Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.


an intrinsic property of a single layer. As T-T
c
the
coherence length always diverges and the supercon-
ductor inherently averages over many layers in a
multilayer. This means that the anisotropic GL mod-
el described here is always applicable and x
z
(T) can
be defined.
The upper critical field of a Type II superconductor
is the field at which superconductivity is destroyed via
a second-order phase transition. Theoretically it can
be obtained by determining the temperature at which
the linearized Eqn. (1),
ac þ
1
2m
_
i
r
e
c
A
2c ¼ 0 ð3Þ
in an applied magnetic field H, along the z direction,
has a nontrivial solution. Using the Landau gauge,
A ¼H
0
y
ˆ
x, and, separating variables, one obtains the
following eigenvalue equation
_
2
2m
d
2
dx
2
þ
e
Hx
c
2
$%
c ¼ac ¼ eðHÞc ð4Þ
where H
c2
(T) is given by the implicit relation e(H) ¼
a(T) ¼_
2
/[2m
x(T)
2
], where x(T) ¼x(0)O(T
c
/T
c
T).
Equation (4) is equivalent to a Schro
¨
dinger equation
of a harmonic oscillator with frequency o
c
¼7e
7H
0
/
m
c and Landau levels. Hence, the lowest eigenvalue,
corresponding mathematically to the highest field at
which superconductivity can nucleate, is
e(H) ¼_e
H=2m
c, from which it follows that
H
c2
¼
F
0
2pxð0Þ
2
T
c
T
T
c
ð5Þ
where F
0
¼hc/e
is the superconducting flux quan-
tum. At H
c2
the density of vortices Bx
2
(T) is such
that the superconductor becomes filled with normal
cores of vortices.
From Eqn. (5) the temperature dependence of H
c2
is linear near T
c
for a three-dimensional supercon-
ductor. Note that it is the coherence lengths in the
two directions perpendicular to the applied field H
that enter into Eqn. (5).
2.2 Superconducting Thin Films
In a superconducting thin film of thickness d, T
c
will
usually decrease as d is reduced due to changes in
electronic interactions and reduced dimensionality.
Neglecting these effects, however, and by treating T
c
and d independently, one can solve Eqn. (3) for the
case of a parallel magnetic field. The appropriate
boundary condition for a free surface is
r
ie
_
c
c
#
n ¼ 0;
where n is the unit vector normal to the surface. One
can apply a solution (see Ketterson and Song 1999,
for example) of the form c ¼ uðzÞe
ik
x
xþik
y
y
and obtain
H
c28
¼
ffiffiffiffiffi
12
p
F
0
2pxðTÞd
ð6Þ
In comparison with Eqn. (5), one x(T) factor is
replaced with d/O12, and therefore the critical field
has a square root temperature dependence instead of
a linear one. These two behaviors are referred to as
2D (square root) and 3D (linear). In the perpendic-
ular direction thin films have the critical field, H
c2>
,
the same as the bulk value of Eqn. (5).
2.3 Superconducting Multilayers and Dimensional
Crossover
In a multilayer superconductivity can have either 2D
or 3D character. These behaviors and their cross-over
can be illustrated with a simple GL model. Lawrence
and Doniach (1971) first described the modified GL
free-energy functional of a series of superconducting
layers which are Josephson coupled—a 1D Josephson
lattice.
