Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.

the tape leader is threaded into the drive over a read/
write head to the take-up reel by a pentagon thread-
ing mechanism. A decoupler column placed near the
cartridge entrance decouples any tape vibrations that
may occur inside the cartridge. The tension is sensed
and controlled by a tension transducer. In a rigid disk
drive as shown in Fig. 3(a), a stack of disks is mounted
on a sealed, grease lubricated ball-bearing spindle. The
disk stack is rotated by a d.c. motor at speeds ranging
from a few thousand rpm to a maximum speed of
about 10 000 rpm. A head slider is supplied for each
disk surface. The slider suspension assembly is actu-
ated by a stepping motor or a voice-coil motor for
read/write operation (Bhushan 1996a, 2000).
Magnetic heads consist either of conventional in-
ductive or inductive/magnetoresistive (MR) devices
(Bhushan 1993, 1996a, 2000). If MR head design is
used, it is only used for read purposes. Air-bearing
surfaces of tape heads are generally cylindrical in
shape (Fig. 2(b)). The tape is slightly underwrapped
over the head surface to generate hydrodynamic lift
during read/write operations. The head sliders used in
rigid disk drives are either two- /three-rail, taper-flat
design or two-rail, step, negative pressure design
(Fig. 3(b)). The sliders are supported by a nonmag-
netic steel leaf spring (flexing) suspension to allow
motion along the vertical, pitch, and roll axes. The
front taper or step pressurizes the air lubricant, while
some air leaking over the side boundaries of the rail
results in a pitch angle.
Magnetic media fall into two categories: (i) partic-
ulate flexible media, where magnetic particles are dis-
persed in a polymeric matrix and coated onto the
polymeric substrate (Fig. 2(c)); and (ii) thin-film media,
where continuous films of magnetic materials are
deposited by vacuum techniques onto the polymeric
substrate or flexible media (Fig. 2(c)) or onto a rigid
substrate such as aluminum, glass, or glass ceramics
(Fig. 3(c)) (Bhushan 1993, Miller and Bhushan 1996).
2. Macrotribology of Magnetic Storage Devices
Friction and wear of two surfaces in relative motion
is governed by the real area of contact and interfacial
adhesion. In addition, for wet contacts with a tiny
amount of liquid present at the interface, ‘‘stiction’’
(or high static friction) can occur owing to meniscus/
viscous effects (Fig. 4). Generally, any liquid that
wets or has a small contact angle on surfaces will
condense from vapor in the form of an annular-
shaped capillary condensate in the contact zone. The
negative (attractive) pressure of the liquid films of the
capillary condensates or pre-existing film of lubricant
can significantly increase the adhesion between solid
bodies. Liquid-mediated adhesive forces can be
divided into two components: meniscus force (F
M
)
owing to surface tension and a rate-dependent vis-
cous force (F
V
). The total tangential force (F) re-
quired to separate the surfaces during sliding is equal
to intrinsic friction force (F
A
) and stiction force (F
S
,a
combination of friction force owing to the meniscus
Figure 3
Schematics of (a) a rigid disk drive, (b) an inductive/MR
thin-film picoslider, and (c) sectional view of a thin-film
rigid disk.
Figure 4
Schematic of a wet interface with meniscus formation,
showing various forces being applied during sliding. F
M
is the meniscus force, W is the normal force, and F
A
and
F
S
are intrinsic friction and stiction forces, respectively.
550
Magnetic Reco rding Devices: Head/Medium Interface
effect and the peak viscous force) (Bhushan 1999b):
F ¼ F
A
þ F
S
¼ m
r
ðW þF
M
ÞþF
V
ð1Þ
where m
r
is the ‘‘true’’ static coefficient of friction and
W is the normal the load. It turns out that since the
surfaces in magnetic storage devices are smooth and
the normal load being applied is relatively low, fric-
tional effects because of meniscus and viscous forces
are significant. Optimization of surface topography
of head and medium and overcoats and lubricant
films needs to be achieved for long-term reliability of
magnetic storage devices.
2.1 Contact Modeling
Numerical techniques are used to solve contact prob-
lems of rough surfaces under dry and wet conditions
(Bhushan 1996b, 1996c, 1998, 1999b, Tian and
Bhushan 1996a, 1996b, Peng and Bhushan 2001).
The numerical model does not require an asperity
model and makes no probabilistic assumptions such
as distribution of asperity heights, slopes, and curva-
tures, as required in analytical models. The numerical
techniques allow analysis of a large number of con-
tact spots that go through elastic and elastic/plastic
deformations. The numerical model provides useful
information on the number of contact spots, their
sizes and distributions, and the spacing between con-
tacts. Furthermore, wet contact analysis for contact
of rough surfaces with a liquid film can be carried
out. In the wet contact analysis, elastic/plastic dry
contact of rough surfaces is first analyzed. In the next
step, a liquid film of known mean thickness is intro-
duced over the deformed rough surfaces. Wetted
areas are determined by selecting the area where as-
perities of both contacting surface touch the liquid.
