magnets is
trivial in the permanent magnetic state: the elemen-
tary carriers of the magnetic moment, the more-or-
less independent grains or particles in these materials,
Figure 9
Ordered domain states in metallic glasses after different
annealing treatments. The ribbon axis is along the
vertical direction in all images. Annealing in a field
transverse to the strip axis results in wide transverse
domains (a). A strongly reduced domain width is
obtained after annealing in fields perpendicular to the
ribbon surface, resulting in an oblique (b) or
perpendicular anisotropy (c) in dependence of the
annealing field strength. The orientation of the narrow
stripe domains in the case of a perpendicular anisotropy
depends on the magnetostriction constant. If the ribbon
is demagnetized in an alternating field along its axis, the
stripes are oriented parallel to the field axis for a low-
magnetostriction material (d) and perpendicular in a
high-magnetostriction material (e). This can be
explained by the compatibility of magnetostrictive
deformations: a positive magnetostriction favors stripe
nucleation perpendicular to the field (sketch e) as
opposed to parallel stripes (sketch d) in which the
deformations are incompatible. The latter is only
possible in low-magnetostriction materials and is
preferred there because a parallel stripe pattern saves
wall energy. The transverse pattern in (f) is obtained by
a ‘‘creep’’ annealing treatment under mechanical load.
At zero field, the magnetization is forced in the
transverse in-plane direction to reduce magnetostatic
energy.
851
Magnets, Soft and Hard: Magnetic Domains
keep saturated along a preferred direction in reman-
ence. Domains are not connected to the permanent
magnet function and should be completely eliminated
to obtain a high coercivity and remanence. They only
play a role during switching. Two main classes of
permanent magnets must be distinguished: Small-par-
ticle magnets and anisotropy-based coarse-grained
magnets (see Hard Magnetic Materials, Basic Princi-
ples of ). Most hard magnetic materials rely on a
strong uniaxial anisotropy with Qb1, as opposed to
soft magnetic materials, which typically have a man-
ifold of easy directions.
3.1 Coarse-grained Magnets
In large-grained sintered magnets, two classes can be
separated: nucleation- and pinning-type magnets. The
difference becomes evident in the initial magnetization
curves. Starting from the thermally demagnetized
state, every grain in a nucleation-type material con-
tains many domain walls that can be displaced easily
(see Fig. 11(a), (b)), leading to a large initial permea-
bility. The permanent magnet properties appear only
when the initial domain walls are driven out in a large
field (Fig. 11(c)) so that also residual domains, which
are a natural consequence of particle shape and local-
ized stray field concentration, are completely annihi-
lated. This requires magnetic fields that may well
exceed the saturation field M
s
along the preferred axis,
in particular for isotropic samples or for misoriented
grains in textured material. The magnet can be de-
magnetized after this step only if new domain walls are
nucleated. In contrast, in a pinning-type magnet, the
domain walls are effectively pinned by precipitates,
leading to a low initial permeability.
Examples of nucleation-type magnets are hexa-
ferrite magnets (see Alnicos and Hexaferrites) and
high-quality rare-earth magnets of the SmCo
5
and
the Nd
2
Fe
14
B types (see Magnets: Sintered). They
consist of highly anisotropic grains in a textured, po-
lycrystalline compound, which is prepared in such a
way that switching of one grain has little influence
on its neighbors. Coercivity is thus primarily given
by some mean switching thresholds of the individ-
ual grains. Both the initial and the final states of
the switching process are essentially saturated in
Figure 10
(a) Ordered domain structure on a nanocrystalline
FeSiBNbCu-based soft magnetic ring core annealed in a
field perpendicular to the tape direction. (b) High-
resolution observation on a zirconium-containing
nanocrystalline ribbon, showing wall and modulated
domain contrast.
Figure 11
Domains on a sintered NdFeB magnet. (a) The
thermally demagnetized state on the basal plane, with
the axis of the textured magnet perpendicular to the
viewing surface. (b) The same state viewed parallel to the
axis reveals the high degree of texture in this material.
The grains are magnetostatically coupled as exchange
interaction is prevented by a nonmagnetic grain
boundary phase. (c) Domain-free, remanent state after
applying a saturation pulse [same location as (a)]. The
character of the branched domains visible in (a) is
displayed in (d) for a large-grained precursor material.
The twin grain boundary acts like a mirror for the
domains, so that ‘‘surface’’ domains (upper part) and
‘‘volume’’ domains (lower part) show up at the same
time (compare Fig. 1(f)).
852
Magnets, Soft and Hard: Magnetic Domains
high-coercivity materials, but the switching process
itself traverses a multidomain state (note that the
grain size is in the 10 mm range, which is much larger
than the single-domain size of about 100 nm).
If the domain walls are narrow due to high crys-
talline anisotropy, the walls may be effectively pinned
by a finely dispersed nonmagnetic phase. The alloy
Sm
2
(CoFe)
17
containing copper-rich Co
5
Sm precipi-
tates is an example for a high-quality permanent
magnet based on this principle. The precipitation
phase has a reduced magnetization and exchange
constant, so that the domain walls prefer to travel
along the platelet-shaped precipitates. The elimina-
tion of residual domains plays no role because these
domains are pinned (like all others) and the coercivity
is determined by the interaction between domain
walls and microstructure.