F ¼
X
n
Z
d
2
r a7c
n
7
2
þ
b
2
7c
n
7
4
þ
1
2m
_
i
r
e
c
A
c
2
$
þ Z7c
nþ1
c
n
7
2
From the free energy functional the GL equation can
be derived by variation as
ac
n
þ b7c
n
7
2
c
n
þ
1
2m
_
i
r
e
c
A
2
c
n
Zðc
nþ1
2c
n
þ c
n1
Þ¼0 ð7Þ
The first part of this equation is the 2D result,
where individual layers are indexed with n. The sec-
ond part represents the Josephson coupling energy,
scaled by the coupling parameter Z. In the limit of
strongly coupled layers, i.e., when c
n
varies slowly
across the layers, the solution is the same as the an-
isotropic GL, with the mass
m
z
¼ _
2
=2s
2
Z
Within the LD model one can solve for arbitrary
coupling of the layers, i.e., not assuming strong cou-
pling. The linearized GL equation in a parallel mag-
netic field becomes
_
2
2m
d
2
dx
2
þ
_
2
m
z
s
2
1 cos
2eHxs
_c
c ¼ac
From this equation the critical field follows as
H
c28
¼
F
0
2ps
2
ð1 s
2
=2x
z
ðTÞ
2
Þ
1=2
m
x
m
z
230
Films and Multilayers: Conventional Superconducting
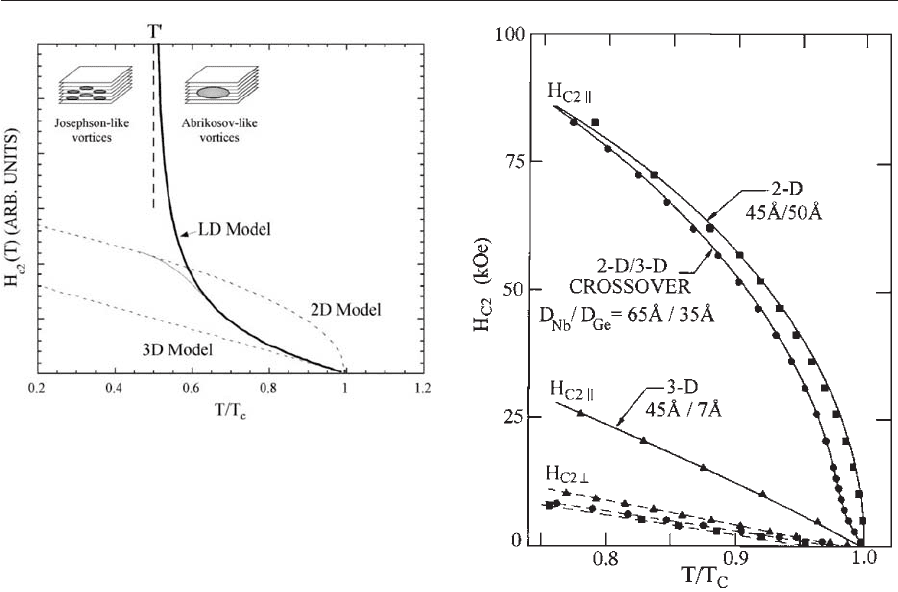
This equation leads to a divergence of the critical field
at T
0
defined by x
z
(T
0
) ¼s/O2 (see Fig. 3).
Above T
0
the multilayer behaves as a 3D aniso-
tropic superconductor. As the temperature is lowered
the coherence length decreases its zero temperature
value. When the coherence length becomes compa-
rable to the spacing of the superconducting layers,
the normal cores of the vortices can fit between the
superconducting layers where they have a reduced
pair-breaking effect. Hence the critical field will in-
crease at this temperature and in the LD model the
critical field becomes infinite. In reality the critical
field will not diverge because it is limited by the single
film value, such as that described above. However,
the essential physics is captured in this simple model
for dimensional crossover. Below T
0
the vortices
change from Abrikosov-like to Josephson-like when
the X layers are nonsuperconducting.
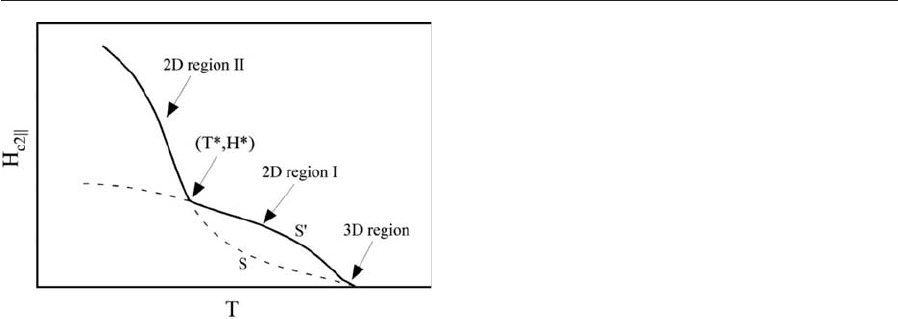
Dimensional crossover was experimentally con-
firmed by a number of experimental reports. The first
most compelling result was by Ruggiero et al. (1980).