The total projected meniscus area is then used for the
calculations of meniscus forces. The model can be
used to develop optimum surface roughness distri-
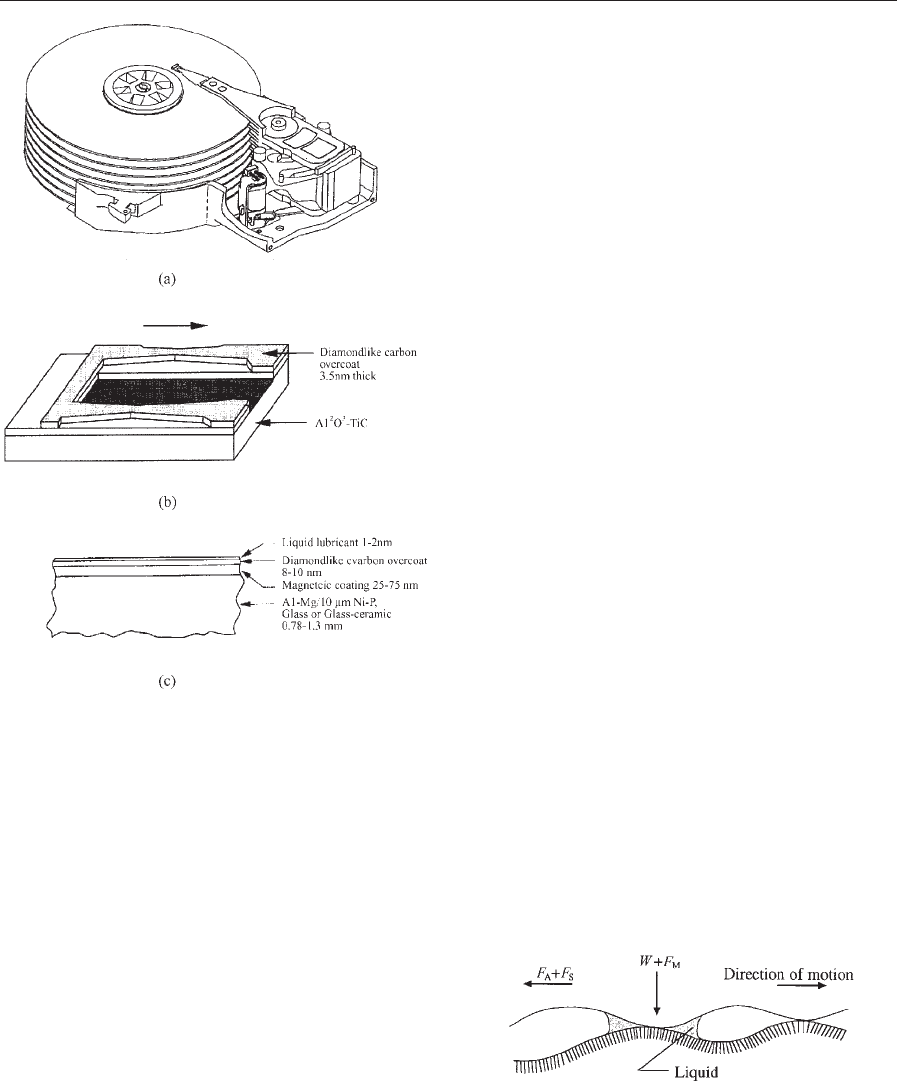
butions for the head and medium surfaces. Figure 5
shows representative contact area and meniscus area
maps for a computer-generated random rough sur-
face in contact with a smooth surface in the presence
of water film. As expected, the meniscus area is larger
than the contact area and the meniscus force is three
times that of the normal force.
Tian and Bhushan (1996b) and Poon and Bhushan
(1996) used the model to predict the effects of relative
humidity and liquid film thickness on the meniscus
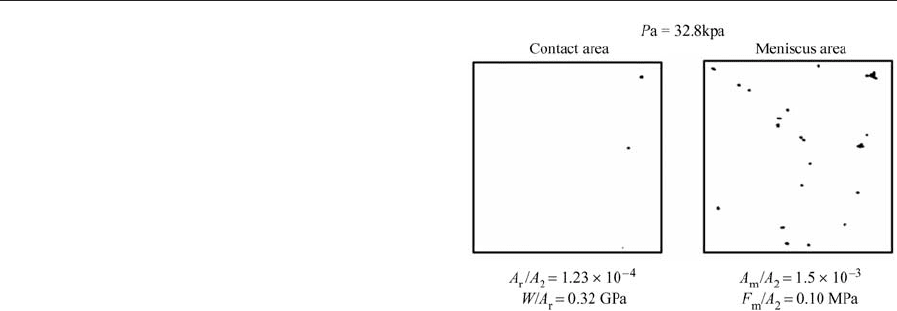
force (see also Bhushan 1998). The effect of relative
humidity on the meniscus force at an interface is
shown in Fig. 6(a). The effect of liquid film thickness
and interface roughness on computer-generated
rough surfaces in contact with a smooth surface is
shown in Fig. 6(b). An increase in either relative hu-
midity or liquid film thickness increases the liquid
present at the interface. The thicker a liquid film, the
more asperities touch the liquid surface and the more
menisci form on a larger number of asperities. In
addition, with a thicker film, a larger volume of liquid
is present around the asperities resulting in a greater
amount of meniscus volume accumulated at the con-
tact interface and a greater meniscus height. These
effects lead to larger meniscus forces. There is a crit-
ical film thickness for a surface with given roughness
above which the meniscus force increases rapidly.
The critical film thickness is of the order of three-
quarters of the composite r.m.s. roughness of the
mating surfaces (s).
Bhushan and Chilamakuri (1996) and Chilamakuri
and Bhushan (1998a) used the numerical model to
study contact between flat rigid surfaces and non-
Gaussian rough surfaces with known skewness (Sk)
and kurtosis (K). Rough surfaces with different skew-
ness and kurtosis were generated using a two-dimen-
sional digital filter technique. Figure 7 shows the
probability density functions of surfaces with various
skewness and kurtosis values. Figure 8(a) shows the
effect of skewness and kurtosis on the fractional real
area of contact (A
r
/A
a
, where A
r
is the contact area
and A
a
is the apparent area) and relative meniscus
force (F
M
/W) at different nominal pressures (p). A
positive skewness between 0 and 0.2 at low pressure
and about 0.2 at higher pressures results in the lowest
real area of contact and meniscus force. Contact area
and meniscus force decrease with an increase in the
kurtosis. Fewer peaks present on a surface with a
range of positive skewness values or high kurtosis can
explain the trends. Figure 8(b) shows the variation of
relative meniscus force with h/s ratio for different
skewness and kurtosis values. Note that the sensitiv-
ity of h/s to the meniscus force decreases at a range of
Figure 5
Contact area and meniscus area for the case of
computer-generated rough surface (s ¼1 nm,
b* ¼0.5 mm) in contact with a smooth, flat surface with
a composite elastic modulus of 100 GPa and a water film
(g ¼73 dyne cm
1
(7.3 10
4
Ncm
1
), y ¼601)
thickness of 1 nm and meniscus height of 1 nm (after
Poon and Bhushan 1996).