Note that both nucleation and pinning type mag-
nets rely on the high energy and narrow width of their
domain walls. A high wall energy makes nucleation
of new domains difficult, a narrow wall width favors
an effective pinning of walls. The finely divided do-
mains in Fig. 11(a), which are of the two-phase
branched type discussed in Sect. 1, can be applied to
measure the wall energy. It was shown that the sur-
face domain width of such a pattern stays constant,
independent of crystal thickness, whereas the internal
domain width increases with thickness. This is deter-
mined by an equilibrium between stray field energy
and wall energy. From the measurement of the sur-
face domain width, the domain wall energy can con-
veniently be determined.
3.2 Small-particle and Fine-crystalline Magnets
If a magnetic body becomes very small, it will not
contain domain walls and the particle becomes single-
domain. This occurs when the expense of wall energy
becomes greater than the gain of stray field energy.
The critical single domain size depends on the
Q-parameter and the particle shape. The single
domain limit rises with increasing elongation of the
particle.
Permanent magnets can also be prepared from
such single-domain particles. If the individual parti-
cles consist of a low-anisotropy material, they must
have an elongated shape to remain single-domain.
For such large particles, the danger of thermally ac-
tivated spontaneous switching (superparamagnetism)
is absent. Elongated single-domain particles are
therefore thermally stable even if they are complete-
ly isolated. Classical examples of this permanent
magnet class are the Alnico alloys, consisting of fine
filaments of a high-saturation FeCo alloy that are
embedded into a nonmagnetic NiAl matrix
(see Alnicos and Hexaferrites).
Modern small-particle magnets are based on high-
anisotropy materials that consist of single-domain
grains. A number of techniques can be used to prepare
fine-crystalline magnets out of precursors such as
Nd
2
Fe
14
B: rapid quenching and subsequent crystalli-
zation, mechanical alloying (see Magnets: Mechani-
cally Alloyed), and the HDDR process (see Magnets:
HDDR Processed). All these methods generate a fine
powder with a particle size around 100 mm, which
then has to be compacted into solid magnets. Oriented
magnets can be produced by ‘‘die-upsetting’’ (see
Textured Magnets: Deformation-induced). Also by the
HDDR process, an anisotropic powder can be pro-
duced which can be oriented in a magnetic field.
But even nonoriented fine-crystalline materials re-
main interesting as a relatively weak exchange inter-
action between very small grains can lead to an
enhanced remanence without a significant loss in
coercivity (exchange-enhanced fine-crystalline mag-
nets). The coupling enhances the remanence above
the average M
r
¼0.5 M
s
of independently oriented
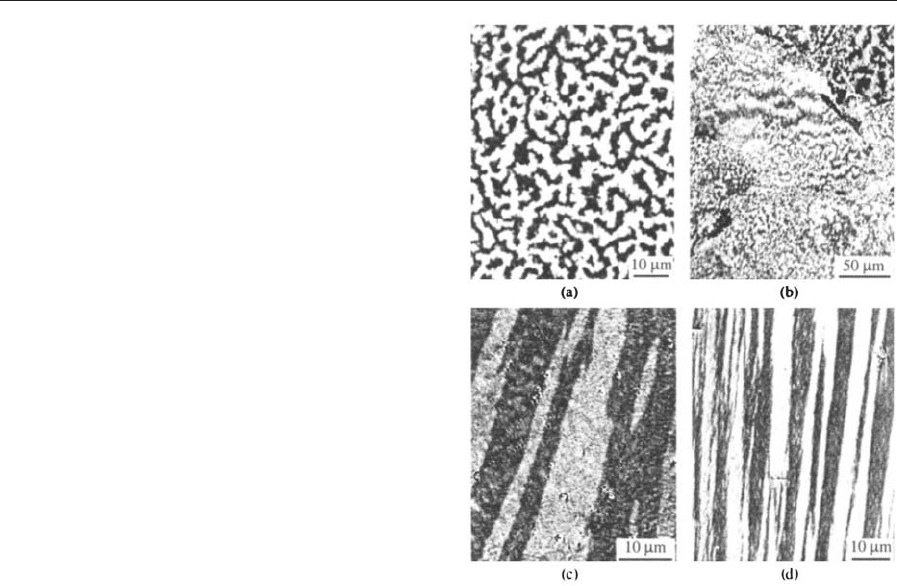
Figure 12
(a) Interaction domains observed on a surface
perpendicular to the preferred axis of textured, fine-
grained NdFeB material prepared by melt spinning and
hot deformation. In the HDDR-processed material (b),
the interaction domain width depends on the degree of
texture. In (c), the interaction domains are observed
parallel to the texture axis in the same material as in (b).
(d) Interaction domains on the side face of an oriented
Alnico crystal.
853
Magnets, Soft and Hard: Magnetic Domains

uniaxial grains. A further possibility for achieving
high remanence in nonoriented small-grain materials
consists of adding a high-saturation soft magnetic
phase, which is strongly exchange-coupled to the ba-
sic hard magnetic phase if the extension of the soft
phase is sufficiently small (see Magnets: Remanence-
enhanced). The coercivity in these two-phase fine-
crystalline magnets is dominated by the hard phase,
whereas the high remanence is primarily a conse-
quence of the soft phase.