Other reports can be found in the reviews mentioned
above. Ruggiero et al. studied Nb/Ge (S/I) multilay-
ers as a function of different layer thicknesses in or-
der to observe dimensional crossover. Figure 4 shows
the upper critical field of several Nb/Ge multilayer
samples with 3D, 2D, and crossover behavior. Later
studies have observed the crossover more elaborately
in S/N systems (see, for example, Banerjee et al.
1983).
2.4 Takahashi–Tachiki Effect in S/S
0
Multilayers
Takahashi and Tachiki (1986a, 1986b) examined the
behavior of the upper critical field in S/S
0
multilayers
within a microscopic theory. For the case of widely
differing diffusion constants, i.e., differing x, but with
similar bulk T
c
of the two superconducting layers,
they observed two possible solutions for nucleation of
superconductivity. One solution is for nucleation of
the superconducting order parameter in the dirty
superconductor, here labeled S, and the other for
nucleation of superconductivity in the clean super-
conductor, or S
0
, layers. The actual critical field will
be the larger of the two solutions.
The Takahashi–Tachiki effect is a discontinuity in
the parallel upper critical field exhibiting an upward
curvature at low temperatures. This happens when
Figure 3
Temperature dependence of the upper critical field for
the Josephson coupled Lawrence–Doniach (LD) model.
Comparison is shown with the 3D GL and 2D GL
models. In reality the critical field will not diverge at T
0
as in the LD model, but will cross over to a 2D behavior.
Below T
0
vortices fit between the superconducting layers
and become Josephson type. Above T
0
vortices are
averaging over several layers and are Abrikosov-type.
Figure 4
Upper critical fields near T
c
for Nb/Ge multilayers. As
the Ge layer thickness increases the system evolves from
3D behavior to ‘‘crossover’’ behavior and finally to
decoupled 2D. Solid lines are fits to the theory (after
Ruggiero et al. 1980).
231
Films and Multilayers: Conventional Superconducting
nucleation of superconductivity switches from S
0
to S
layers. Figure 5 illustrates the behavior of the critical
field for a particular set of material parameters where
this effect may be observed. The effect is only ob-
served for a ratio of diffusion constants above a cer-
tain value, or equivalently above a minimum x
S
0
/x
S
.
For temperatures very close to T
c
the parallel crit-
ical field has the usual linear 3D behavior. At some
temperature below T
c
there is a crossover to 2D be-
havior with nucleation in the S
0
layers. As the tem-
perature decreases further and the coherence length
becomes shorter than d
S
, the order parameter is con-
strained inside the S layers and superconductivity
behaves as it would in the bulk S material which has a
high critical field. At T* superconductivity crosses
over from being nucleated in the S
0
to the S layers and
there is a discontinuity in the critical field.
The theoretical prediction of the effect was subse-
quently experimentally confirmed in the Nb/NbTi
multilayer system by Karkut et al. (1988). For the
proper choice of materials, as in Nb
0.6
Ti
0.4
and Nb,
the two layers have similar T
c
but their diffusivities
differ by a factor of 20. Karkut et al. observed an
upturn in the parallel critical field at a particular
temperature T* as predicted by the Takahashi–
Tachiki theory.
See also: Superconducting Materials, Types of;
Superconducting Thin Films: Materials Preparation
and Properties; Superconducting Thin Films: Multi-
layers
Bibliography
Banerjee I, Yang Q S, Falco C M, Schuller I K 1983 Aniso-
tropic critical fields in superconducting superlattices. Phys.