551
Magnetic Reco rding Devices: Head/Medium Interface
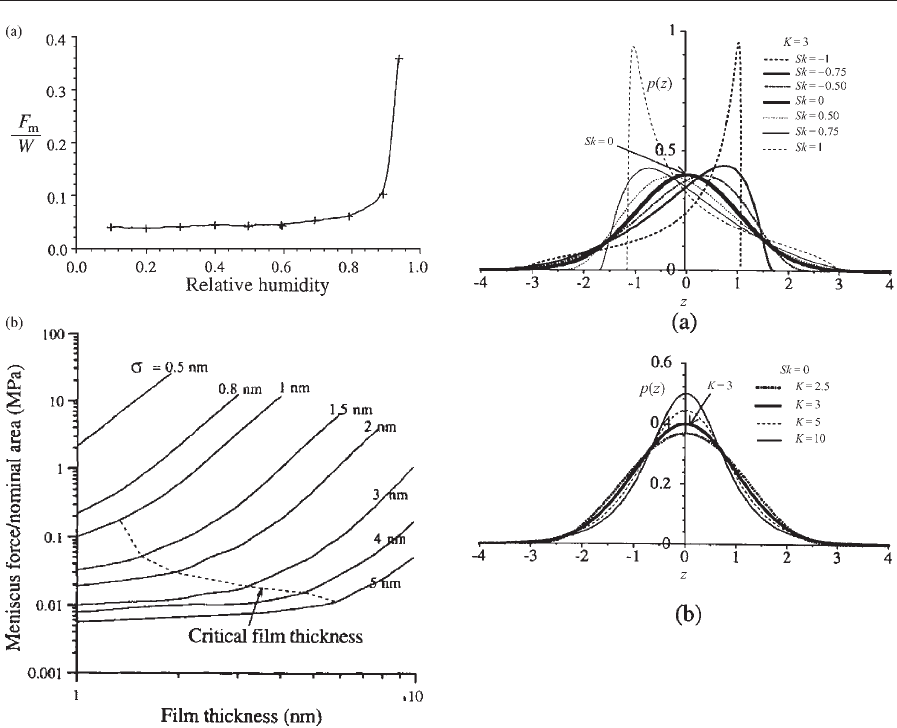
positive skewness of 0–0.2, and kurtosis of about five
or larger is optimum.
Laser-textured disks are becoming increasingly
popular in the design and manufacture of rigid disks.
In the laser-texturing technique, discrete topographic
features with either rounded, dome-like protrusions
(sombrero shaped) or craters (V or W types or donut
shaped) usually known as bumps are generated over
the disk surface. In laser-textured disk surfaces, these
microscale features are placed in the landing zone to
reduce friction/stiction during the start and stop ope-
rations. Typical sombrero-, V-, and W-type bumps
have about 10–15 mm rim diameter and height of
about 15–25 nm with a 50 mm 50 mm pitch. This
gives of the order of 4 10
5
bumps in the landing
zone of a 95 mm diameter disk. V- or W-type bumps
are more commonly used because they can be rep-
roducibly manufactured. The stiction phenomena at
the head/disk interface can be controlled by control-
ling the size and shape of the laser bumps. Studies to
identify an optimum bump shape and the relationship
between the optimum number of bumps as a function
of the bump geometry for a given bump shape have
been conducted (Chilamakuri and Bhushan 1997,
1998b, 1999a, 1999b, Chilamakuri et al. 2000).
The following design methodology has been used to
determine an optimum number of bumps. For a given
size and shape, the meniscus force increases with an
increase in the number of bumps, so it is important to
minimize the number of bumps to control the stiction
phenomena. However, too few bumps can result in
Figure 6
(a) Effect of relative humidity on the relative meniscus
force for a glass/ceramic disk substrate in contact with a
smooth surface (after Tian and Bhushan 1996b). (b)
Effect of water film thickness and surface roughness on
the relative meniscus force for computer-generated
Gaussian surfaces (correlation distance b* ¼0.5 mm) in
contact with a smooth surface. The dotted line defines
the critical film thickness for different s (after Poon and
Bhushan 1996).
Figure 7
Probability density functions for surfaces with (a)
different skewness and (b) different kurtosis values (after
Chilamakuri and Bhushan 1998a).
552
Magnetic Reco rding Devices: Head/Medium Interface
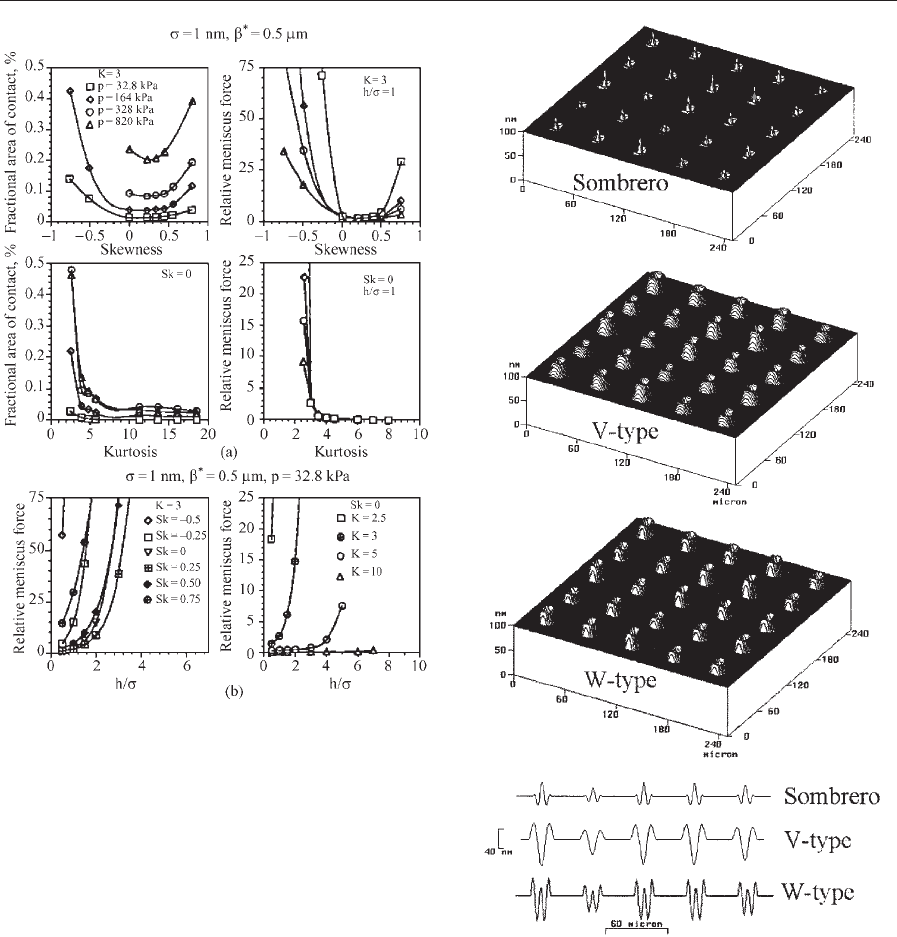
the failure of the disk surface owing to plastic defor-
mation and wear. To prevent the bumps from yield-
ing, the number of bumps on the disk surface should
be greater than a certain minimum, which would
also give a lower bound for the stiction. Numerical
analyses of computer-generated surfaces are used to
predict an optimum number of bumps. Figure 9
shows typical computer-generated surfaces with var-
ious bump types and variable heights. The three-
dimensional numerical contact model mentioned
earlier is used to perform contact analysis of a com-
puter-generated laser-textured surface against a flat
surface. Figure 10 shows an optimum number of
bumps, contact area, and meniscus force as a func-
tion of yield stress at different values of coefficient
of friction. As expected, the optimum number of
bumps increases with an increase in the coefficient of
Figure 8
(a) Fractional real area of contact and relative meniscus
force as a function of skewness and kurtosis at various
nominal pressures, and (b) relative meniscus force as a
function of h/s for different skewness and kurtosis
values, for an interface in the presence of
perfluoropolyether film (g ¼25 dyne cm
1
(2.5 10
4
Ncm
1
), y ¼101) (after Chilamakuri and
Bhushan 1998a).