The magnetization of the grains in fine-grained
permanent magnets is strongly correlated. An irreg-
ular magnetic microstructure is displayed in the de-
magnetized state as shown in Fig. 12(a)–(c). The
black-and-white contrasts correspond to grain neigh-
borhoods that are magnetized along a certain net
magnetization direction. These patterns are predo-
minantly determined by dipolar interactions between
the fine grains—an assumption which is justified by
the fact that this kind of domains is also observed in
small-particle magnets like Alnico (Fig. 12(d)) for
which the presence of exchange interaction between
the needles can be excluded. To indicate their special
nature, they were called ‘‘magnetostatic interaction
domains.’’ A characteristic feature of interaction
domains is their elongated shape extending along
the preferred axis of the material as is visible in
Fig. 12(c). The ‘‘domain boundaries’’ of the interac-
tion domains can move laterally to some extent by
the successive switching of neighboring grains (or
particles in case of Alnico), thus providing a certain
permeability for the demagnetized state.
3.3 Conclusions
The domain patterns in a variety of soft and hard
magnetic (mainly bulk) materials have been reviewed.
In both material classes, magnetic domains are
formed to reduce the magnetostatic energy of finite
samples. The interactions between physical micro-
structure, easy axes, applied fields, surface orienta-
tions, and mechanical stress leads to an enormous
variety of domain patterns, only a small fraction of
which could be presented by focusing on selected,
undisturbed samples.
Although in soft magnetic materials the avoidance
of magnetic charges is the dominating principle, the
domain arrangement is strongly influenced by an-
isotropy. The orientation of the main surfaces with
respect to the easy directions is decisive. If a surface
contains easy directions, it is no problem to reconcile
pole avoidance with avoiding anisotropy energy by
employing the surface-parallel easy directions only.
Grain-oriented transformer steel belongs to this class.
Here the degrees of freedom for domain formation
are strongly reduced and the basic domain patterns
are related to those of ideally oriented grains even in
the case of small misorientation.
Due to the near-single-crystal nature of this ma-
terial, its magnetic microstructure is conveniently ob-
servable and well understood, so that the losses of
transformer steel are steadily reduced by studying
and controlling the domain structure. Domain con-
trol is also possible in amorphous and nanocrystalline
ribbons if the influence of mechanical stress can be
avoided. For most other bulk soft magnetic materi-
als—the nonoriented polycrystalline SiFe, NiFe, and
ferrite materials—similar possibilities are not availa-
ble. Observing the complex, often heavily branched
domains at the surface of such materials offers only a
glimpse at the magnetization processes in the volume.
Domains in hard magnetic materials are only im-
portant during switching. In coarse-grained material
they are determined by a strong uniaxial anisotropy,
and in fine-grained magnets the magnetostatic inter-
action between small grains results in an inhomoge-
neous magnetic microstructure called interaction
domains.
See also : Coercivity Mechanisms; Ferrite Magnets:
Improved Performance; Magnetic Films: Hard
Bibliography
Cullity B D 1972 Introduction to Magnetic Materials. Addison-
Wesley, Reading, MA
Chikazumi S 1997 Physics of Ferromagnetism. Clarendon,
Oxford
Hubert A, Scha
¨
fer R 1998 Magnetic Domains. The Analysis of
Magnetic Microstructures. Springer, Berlin
O’Handley R 2000 Modern Magnetic Materials. Principles and
Applications. Wiley, New York
Van den Berg H 1986 Self-consistent domain theory in soft-
ferromagnetic media II. Basic domain structures in thin film
objects. J. Appl. Phys. 60, 1104–13
R. Scha
¨
fer
Institut fu
¨
r Metallische Werkstoffe, Dresden, Germany
Magnets: Biomedical Applications
This article deals with magnetic substances used in-
side living beings, particularly humans. The applica-
tion of magnets in medicine has been known for more
than 2500 years. Probably the earliest document
dates from about 600 bc and concerns the extraction
of an iron arrow tip by means of an extracorporally
applied loadstone (Hirschberg 1899). Several publi-
cations of pre-Christian centuries described the ap-
plication of magnetic material as a remedy for a
variety of diseases (Ha
¨
feli 1998). Nevertheless, it was
not before the middle of the twentieth century that
the number of different magnetic materials for bio-
medical applications considerably increased.
854
Magnets: Biomedical Applications

1. Natural Magnetic Substances in Humans
The existence of magnetic material, mainly magnetite
(Fe
3
O
4
), in living beings was found for the first time
in 1962 (Lowenstam 1962) and later recognized as
being responsible for the orientation capabilities of a
number of animals. The discovery of small particles
consisting of magnetite and maghemite (g-Fe
2
O
3
)in
human tissue was reported for the first time by
Kirschvink et al. (1992). There is evidence for some
influence of this material with reference to magnetic
resonance imaging (MRI), where it may be respon-
sible for contrast alterations. Human tissue is almost
completely diamagnetic. There are, however, some
paramagnetic substances with high values of suscep-
tibility: hemosiderin and ferritin. These substances
are responsible for storage and transport of iron in
organisms and can be used for the noninvasive ex-
amination of the iron content in the liver (Nielsen
et al. 1998). Finally, it is worth noting that the mag-
netic susceptibility of hemoglobin, an important part
of red blood cells, changes depending on its oxygen-
ation state. This behavior is used for the so-called
blood oxygenation level dependent (BOLD) contrast
in functional MRI (Schreiber et al. 1998).