Rev. B 28, 5037–40
Buzdin A I, Bulaevskii L N, Panyukov S V 1982 Critical-cur-
rent oscillations as a function of the exchange field and
thickness of the ferromagnetic metal (F) in an S–F–S Jose-
phson junction. JETP Lett. 35, 178–80
Fulde P, Ferrell R A 1964 Superconductivity in a strong spin-
exchange field. Phys. Rev. 135A, 550–63
Huebener R P 1979 Magnetic Flux Structures in Superconduc-
tors. Springer-Verlag, Berlin
Jiang J S, Davidovic D, Reich D H, Ghien C L 1995 Oscillating
superconducting transition temperature in Nb/Gd multilay-
ers. Phys. Rev. Lett. 74, 314–7
Karkut M G, Matijasevic V, Antognazza L, Triscone J -M,
Missert N, Beasley M R F, 1988 Anomalous upper critical
fields of superconducting multilayers: verification of the
Takahashi–Tachiki effect. Phys. Rev. Lett. 60, 1751–4
Ketterson J B, Song S N 1999 Superconductivity. Cambridge
University Press, Cambridge, UK
Larkin A I, Ovchinnikov Yu N 1964 Inhomogeneous state of
superconductors. Zh. Eksp. Teor. Fiz. 47, 1136 [Sov. Phys.
JETP 20, 762 (1965)]
Lawrence W E, Doniach S 1971 Theory of layer-structure su-
perconductors. In: Kanda E (ed.) Proc. 16th Int. Conf. on
Low Temperature Physics, pp. 361–2
Matijasevic V, Beasley M R 1987 Superconductivity in super-
lattices. In: Shinjo T, Takada T (eds.) Metallic Superlattices:
Artificially Structured Materials. Elsevier Science, The Neth-
erlands
Mu
¨
hge Th, Garif’yanov N N, Goryunov Yu V, Khaliullin G G,
Tagirov L R, Westerholt K, Garifullin I A, Zabel H 1996
Possible origin for oscillatory superconducting transition
temperature in superconductor/ferromagnet multilayers.
Phys. Rev. Lett. 77, 1857–60
Radovic Z, Dobrosavljevic-Grujic L, Buzdin A I, Clem J R
1988 Upper critical fields of superconductor–ferromagnet
multilayers. Phys. Rev. B 38, 2388–93
Ruggiero S T, Barbee T W, Beasley M R 1980 Superconduc-
tivity in quasi-two-dimensional layered composites. Phys.
Rev. Lett. 45, 1299–302
Ruggiero S T, Beasley M R 1984 Synthetically layered super-
conductors. In: Chang L L, Giessen B C (eds.) Synthetic
Modulated Structures. Academic Press, New York
Ryazanov V V, Oboznov V A, Rusanov A Yu, Veretennikov A
V, Golubov A A, Aarts J 2001 Coupling of two supercon-
ductors through a ferromagnet: evidence for a p-junction.
Phys. Rev. Lett. 86, 2427–30
Schuller I K, Guimpel J, Bruynseraede Y 1990 Artificially lay-
ered superconductors. MRS Bull. 15, 29–36
Takahashi S, Tachiki M 1986a Theory of the upper critical field
of superconducting superlattices. Phys. Rev. B 33, 4620–31
Takahashi S, Tachiki M 1986b New phase diagram in super-
conducting superlattices. Phys. Rev. B 34, 3162–4
V. Matijasevic
Oxxel GmbH, Germany
Fullerene Formation
Diamond and graphite constitute two natural crys-
talline forms of pure carbon. Their properties and
characteristics are completely different, a fact that
can be explained in terms of the way carbon atoms
Figure 5
Schematic of the Takahashi–Tachiki effect (after
Takahashi and Tachiki (1986b).
232
Fullerene Formation
bond to each other within the structure. For dia-
mond, sp
3
hybridization prevails, four bonds from
each carbon atom being directed towards the corners
of a regular tetrahedron. The resulting three-dimen-
sional network is extremely rigid, which is one reason
for the hardness of diamond. In graphite, sp
2
hy-
bridization results in three bonds per carbon atom,
distributed evenly (1201) in the xy plane, with a weak
p bond along the z axis. The C–C bond length is
1.42 A
˚
, and the sp
2
set creates the honeycomb (hex-
agonal) lattice typical of a single sheet of graphite.
The p (van der Waals) bond is responsible for weak
interactions between the layers, which are 3.35 A
˚
apart.