Figure 9
Three-dimensional surface plots and two-dimensional
line plots of sombrero-, V-, and W-type laser surfaces
(R
rim
¼7.5 mm, H
mean
¼25 nm, s ¼5 nm) with a
Gaussian height distribution.
553
Magnetic Reco rding Devices: Head/Medium Interface
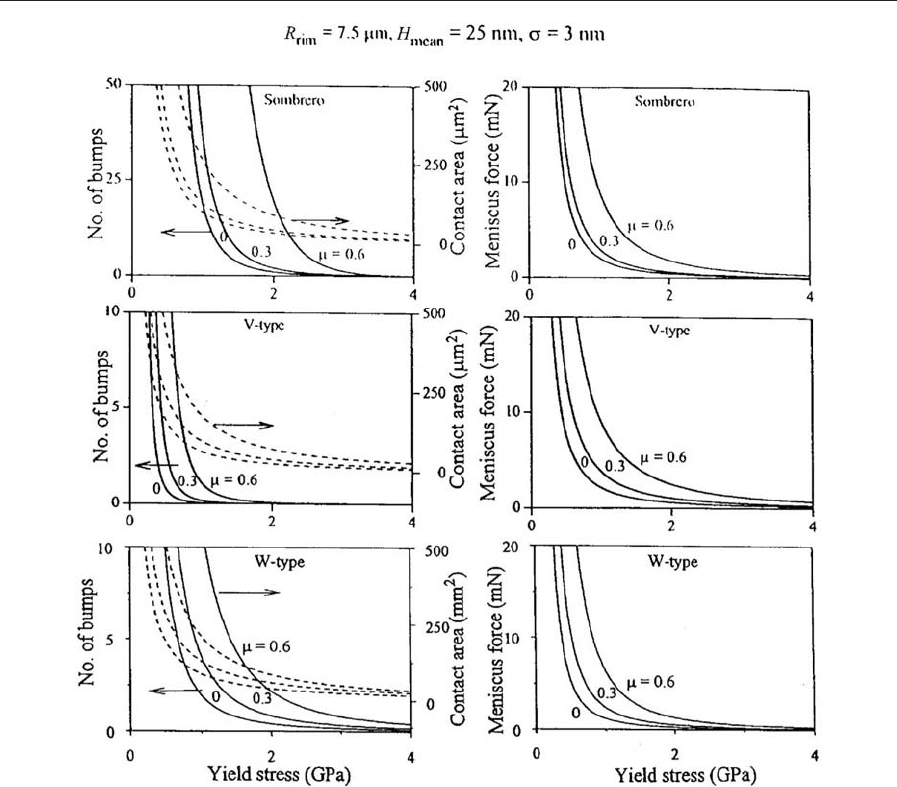
friction. Contact area and meniscus force also
follow similar trends. Measurements made on exper-
imental disks verify numerical predictions (Bhushan
2000).
2.2 Overcoats and Lubricants
The carbon overcoat is almost exclusively used as an
overcoat for thin-film disks and metal tapes (Bhushan
1999c). It has to be chemically active in order to attach
to lubricant molecules, whereas the lubricant mole-
cules should be hydrophobic so that they repel water
from the environment. Lubricants can attach to the
carbon surface by one or more of the following
mechanisms: physisorption, conformational rearrange-
ment, and chemisorption. The type and degree of
attachment depends on the molecular structure and
end groups of the lubricants and surface groups on
the carbon surface. A hydrogenated carbon surface is
highly functional and consists of many oxygen-
containing surface groups which include –C–O–C–
(ether), 4CQO (carbonyl), –COOR (ester), –COOH
(carboxyl), and –COH (hydroxyl) groups, as well as
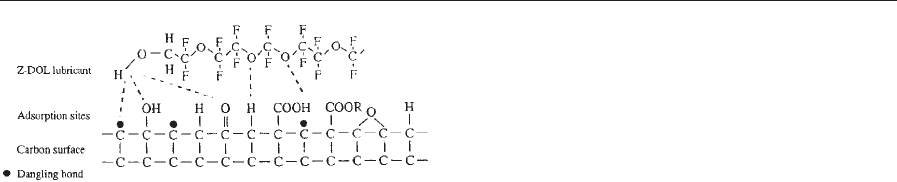
unpaired electrons (dangling bonds). Figure 11 shows
schematically the adsorption of a polar perfluoro-
polyether (PFPE) (Z-Dol) molecule on a carbon sur-
face at 20 1C. The CQO bond and the hydroxyl
groups in the case of Z-Dol interact with functional
groups and dangling bonds on the carbon surface. The
Figure 10
Effect of coefficient of friction, m, on number of bumps, contact area, and meniscus force for sombrero-, V-, and
W-type bumps at s ¼3 nm.
554
Magnetic Reco rding Devices: Head/Medium Interface
functional groups behave as an anchor, i.e., an im-
mobile fraction of the lubricant film. The addition of
an alcohol (–OH) end group to a lubricant molecule
adds an interaction energy of the order of
20 kJmol
1
, even if no chemical reaction occurs with
the surface. The attachment of lubricant molecules
with or without functional groups is enhanced after
sliding as long as the lubricant chains are exposed to
the disk surface (Zhao et al. 1999).