2. Bulk Magnets
Important properties of bulk magnets positioned in-
side a living being concern the force or the torque
exerted in magnetic fields on appropriately designed
pieces of the material, the magnetic stray field that
may be used for monitoring the position of the mag-
net, and the magnetic losses arising in alternating
magnetic fields. All the substances have to be non-
toxic. This can be achieved either by the choice of
nonpoisonous magnetic materials, as is the case with
magnetite, or by covering the magnet with suitable
coatings (plastic, gold film, etc.).
2.1 Bulk Magnets for Force or Torque Applications
A magnetic field may be generated by permanent
magnets inside the body. Another way is to use fields
generated outside the body by coils or permanent
magnets. An example of permanent magnets used
inside the body is the application in veterinary med-
icine (Fahlenbrach and Fahlenbrach 1970) where
cows are forced to swallow rods of Alnico, a perma-
nent magnetic material, covered with plastic to pre-
vent both the magnet from corrosion and tissues
from toxic effects. The magnet remains in one of the
stomachs and attracts steel parts often swallowed by
the animals together with green fodder or hay. In this
way the stomach and intestines are protected against
being damaged. The same principle is sometimes also
used in human medicine to remove pins, nails, and
other steel parts that have been swallowed (Ha
¨
feli
1998). Another example concerns the fixation of tem-
porary pacemaker electrodes by hand-held perma-
nent magnets in emergency cases (Paliege et al. 1979).
Pieces of gold-plated iron wires have been used as tips
of flow-in stimulation catheters for this end. Inter-
esting experiments have been performed with certain
kinds of catheters that are able to move through
blood vessels driven by a combination of a d.c. mag-
netic field and an a.c. field. These parts consist of a
small permanent magnet (barium–ferrite cylinders of
a few cubic millimeters) and a flexible plastic tail
(Frei et al. 1966).
In spite of this fascinating idea these catheters
could obviously not be introduced into general med-
ical practice. Guidance of small magnetic probes is
being investigated in terms of the so-called magnetic
stereotaxis, i.e., the minimally invasive manipulation
of a permanent magnet inside the human brain along
a path of minimum damage to a position where it can
be used to heat a well-defined small region of tissue
(e.g., a brain tumor) or to act as a catheter for other
purposes. In vivo experiments have been successfully
performed with animals (Grady et al. 1990) and ap-
plication in humans, e.g., using multiple supercon-
ducting coils, looks promising (Meeker et al. 1996).
Equipment for generating fields outside the body is
generally less complicated. However, it was not until
the mid-1990s that a piece of permanent magnetic
material (Nd–Fe–B disk of 5 mm diameter and 2 mm
thickness) was positioned in the stomach and kept
there for several hours simply by the force exerted by
an external fixed permanent magnet (Gro
¨
ning et al.
1996). The magnetic disk covered by a layer of car-
nauba wax was part of a tablet designed for osmo-
tically controlled drug release. An interesting appli-
cation is the magnetic stoma seal for colostomy. It
consists of an implanted permanent magnetic ring
(Sm–Co covered with titanium) and a suitably
formed lid. At first glance this system seems to be
very attractive. A critical review of about five years’
practice, however, showed a relatively high percent-
age of incontinence (Schwemmle 1982). Therefore,
the conventional technique is still widely used.
2.2 Bulk Magnets for Monitoring
For many years there have been attempts to inves-
tigate the motility of the gastrointestinal tract by
magnetic monitoring (Forsman 1998) with the aim of
avoiding exposure to x rays or other ionizing radi-
ation. However, it was not before 1997 that satisfying
results were presented. Weitschies et al. (1997) pub-
lished reliable images of magnetic marker monitoring
with a spatial resolution in the millimeter range and a
temporal resolution down to 4 ms. The technique uses
markers consisting of sucrose pellets coated with a
mixture of magnetite and polymethyl methacrylate
and enclosed in silicone rubber. After ingestion of
855
Magnets: Biomedical Applications

these markers the magnetic stray field outside the
body is measured by means of a superconducting
quantum interference device (SQUID) and can be
used to calculate the actual position of the marker.
However, the technique is a rather expensive one
(SQUID array cooled by liquid helium, sophisticated
electronics, and a shielded room to reduce background
fields by several orders of magnitude). A simpler novel
technique is where an extracorporally applied pulsed
magnetic field is used to repeatedly align the marker
with its magnetic axis. The stray field is measured
during the impulse pauses after successively alternat-
ing pulses. In this way two essential advantages result:
the use of much cheaper conventional field sensors
and working without any magnetic shielding, i.e., in a
normal environment (Andra
¨
et al. 2000). It is worth
noting that the same principle can be used to monitor
other objects, e.g., the tip of a heart catheter.