For generations, diamond and graphite were the
only known allotropes of carbon. However, in 1985 a
new form of carbon, buckminsterfullerene (C
60
)
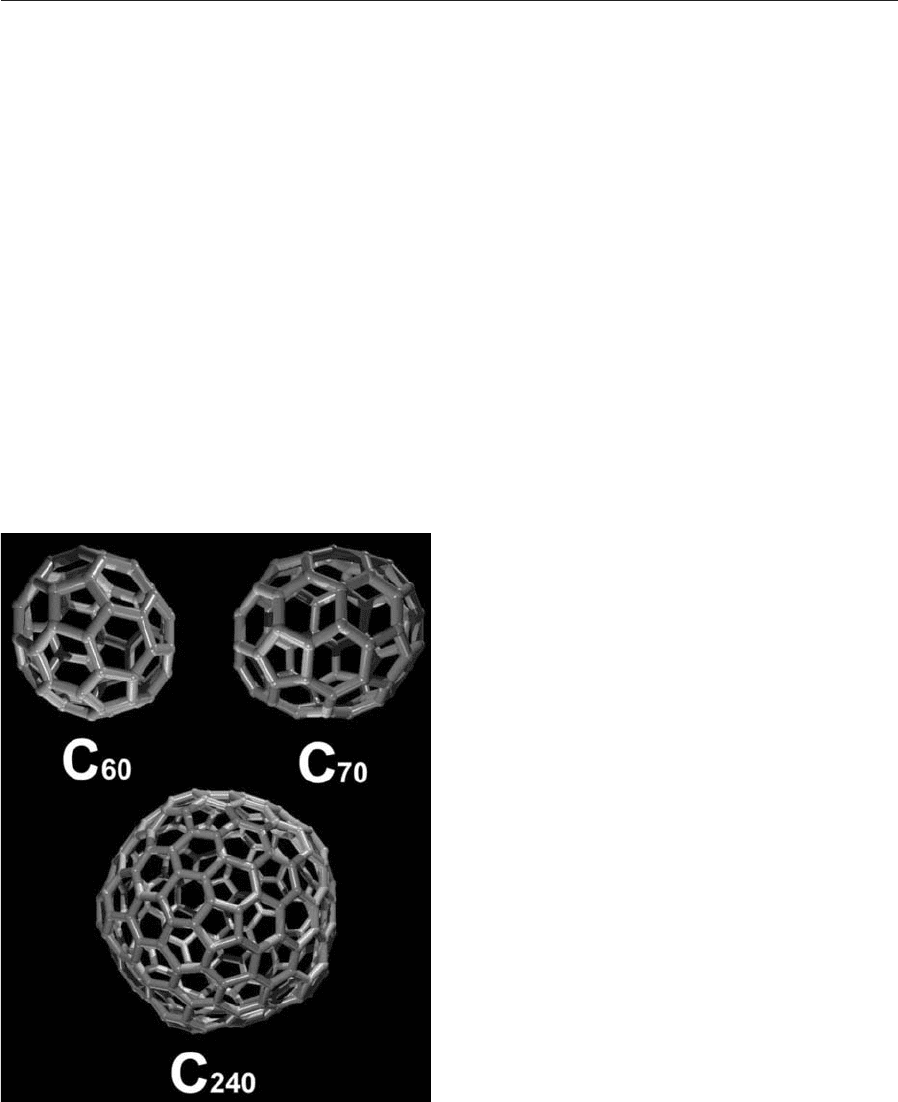
(Kroto et al. 1985), appeared on the scene.
C
60
is a soccer-ball-like cage composed of 60 car-
bon atoms with a diameter of 7.1 A
˚
. The shape and
symmetry of C
60
is that of a truncated icosahedron,
consisting of 20 hexagons and 12 pentagons (Fig. 1).
Curvature is due to the presence of the pentagons.
The relationship between the vertices, faces, and edg-
es of C
60
finds expression in Euler’s law (see Sect. 3).
C
60
is but one example of closed structures made up
of any number of hexagons (except one) and 12 pen-
tagons now styled fullerenes.
1. Discovery of C
60
One part of this story starts in the 1970s, when
the chemists Harry Kroto and David Walton at
Sussex University were studying cyanopolyynes
H(CC)
n
CN (chain-like molecules, in which car-
bon atoms are linked by alternating single and triple
bonds, being end-capped with hydrogen and nitro-
gen). These compounds display characteristic micro-
wave spectra commensurate with their linear dipole
structure (Kroto 1992).