The PFPE backbone mainly interacts with surfaces
through van der Waals forces. The van der Waals
interaction energy of PFPE molecules with a DLC
surface is of the order of 2 kJmol
1
or less. This en-
ergy is sufficiently high so that molecules lie flat on
the surface when the lubricant film thickness is com-
parable to the polymer chain diameter of 0.7 nm.
There are two approaches for chemisorption that
have been shown to be successful in chemical bonding
of the liquid monolayer to the overcoat. The first re-
lies on exposure of the disk lubricated with neutral
PFPE to various forms of radiation, such as low-
energy x rays, electron or ion beam, nitrogen plasma,
or far UV. The improved affinity of the lubricant for
the surface by obtained by UV radiation is believed
to result from enhanced bonding by photochemical
alteration of the lubricant, the substrate, or the air-
deposited organics. The PFPEs undergo dissociative
electron attachment caused by photoelectrons that
are generated by the interaction of the UV light with
the substrate. This dissociative electron attachment
results in the formation of a negative ion and a rad-
ical. It is suggested that subsequent radical propaga-
tion and termination steps cross-link the PFPE and
bond it to the substrate. Another approach uses
chemically active PFPE molecules through either the
above-mentioned treatments or a post-thermal treat-
ment, in which the various functional end groups
offer the opportunity of strong attachments to a
specific interface if a high enough temperature is ap-
plied to overcome the activity barrier (Zhao and
Bhushan 1996, Zhao et al. 2000). The activation en-
ergy of the thermal attachment of Z-Dol to a carbon
surface is of the order of 30 kJmol
1
. Surface clean-
liness and chemical activity of the overcoat affect
the degree of bonding. The length of the thermal
treatment and the temperature for PFPE lubricants
with polar end groups are important factors in de-
termining the adsorption level of lubricants to the
disk surface (Zhao and Bhushan 1996).
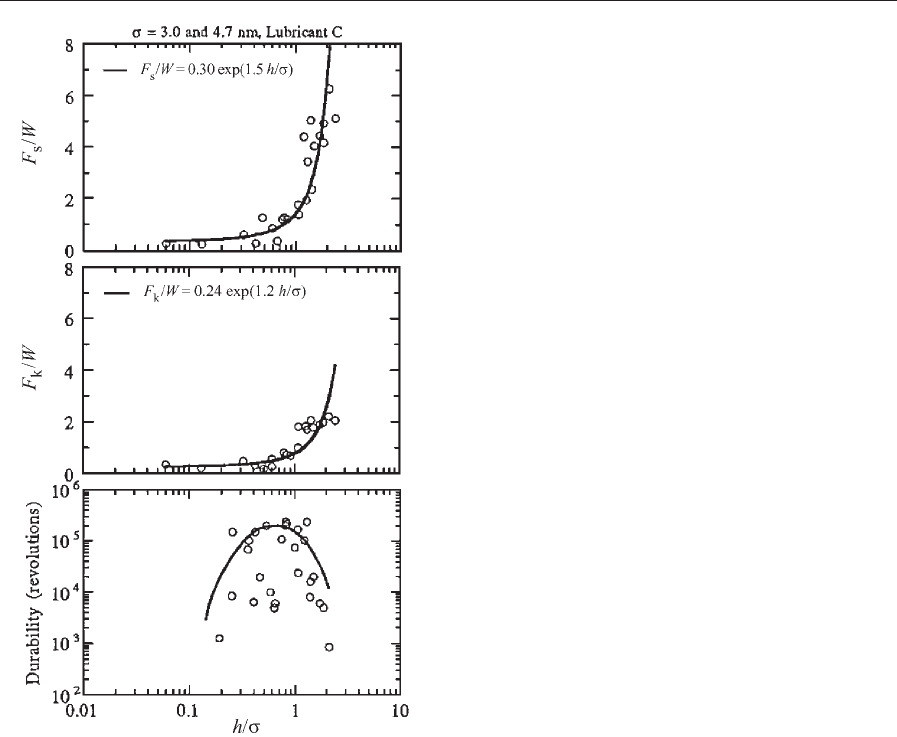
The lubricant film thickness (h) and composite
roughness (s) are generally known to have an oppo-
site effect on static and kinetic friction and durability
in terms of desirability, i.e., an increase in h or a
decrease in s results in an increase in the values of
F
s
/W, F
k
/W, and durability. F
s
/W, F
k
/W, and dura-
bility data obtained on various disks with a lubricant
C (Z-Dol) as a function of h/s is shown in Fig. 12
(Bhushan and Zhao 1999). In Fig. 12, h represents a
mobile fraction of the total lubricant film thickness in
static and kinetic friction because only the mobile
fraction forms menisci; h is a total film thickness in
durability because both mobile and immobile frac-
tions affect wear life. The Z-Dol lubricants used are
untreated, partially bonded, and fully bonded. The
normalized static friction force (F
s
/W) and normal-
ized kinetic friction force (F
k
/W) increase rapidly
above a critical value of h/s (B0.7) and durability
decreases above the critical film thickness. Trends in
experimental results for static friction force are com-
parable with the results of the numerical contact
model presented earlier (see Fig. 6(b)). A rapid de-
crease in durability above a critical film thickness
occurs because of a large meniscus being formed
around slider edges and the presence of the stick/slip
phenomena (Bhushan and Zhao 1999).
Zhao et al. (2000) and Kajdas and Bhushan (1999)
have presented detailed discussion of various degra-
dation mechanisms of Z-Dol lubricant which include
thermal decomposition, catalytic degradation, elec-
tron-mediated degradation, and mechanical degra-
dation processes. Comparing various degradation
mechanisms it is believed that the catalytic degra-
dation mechanism proposed in the literature is not
relevant because kinetics is slow at asperity temper-
atures. Based on a wide variety of experimental data
it is reasonable to emphasize that anionic inter-
mediates (negative ions and/or negative-ion/radical
species) produced by low-energy electrons play an
important part in both (i) the electron-mediated deg-
radation process of PFPE lubricants, and (ii) chem-
ical bonding of PFPE lubricant films with DLC
surfaces under sliding conditions.