2.3 Bulk Magnets for Tissue Heating
One kind of tumor therapy already introduced in
clinical practice is the combined treatment by radi-
ation and heat. Here, heating can be realized by eddy
currents induced in metallic pieces, so-called thermo-
seeds, implanted in the tumor region as an array of
appropriately positioned needles. Magnetic metals
offer the possibility of using magnetic losses with the
important advantage of having a mechanism for self-
regulation of the maximum temperature by means of
the appropriately chosen Curie temperature (Burton
et al. 1971). In contrast to most other medical appli-
cations of magnets, the materials of thermally self-
regulating implants are specially designed and consist
of nickel alloys with copper, silicon, and palladium,
or alloys of Fe–Pt and Pd–Co. A novel principle
(Cetas et al. 1998) makes use of the field concentra-
tion due to the high permeability of a specially com-
posed ferrite material (e.g., containing manganese,
zinc, and copper) with a Curie temperature in the
range 45–60 1C. The rod-shaped ferrite core is sur-
rounded by a nonmagnetic electrically conductive
sheath (e.g., stainless steel), which simultaneously
provides biocompatibility.
3. Magnetic Par ticles
The biomagnetic application of magnetic particles has
been known since the late 1950s (Gilchrist et al. 1957)
but there is now renewed interest. The fields of interest
concern contrast media, vascular occlusion, localized
heating, targeting of drugs and radiation sources, and
cell separation. Most of the modern literature can be
found under the keyword ‘‘magnetic carriers.’’
3.1 Magnetic Contrast Agents
An early attempt to use magnetic powders as contrast
agents for x-ray investigations was made by Frei et al.
(1968). Satisfactory visualization was achieved of the
gastrointestinal tract by means of commercially avail-
able powders (e.g., consisting of magnesium ferrite)
even with human volunteers. These investigations
were not continued in spite of an advantage over
conventional contrast agents, namely the possibility
of manipulating the material by external fields during
the x-ray investigation. Other studies were concerned
with MRI of the abdomen using orally administered
superparamagnetic particles consisting of polymer
spheres surrounded by iron oxide crystals, each crys-
tal measuring less than 50 nm (Boudghe
`
ne et al.
1993). Dextran-coated superparamagnetic iron oxide
particles (presumably magnetite) can be used for cell
labeling (Yeh et al. 1993), particularly after coupling
to special monoclonal antibodies. Other magnetite
nanoparticles have already been approved for imag-
ing of liver metastases.
3.2 Vascular Occlusion
Several investigations have been performed with re-
spect to vascular occlusion by means of magnetic
particles. One of the first publications deals with the
occlusion of intracranial aneurisms (Roth 1969). Iron
particles were used and a clinical experiment was
performed generating a magnetic thrombus with in-
jected particles exposed to a localized magnetic field
of implanted magnets. Vascular occlusion with mag-
netic particles has also been investigated as a tech-
nique for selective infarction of tumors and organs.
Mosso and Rand (1973) injected mixtures of car-
bonyl–iron microspheres with liquid silicone into the
renal arteries of dogs. After a certain time, during
which the material was held in place by an extra-
corporally applied magnetic field, vulcanization oc-
curred. Other authors have reported experiments
performed without silicone and also found agglo-
meration by means of the magnetic field.
3.3 Localized Heating
Many attempts have been made to use the heating
of magnetic particles in high-frequency magnetic
fields for selective treatments of cancer cells within
unaffected normal tissue. Gilchrist et al. (1957) de-
scribed this idea and reported on promising ex-
perimental results obtained with g-Fe
2
O
3
particles
(20–100 nm diameter) administered by injection into
different kinds of tissue of dogs from where the par-
ticles were carried into the lymph nodes. The hope
was to heat the nodes and in this way to destroy
lymphatic metastases.
Similarly, the treatment of other tumors seemed to
be possible either by direct injection or by transport
via blood vessels, particularly the vessels formed by
neo-vascularization near growing tumors. A great
number of both in vitro and in vivo experiments has
856
Magnets: Biomedical Applications

been carried out since the pioneering work. Magnetic
particles of different size (diameters ranging from the
so-called multidomain state down to the superpara-
magnetic region) and different material (magnetite,
maghemite, magnetic glass-ceramic, Ba–Co–ferrite,
etc.) have been tested. The particles are covered or
composed with different organic material or used in
the bare state. Reviews of the results (Jordan et al.
1993, 1999, Hergt et al. 1998, Andra
¨
1998) show that
clinical application cannot yet be applied and several
essential problems are still to be solved.
3.4 Magnetic Targeting of Drugs and
Radiation Sources
Magnetic drug targeting, i.e., guidance and retention
of drug-carrying magnetic particles by means of ex-
ternally applied magnetic fields, has been investigated
since the 1970s. The first clinical experiences with hu-
man patients were reported by Kuznetsov et al.(1997)
who used Fe–C particles combined with anticancer
drugs. Lu
¨
bbe and Bergemann (1997) used magnetic
carriers of multidomain particles of Fe
3
O
4
coated with
starch derivatives and loaded with epirubicin. A con-
centration of the particles was achieved by means of
suitably arranged permanent magnets outside the
body of the patient. Magnetic drug targeting may al-
so be used for the localized treatment of thrombosis,
as demonstrated by Inada et al.(1987)whoperformed
in vitro experiments with magnetic enzyme particles
consisting of magnetite and polyethylene glucol cou-
pled to urokinase. A further kind of magnetic target-
ing is the application of particles for the transport of
radioisotopes, e.g.,
90
Y, to the region where the radi-
ation is to be applied. This novel technique was tested
in animal experiments by Ha
¨
feli et al. (1997) who used
microspheres (10–30 mm in diameter) consisting of a
mixture of polylactic acid and magnetite.