The two Sussex scientists together with Anthony
Alexander (a BSc-by-thesis student) succeeded in
preparing HC
5
N and, with Colin Kirby (a research
student), HC
7
N. Subsequently, with Canadian as-
tronomers Takeshi Oka and co-workers, radio waves
emitted from HC
n
N(n ¼5, 7, 9) in the center of our
galaxy were detected (Kroto 1992).
In 1984 Kroto visited Robert Curl at Rice Univer-
sity (Houston, Texas) and was introduced to Richard
Smalley, who was generating clusters of atoms by la-
ser-vaporizing solids. Kroto was interested in subject-
ing graphite to this treatment in order to simulate the
conditions thought to prevail in red giant stars.
In late August 1985 Kroto traveled to Houston in
order to participate in the graphite experiment, which
involved evaporating carbon from the surface of a
rotating graphite disk in the presence of a high density
helium flow. The resulting carbon clusters were al-
lowed to expand though a nozzle and were detected by
a time-of-flight mass spectrometer (Kroto et al.1985).
The mass-spectral profile for carbon, obtained in
these experiments, had in fact already been observed
by Rohlfing et al. (1984) and consisted of two main
groups of clusters: (a) those with low mass (o30
carbon atoms) consisting of odd numbers of carbon
atoms and (b) high-mass clusters (436 atoms) con-
taining only even numbers of carbon atoms (Fig. 2).
Rohlfing et al. (1984) proposed that clusters with less
than nine carbon atoms were linear chains, and that
the odd masses between 11 and 23 were due to rings.
No comment was made regarding the structure of
higher clusters other than that they were exceptional
and possibly carbyne-like (consisting of linear carbon
chains with alternating single and triple bonds, i.e.,
polyynes).
In the experiments carried out at Rice University,
the dominant role played by the cluster of 60 carbon
atoms was noted and its stability was ascribed to the
unique nature of the truncated icosahedral cage (see
above) without ‘‘dangling’’ bonds (Fig. 1). C
60
was
named buckminsterfullerene by the Sussex/Rice
Figure 1
(a) Buckminsterfullerene (C
60
), the third carbon
allotrope, discovered in 1985. (b) C
70
with D
5h
symmetry. (c) C
240
, an icosahedral giant fullerene.
233
Fullerene Formation
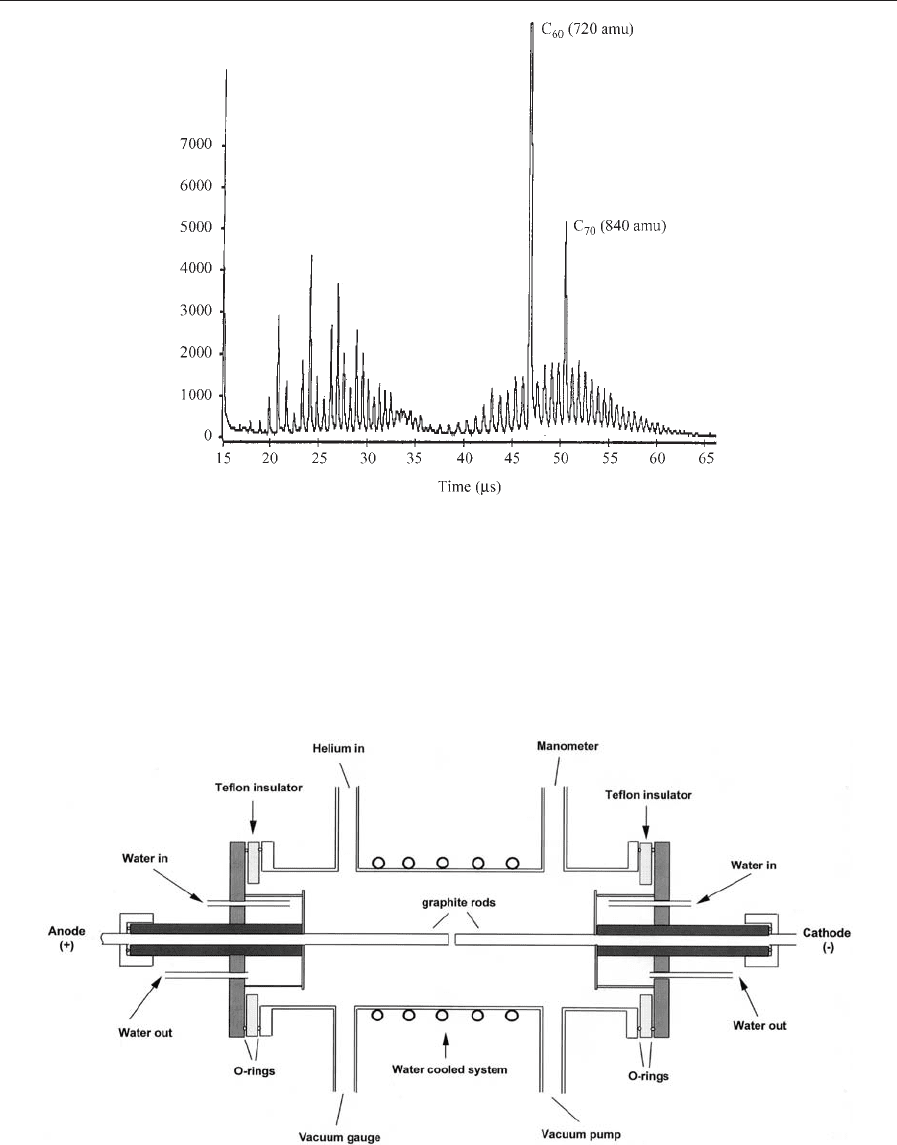
Figure 2