3. Microtribology of Magnetic Storage Devices
At most interfaces of technological relevance, contact
occurs at multiple asperity contacts. Consequently,
the importance of investigating single-asperity con-
tacts in studies of the fundamental micromechanical
and tribological properties of surfaces and interfaces
has long been recognized. The emergence and prolif-
eration of proximal probes, in particular tip-based
microscopy (the atomic force microscope/friction
Figure 11
Schematic of Z-Dol molecules adsorbed on a carbon
surface at 20 1C.
555
Magnetic Reco rding Devices: Head/Medium Interface
microscope (AFM/FFM)), and of computer simula-
tions of tip/surface interactions and interfacial prop-
erties, provide nanoscale realizations of single-
asperity contacts, and allow systematic investigations
of interfacial problems of materials with high reso-
lution as well as ways and means for modifications
and manipulations of nanoscale structures. These
advances provide the impetus for intensive research
endeavors aimed at fundamental understanding of
the nature and consequences of the interactions
between materials on the atomic scale, and guide
the ‘‘rational’’ design of material for technological
applications. The advent of these techniques and
methodologies has led to the development of the field
of micro/nanotribology and micro/nanomechanics
(Bhushan 1999a, Bhushan et al. 1995). This field per-
tains to experimental and theoretical investigations of
processes ranging from the atomic and molecular
scales to the microscale, that arise as a consequence
of interfacial interactions.
3.1 Friction
Friction has been measured on a microscale (Bhush-
an 1999a), and it has been reported that the coeffi-
cient of friction on a microscale is lower than the
macrofriction. Table 1 gives typical values of coeffi-
cients of friction of metal-particle (MP) and metal-
evaporated (ME) tapes, poly(ethylene terephthalate)
(PET) tape substrate, and thin-film rigid disks. Fric-
tion values on the microscale are much lower than
those on the macroscale, which is believed to be be-
cause of a lower plowing contribution (almost no
damage on the disk surface as measured). When
measured for the small contact areas and very low
loads used in microscale studies, mechanical proper-
ties are higher than at the macroscale, this reduces the
degree of wear. In addition, the apparent area of
contact reduces the number of particles trapped at
the interface, and thus minimizes the plowing contri-
bution to the friction.
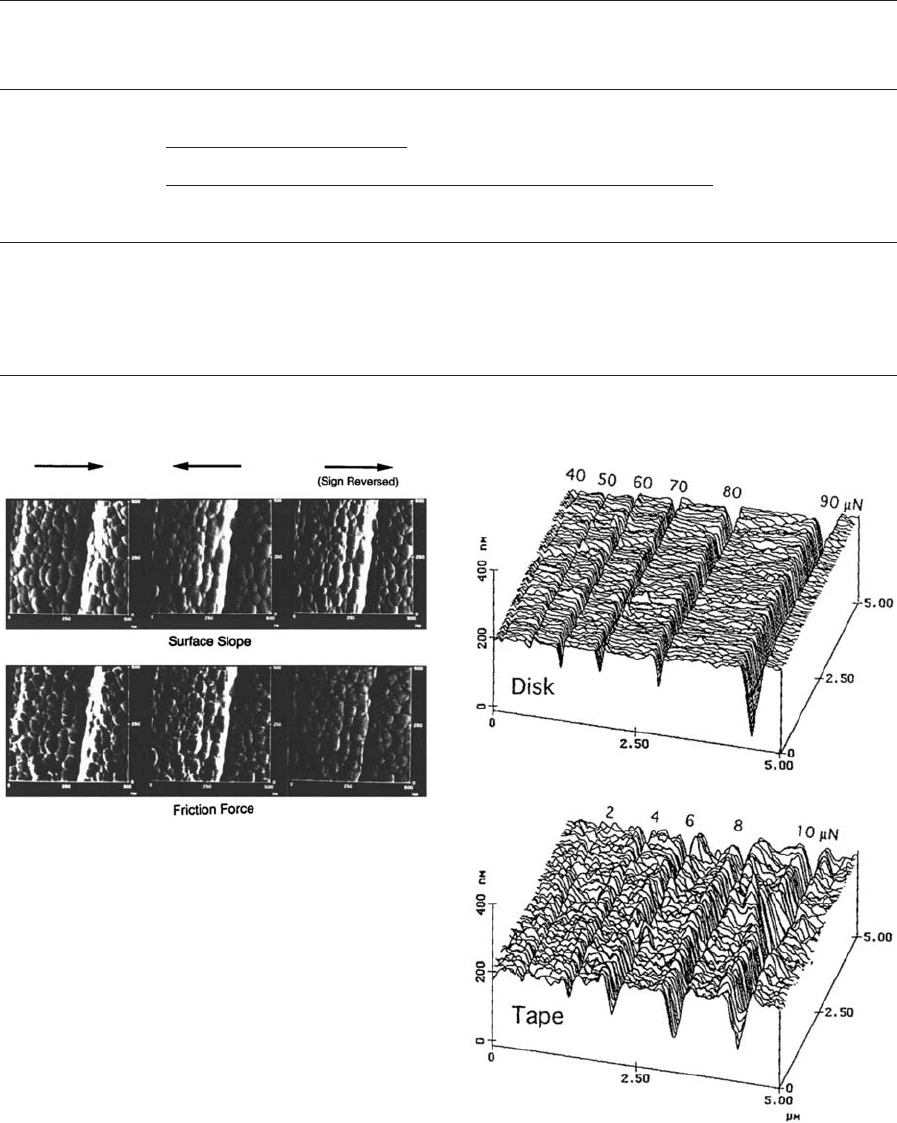
Gray-scale plots of coefficients of local friction of a
thin-film disk as the FFM tip is scanned in either
direction are shown in Fig. 13. Corresponding gray-
scale plots of slope of roughness profiles are also
shown in Fig. 13. Since the sign of the friction and the
slope reverses with the change in the scanning direc-
tion, the sign of one set of images needs to be reversed
before data can be compared. The left-hand sections
correspond to the sample sliding from the left to-
wards the right, the center sections correspond to the
sample sliding from the right toward the left, and the
right-hand sections correspond to the first set with
the sign of slope and friction values reversed. We note
a general correlation between the slope and friction
profiles. We note that generally the points that have
high friction in the left to right scan also have high
friction as the sliding direction is reversed (after sign
change). This relationship is not true at some loca-
tions. We observe some differences in the friction
profiles that may result from the asymmetrical as-
perities and/or asymmetrical transfer of wipe material
during manufacturing of the disk. The directionality
effect in friction on a macroscale has been observed in
some magnetic tapes (Bhushan 1999a).