3.5 Magnetic Cell Separation
An already commercially introduced technology is
the selective cell separation by means of polymer
spheres containing small magnetic particles, so-called
magnetic beads. The surface of the beads is coated
with different kinds of monoclonal antibodies de-
signed for special selective binding with appropriately
prepared biological objects. In this way blood cells,
bacteria, or even specific nucleic acid sequences can
be isolated and manipulated in special magnetic sep-
aration equipment. The magnetic component of the
beads in most cases is magnetite. A review of this field
can be found in Ugelstad et al. (1997).
See also: DNA Microarrays Using Magnetic Label-
ing and Detection; MRI Contrast Agents; Permanent
Magnetic Devices in Otiatria; SQUIDs: Biomedical
Applications
Bibliography
Andra
¨
W 1998 Magnetic hyperthermia. In: Andra
¨
W, Nowak H
(eds.) Magnetism in Medicine. Wiley-VCH, Berlin
Andra
¨
W, Danan H, Kirme W, Kramer H-H, Saupe P,
Schmieg R, Bellemann M 2000 A novel method for real time
magnetic marker monitoring in the gastrointestinal tract.
Phys. Med. Biol. 45, 3081–93
Boudghe
`
ne F P, Bach-Gansmo T, Grange J -D, Lame S,
Nantois C, Wallays C, Bigot J-M 1993 Contributions of oral
magnetic particles in MR imaging of the abdomen with spin-
echo and gradient-echo sequences. JMRI 3, 107–12
Burton C, Hill M, Walker E 1971 The RF thermoseed—a
thermally self-regulating implant for the production of brain
lesions. IEEE Biomed. Eng. BME-18, 104–9
Cetas T C, Gross E J, Contractor Y 1998 A ferrite core/metallic
sheath thermoseed for interstitial thermal therapies. IEEE
Trans. Biomed. Eng. 45, 68–77
Fahlenbrach B, Fahlenbrach H 1970 Der Magnetismus in
Medizin und Biologie. Umschau 14, 429–33
Forsman M 1998 Magnetism in gastroenterology. In: Andra
¨
W,
Nowak H (eds.) Magnetism in Medicine. Wiley-VCH, Berlin
Frei E H, Driller J, Neufeld H N, Barr I, Bleiden L, Askenazy H
M 1966 The POD and its applications. Med. Res. Eng. 5, 11–8
Frei E H, Gunders E, Pajewsky M, Alkan W J, Eshchar J 1968
Ferrites as contrast material for medical X-ray diagnosis. J.
Appl. Phys. 39, 999–1001
Gilchrist R K, Medal R, Shorey W D, Hanselman R C, Parrott
J C, Taylor C B 1957 Selective inductive heating of lymph
nodes. Ann. Surgery 146, 596–606
Grady M S, Howard III M A, Molloy J A, Ritter R C, Quate E
G, Gillies G T 1990 Nonlinear magnetic stereotaxis: three-
dimensional, in vivo remote magnetic manipulation of a small
object in canine brain. Med. Phys. 17, 405–15
Gro
¨
ning R, Werner M, Berntgen M, Georgaratis M 1996 Per-
oral controlled release dosage forms with internal magnets
and extracorporal magnetic guidance—investigations into
the renal elimination of riboflavin. Eur. J. Pharm. Biopharm.
42, 25–8
Ha
¨
feli U 1998 The history of magnetism in medicine. In: Andra
¨
W, Nowak H (eds.) Magnetism in Medicine. Wiley-VCH,
Berlin
Ha
¨
feli U, Pauer G, Macklis R M 1997 Magnetically targeted
microspheres for intracavity and intraspinal Y-90 radiother-
apy. In: Ha
¨
feli U, Schu
¨
tt W, Teller J, Zborowsky M (eds.)
Scientific and Clinical Applications of Magnetic Carriers. Ple-
num, New York
Hergt R, Andra
¨
W, d’Ambly C-G, Hilger I, Kaiser W A,
Richter U, Schmidt H-G 1998 Physical limits of hyper-
thermia using magnetite fine particles. IEEE Trans. Magn.
34, 3745–54
Hirschberg J 1899 Geschichte der Augenheilkunde Engelmann,
Leipzig, Vol. 12
Inada Y, Ohwada K, Yoshimoto T, Kojima S, Takahashi K,
Kodera Y, Matsushima A, Saito Y 1987 Fibrinolysis by
urokinase endowed with magnetic property. Biochem. Bio-
phys. Res. Commun. 148, 392–6
Jordan A, Scholz R, Wust P, Fa
¨
hling H, Felix R 1999 Magnetic
fluid hyperthermia (MHF): cancer treatment with AC mag-
netic field induced excitation of biocompatible superpara-
magnetic nanoparticles. J. Magn. Magn. Mater. 201, 413–9
Jordan A, Wust P, Fa
¨
hling H, John W, Hinz A, Felix R 1993
Inductive heating of ferrimagnetic particles and magnetic
fluids: physical evaluation of their potential for hype-
rthermia. Int. J. Hyperthermia 9, 51–68
857
Magnets: Biomedical Applications

Kirschvink J L, Kobayashi-Kirschvink A, Woodford B J 1992
Magnetite biomineralization in the human brain. Proc. Natl.