Mass-spectral profile obtained by the Sussex/Rice team, showing C
60
and C
70
as the most intense peaks. Additionally,
they observed two groups of clusters: those with lower mass (o30 carbon atoms) consisting of odd numbers of
carbon atoms and high-mass clusters (436 atoms) exhibiting only even numbers of carbon atoms (courtesy of
H. W. Kroto).
Figure 3
Schematic diagram of a d.c. arc discharge chamber used for fullerene production.
234
Fullerene Formation
team, in recognition of the US architect R. Buckm-
inster Fuller, who designed geodesic domes with sim-
ilar topologies.
In order to verify the structure of C
60
, bulk quan-
tities were needed. However, at the time only pico-
gram amounts were available.
2. Bulk Production and Characterization of C
60
In 1990 Kra
¨
tschmer and Huffman succeeded in pre-
paring milligram quantities of C
60
, accompanied by
smaller amounts of higher fullerenes (e.g., C
70
,C
76
,
etc.) by extracting soluble material from soot deposits
formed in an arc discharge between graphite elec-
trodes in a helium atmosphere (Fig. 3). Subsequently,
C
60
and C
70
were separated and characterized by the
Sussex team using conventional chromatography and
NMR techniques. Fullerenes have also been obtained
by combustion and by pyrolysis of hydrocarbons
such as naphthalene.
In the solid state, C
60
crystallizes as a cubic struc-
ture with a lattice constant of 14.17 A
˚
, a nearest-
neighbor C
60
–C
60
distance of 10.02 A
˚
, and a density
of 1.72 g cm
3
(Fig. 4(a)). At room temperature, C
60
clusters rotate rapidly in an f.c.c. structure about
their lattice positions (Tables 1 and 2). However, a
transition to a simple cubic phase (a ¼14.17 A
˚
con-
sisting of four C
60
clusters per unit cell) occurs below
261 K (Tanigaki and Prassides 1995).
The first fullerene isolated other than C
60
, was C
70
(isolated by Taylor et al.), which consists of two C
60
hemispheres connected by a ring of 10 carbons (re-
sembling a rugby ball; Fig. 1). Crystalline C
70
has
different crystallographic arrangements depending on
the temperature: the most stable phases are the
f.c.c. structure with a lattice constant of 15.01 A
˚
and
the hexagonal phase with a ¼b ¼10.56 A
˚
and
c ¼17.18 A
˚
. Higher fullerenes such as C
76
,C
78
,C
82
,
C
84
, were subsequently isolated and characterized.