3.2 Scratching and Wear
Microscratches made on MP tape and on an unlu-
bricated thin-film rigid disk at various loads are
Figure 12
Normalized static and kinetic friction forces and
durability in revolutions as a function of the ratio of the
lubricant film thickness to composite roughness (h/s).
Data on untreated, partially bonded, and fully bonded
Z-Dol lubricants are presented. For static and kinetic
friction data, h used is a mobile fraction; for durability, h
used is a total film thickness (after Bhushan and Zhao
1999).
556
Magnetic Reco rding Devices: Head/Medium Interface
shown in Fig. 14. All scratches are made with 10
cycles. We notice that the scratch depth increases
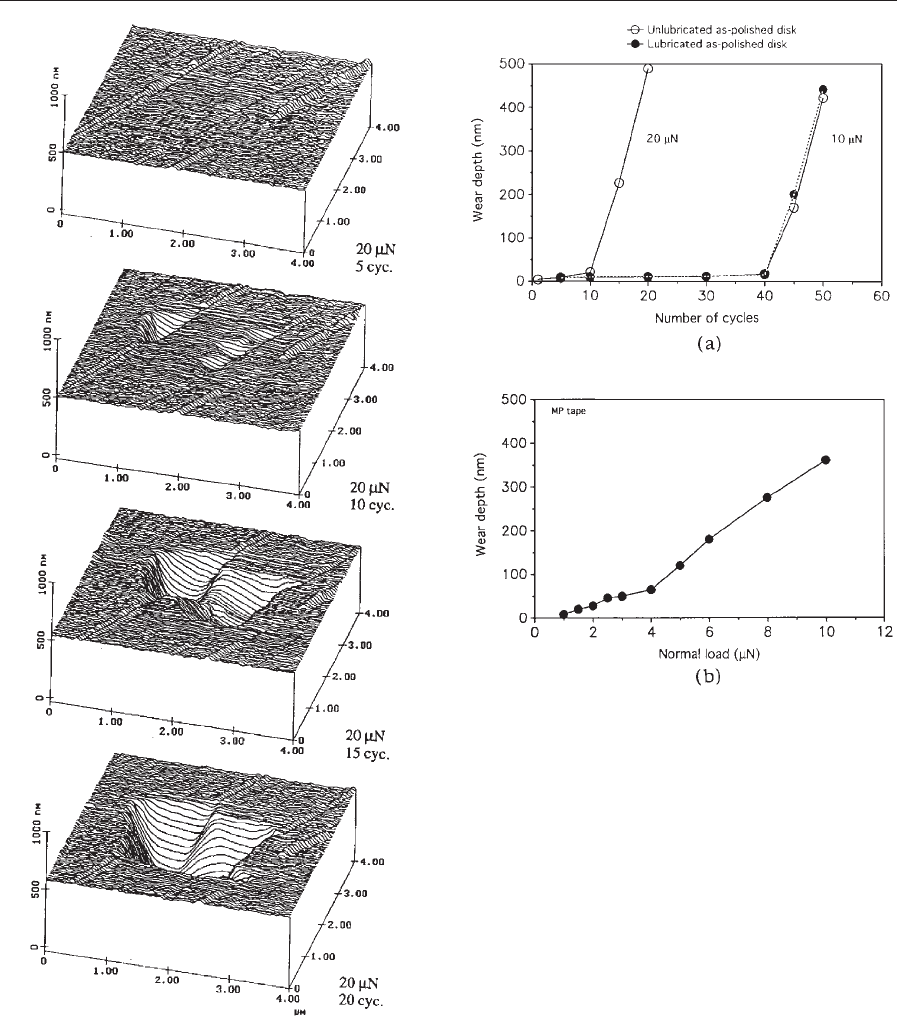
with an increase in the normal load. Figure 15 shows
the microwear profiles at 20 mN load and at various
cycles of an unlubricated thin-film disk. We note that
wear is not uniform and is largely initiated at the
texture marks present on the disk surface. This sug-
gests that surface defects act as initiated sites for
wear. Figure 16(a) shows the wear depth as a function
of the number of cycles for unlubricated and lubri-
cated thin-film disks at 10 mN and 20 mN loads. Wear
Figure 13
Gray-scale plots of the slope of the surface roughness
and the friction force profiles for a lubricated disk with
FFM tip sliding in different directions. Higher points are
shown by lighter color (after Bhushan 1999a).
Figure 14
Surface profiles for scratched, unlubricated thin-film
rigid disk and MP tape (after Bhushan 1999a).
Table 1
Surface roughness (r.m.s.), microscale and macroscale friction, and nanohardness data of thin-film magnetic rigid
disk, magnetic tape, and magnetic tape substrate (PET) samples.
Roughness (r.m.s.) (nm)
Coefficient of
microscale
friction
Coefficient of
macroscale
friction
NOP
a
AFM
b
Sample
250 mm
250 mm
c
1 mm
1 mm
c
10 mm
10 mm
c
1 mm
1 mm
c
10 mm
10 mm
c
Mn–Zn
ferrite
Al
2
O
3
–
TiC
Nanohardness/normal
load (GPa)/(mN)
Unlubricated disk 2.2 3.3 4.5 0.05 0.06 0.26 21/100
Lubricated disk 2.3 2.3 4.1 0.04 0.05 0.19
Metal-particle tape 6.0 5.1 12.5 0.08 0.06 0.19 0.30/50
Metal-evaporated
tape
9.3 4.7 5.1 0.05 0.03 0.18 0.7 to 4.3/75
PET tape substrate 33 5.8 7.0 0.05 0.04 0.55 0.3/20 and 1.4/20
d
a Noncontact optical profiler. b Atomic force microscope. c Scan area. d Polymer and particulate regions, respectively.
557
Magnetic Reco rding Devices: Head/Medium Interface
initially takes place slowly with a sudden increase
after about 40 cycles at 10 mN and after about
10 cycles at 20 mN. The rapid increase is associated
with the breakdown of the carbon coating. Similar
behavior has been reported for ME tapes. Wear rates
for particulate tapes (Fig. 16(b)) and PET tape sub-
strates are approximately constant for various loads
and number of cycles (Bhushan 1999a).
We note that scratches and wear profiles can be
produced with very shallow depths, thus the AFM
technique can be used to measure scratch resistance
and wear resistance of ultrathin films.