Acad. Sci. USA 89, 7683–7
Kuznetsov A A, Harutyunyan A R, Dobrinsky E K, Filippov V
I, Malenkov A G, Vanin A F, Kuznetsov O A 1997 Ferro-
carbon particles: preparation and clinical application. In:
Ha
¨
feli U, Schu
¨
tt W, Teller J, Zborowsky M (eds.) Scientific
and Clinical Applications of Magnetic Carriers. Plenum, New
York
Lowenstam H A 1962 Magnetite in denticle capping in recent
chitons (Polyplacophora). Geol. Soc. Am. Bull. 73, 435–8
Lu
¨
bbe A S, Bergemann C 1997 Selected preclinical and first
clinical experiences with magnetically targeted 4
0
-epidoxo-
rubicin in patients with advanced solid tumors. In: Ha
¨
feli U,
Schu
¨
tt W, Teller J, Zborowsky M (eds.) Scientific and Clin-
ical Applications of Magnetic Carriers. Plenum, New York
Meeker D C, Maslen E H, Ritter R C, Creighton F M 1996
Optimal realization of arbitrary forces in a magnetic stereo-
taxis system. IEEE Trans. Magn. 32, 320–8
Mosso J A, Rand R W 1973 Ferromagnetic silicone vascular
occlusion: a technique for selective infarction of tumors and
organs. Ann. Surg. 178, 663–8
Nielsen P, Fischer R, Engelhardt R, Dresow B, Gabbe E E 1998
Neue Mo
¨
glichkeiten in der Diagnose der heredita
¨
ren Ha
¨
mo-
chromatose. Deutsches A
¨
rzteblatt 95, B-2262–8
Paliege R, Volkmann H, Andra
¨
W 1979 Magnetische lagefixie-
rung einschwemmbarer Elektrokatheter zur tempora
¨
ren
Schrittmachertherapie. Dt. Gesundh.-Wesen 50, 2514–8
Roth D A 1969 Occlusion of intracranial aneurisms by ferro-
magnetic thrombi. J. Appl. Phys. 40, 1044–5
Schreiber A, Kraemer F M, Janz C, Hennig J 1998 Functional
magnetic resonance imaging. In: Andra
¨
W, Nowak H (eds.)
Magnetism in Medicine. Wiley-VCH, Berlin
Schwemmle K 1982 Colostomie mit Magnetverschlu. Chirurg
53, 547–50
Ugelstad J, Prestvik W, Stenstad P, Kilaas L, Kvalheim G 1997
Selective cell separation with monosized magnetizable poly-
mer beads. In: Andra
¨
W, Nowak H (eds.) Magnetism in
Medicine. Wiley-VCH, Berlin
Weitschies W, Ko
¨
titz R, Cordini D, Trahms L 1997 High-res-
olution monitoring of the gastro-intestinal transit of a mag-
netically marked capsule. J. Pharmaceut. Sci. 86, 1218–22
Yeh T-C, Zhang W, Ildstad S T, Ho C 1993 Intracellular labe-
ling of T-cells with superparamagnetic contrast agents.
Magn. Reson. Medicine 30, 617–25
W. Andra
¨
and R. Hergt
Institut fu
¨
r Physikalische Hochtechnologie e.V.
Jena, Germany
Magnets: Bonded Permanent Magnets
Bonded magnets are generally prepared by blending
coercive powder with a binder followed by compacting
or molding the material to the final shape. Because
of the presence of the binder the energy product of
bonded magnets is always lower than that of their
sintered counterparts. However, two great advan-
tages of bonded magnets are their superior mechani-
cal properties and the fact that the magnets can be
prepared under net shape conditions. Bonded and sin-
tered magnets of ferrites have been known for many
decades. Both types of magnets have very low energy
products when compared to sintered rare earth-based
magnets. The excellent properties of rare earth-based
magnets are, however, accompanied by a high price.
The last decade of the twentieth century saw an
increased use of actuators and small electromotors
not only of low weight but also of low energy con-
sumption, as in battery-driven motors in particular.
There has also been an increased use of electronically
controlled, electrically actuated systems in automo-
biles, especially for critical vehicle control functions.
All this and the increased use of electromagnetic de-
vices in the electronic and electric industry has led to
an enhanced pressure to find improved or alternative
permanent magnet materials lying between the low-
cost low-performance ferrite magnets and the high-
cost high-performance sintered NdFeB magnets.
Bonded NdFeB magnets meet most of the criteria
mentioned and their use is growing steadily. For this
reason bonded types of rare earth permanent mag-
nets will be discussed below in somewhat more detail.
1. Bonding Technologies
Bonded magnets are generally prepared by blending
coercive powder with a binder or by encapsulating
coercive powder in a binder, followed by compacting
or molding the material to the final shape. The pow-
der of the magnetic materials used in bonded magnets
may be ferrites, alnico, samarium cobalt or neodym-
ium–iron–boron. In some cases also hybrid powders
are used.
Depending on the binder, the magnets produced
may be flexible or rigid. Nitrile rubber or vinyl are
commonly used for the former type whereas for the
latter type nylon, teflon, polyester, or thermoset epox-
ies are employed.