3.3 Lubrication
To study lubricant depletion during microscale meas-
urements, the microscale friction as a function of
Figure 15
Surface profiles of unlubricated thin-film rigid disk
showing the worn region (center 2 mm 2 mm). The
normal load and the number of test cycles are indicated
(after Bhushan 1999a).
Figure 16
Wear depth as a function of number of cycles for
(a) unlubricated and lubricated thin-film rigid disks at
10 mN and 20 mN loads, and (b) MP tape at a normal
load of 2 mN (after Bhushan 1999a).
558
Magnetic Reco rding Devices: Head/Medium Interface
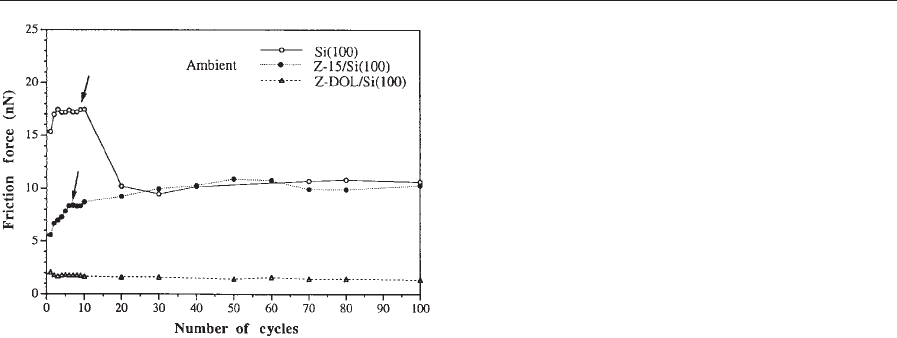
number of cycles of a virgin Si(100) surface and a
silicon surface lubricated with about 2 nm thick Z-15
and Z-Dol PFPE lubricants are shown in Fig. 17.
These experiments were conducted in an AFM/FFM
by sliding a Si
3
N
4
tip against the samples. Z-Dol is a
PFPE lubricant with hydroxyl end groups. Its lubri-
cant film was thermally bonded at 150 1C for 30
minutes and washed off with a solvent to provide a
chemically bonded layer of the lubricant film. We
note from Fig. 17 that the friction force in a virgin
silicon surface decreases after a few cycles after the
natural oxide and other contaminant film present
on the silicon surface gets removed. In the case of the
Z-15-coated silicon sample, the friction force is
initially low and then approaches that of an unlubri-
cated silicon surface after a few cycles. The increase
in friction of the lubricated sample suggests that the
lubricant film gets worn and the silicon underneath is
exposed.
In the case of the Z-Dol-coated silicon sample, the
friction force starts out low and remains low during
the 100 cycles of the test. It suggests that Z-Dol does
not get displaced/depleted as readily as Z-15. Fur-
thermore, the initial friction value for Z-Dol is low as
compared to that of Z-15. Z-15 gets displaced locally
by condensed water molecules permeated through the
lubricant film or condensed over exposed areas,
whereas Z-Dol does not get displaced as readily by
water because it is chemically bonded to the subst-
rate. The absence of mobile lubricant and lack of
water molecules minimize the possibility of formation
of meniscus bridges, which is responsible for low
friction in the case of Z-Dol. From this example, it is
clear that AFM methods can be used to study the
performance of lubricants in simulated single-asper-
ity contacts.
4. Closure
The magnetic recording process involves relative
motion between a magnetic medium and a station-
ary or rotating read/write magnetic head. Under
steady operating conditions, a load-carrying air film
is formed. There is a physical contact between the
medium and the head during starting and stopping.
In modern high-end computer tape and disk drives,
the surfaces have roughness of the order of 2–5 nm
r.m.s. and the head-to-medium separation is of the
order of 25–50 nm. The need for higher recording
densities requires the surfaces be as smooth as pos-
sible and the flying height be as small as possible. The
very smooth surfaces and ultralow flying heights re-
quired present challenges and opportunities unparal-
leled in any other industry.
Analytical models and experimental data available
of the tribology of the head/medium interface are
plentiful. Understanding of tribology on nanoscales
is beginning to emerge. Understanding of micro/
nanotribology of ultrasmooth surfaces and small
components in future head/medium interfaces is lack-
ing. AFM/FFM studies are expected to play a major
role in computer tribology.
See also: Magnetic Recording: Rigid Media, Tribo-
logy; Magnetic Recording Technologies: Overview
Bibliography
Bhushan B 1993 Magnetic slider/rigid disk substrate materials
and disk texturing techniques—status and future outlook.
Adv. Info. Storage Syst. 5, 175–209
Bhushan B 1996a Tribology and Mechanics of Magnetic Stor-
age Devices, 2nd edn. Springer, New York
Bhushan B 1996b Contact mechanics of rough surfaces in
tribology: single asperity contact. Appl. Mech. Rev. 49,275–98
Bhushan B 1996c Methodology for roughness measurement
and contact analysis for optimization of interface roughness.
IEEE Trans. Magn. 32, 1819–25
Bhushan B 1998 Contact mechanics of rough surfaces in
tribology: multiple asperity contact. Tribol. Lett. 4, 1–35
Bhushan B (ed.) 1999a Handbook of Micro/Nanotribology 2nd
edn. CRC Press, Boca Raton, FL
Bhushan B 1999b Principles and Applications of Tribology.
Wiley, New York
Bhushan B 1999c Chemical, mechanical and tribological char-
acterization of ultra-thin and hard amorphous carbon coat-
ings as thin as 3.5 nm: recent developments. Diamond Relat.
Mater. 8, 1985–2015
Bhushan B 2000 Mechanics and Reliability of Flexible Magnetic
Media, 2nd edn. Springer, New York
Bhushan B, Chilamakuri S K 1996 Non-Gaussian surface
roughness distribution of magnetic media for minimum fric-
tion/stiction. J. Appl. Phys. 79, 5794–6
Bhushan B, Israelachvili J N, Landman U 1995 Nanotri-
bology: friction, wear and lubrication at the atomic scale.
Nature 374, 607–16
Bhushan B, Zhao Z M 1999 Macroscale and microscale tribo-
logical studies of molecularly thick boundary layers of
Figure 17
Friction force as a function of number of cycles for
unlubricated and lubricated silicon samples (after
Bhushan 1999a).
559
Magnetic Reco rding Devices: Head/Medium Interface