There are four main manufacturing routes for
bonded magnets: calendering, injection molding, ex-
trusion, and compression bonding.
(i) Calendering involves processing the material
between rollers and leads to continuous strips of ad-
justable thickness and length. This process is sche-
matically shown in Fig. 1. The material used for
calendering is usually a mixture of the magnetic pow-
der and a thermoplastic binder. This mixture is pro-
cessed between rollers forming a continuous strip that
may be up to 100 m in length. Magnetic loading can
be up to 70% by volume.
(ii) The injection molding process is schematically
represented in Fig. 2. This process involves forcing
the heated mixture of magnetic powder and thermo-
plastic binder via tubes into a mold where it is al-
lowed to cool and harden. In this case too, magnetic
loading can reach 70%, especially when using powder
of spherical shape (Ma et al. 2002).
858
Magnets: Bon ded Permanent Magnets
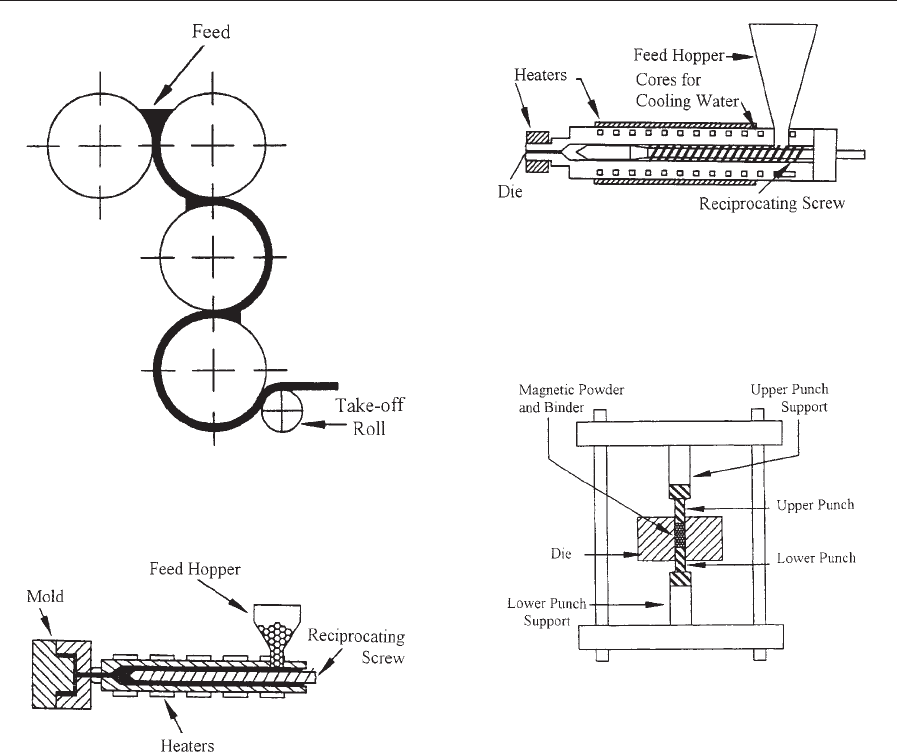
(iii) Extrusion makes use of an orifice through
which the compounded material is squeezed, as illus-
trated in Fig. 3. During the extrusion process the
orifice is heated and the profile is controlled as
the compounded material cools. Depending on the
binder, the cooled product can be firm, as for flexible
end products, or rigid if a nonflexible end product is
desired. The loading can reach slightly higher values
(up to 75% by volume) than in the two previous
cases.
(iv) Compression bonding uses magnetic powder
blended with an epoxy that has been liquefied by dis-
solution in an organic solvent. The latter is evaporated
during the blending process, leaving the magnetic
particles in an epoxy-encapsulated state. After drying,
the coated powder is flowed into a conventional pow-
der press (Fig. 4) and compacted into the desired
shape. Finally, the compacted magnet is cured in an
oven at temperatures of around 150–175 1C. This
process can reach magnetic loadings up to 80%.
Because of the presence of the binder, the magnetic
remanence is always lower than that of the fully dense
magnetic material, as obtained, for instance, by sinte-
ring. The situation is even less favorable for the energy
product, being roughly proportional to the remanence
squared. There are, however, obvious advantages.
The major advantage is that bonded magnets can be
Figure 1
Schematic diagram showing the manufacturing of
magnetic strips by calendering (after Ormerod and
Constantinides 1997).
Figure 2
Schematic representation of the injection molding
process. This process involves forcing the heated mixture
of magnetic powder and thermoplastic binder via tubes
into a mold where it is allowed to cool and harden (after
Ormerod and Constantinides 1997).
Figure 3
Schematic representation of the extrusion process. The
compounded magnetic material is squeezed through an
orifice. During the extrusion process the orifice is heated
and the profile is controlled as the compounded material
cools (after Ormerod and Constantinides 1997).
Figure 4
Diagram showing the compression bonding process. The
magnetic powder is blended with an epoxy that has been
dissolved in an organic solvent. The latter is evaporated
during the blending process, leaving the magnetic
particles in an epoxy-encapsulated state. The coated
powder is flowed into a conventional powder press and
compacted into the desired shape. The compacted
magnet is finally cured in an oven (after Ormerod and
Constantinides 1997).
859
Magnets: Bonded Permanent Magnets