3. Geometry of Fullerenes
Fullerenes are closed polyhedrons made of carbon,
and understanding their geometry may eventually
shed light on the way they are constructed. As men-
tioned above, Euler’s law expresses the proportions
of polygon types (pentagons and hexagons) needed to
form C
60
. For any polyhedron FE þV ¼w, where F
is the number of faces, E is the number of edges, V is
the number of vertices, and w is the Euler character-
istic, which is a geometric invariant that describes
structures with the same shape or topology. For all
closed polyhedrons w ¼2. For example, in C
60
, F ¼32
(12 pentagons and 20 hexagons), E ¼90, V ¼60, and
w ¼2; for C
70
, F ¼37 (12 isolated pentagons, 25 hex-
agons, and D
5h
symmetry), E ¼105, V ¼70, and
w ¼2. All closed polyhedrons have the same topology
as the sphere, which means that any polyhedron can
be transformed into a sphere by bending and stretch-
ing without cutting or tearing. In addition, no full-
erene can be formed from an odd number of atoms.
For a ‘‘graphitic’’ network, considering only pen-
tagonal, hexagonal, heptagonal, and octagonal rings
Figure 4
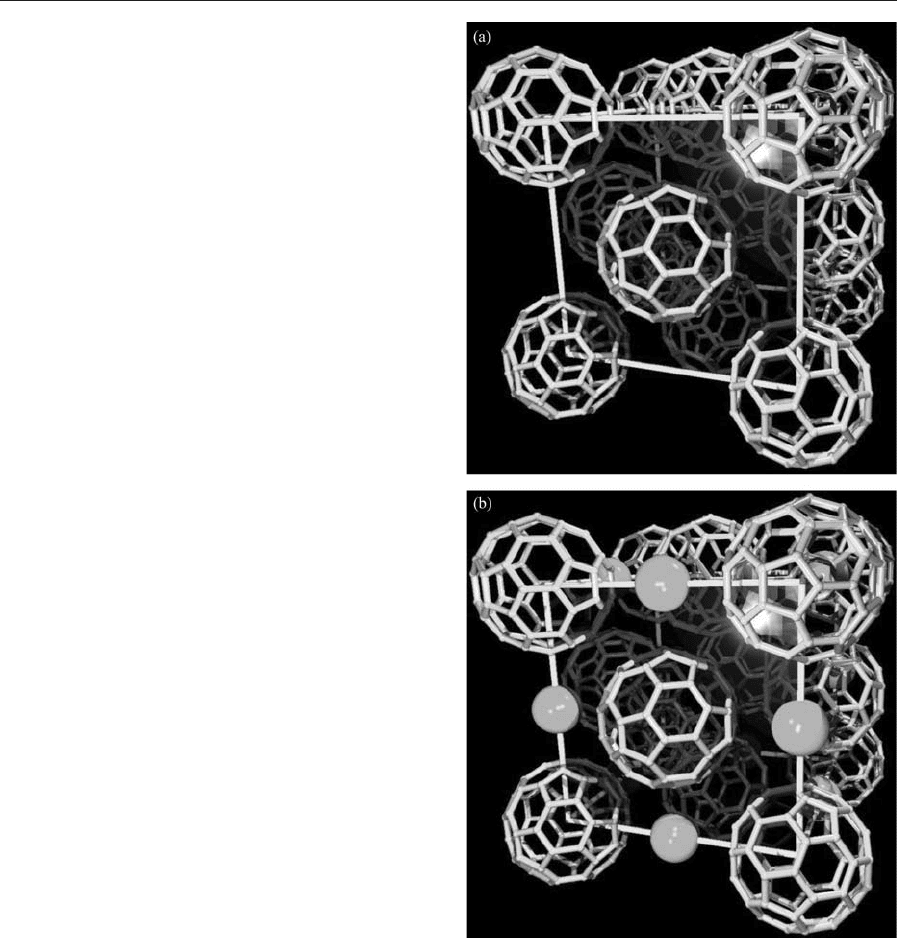
(a) F.c.c. crystal structure of C
60
. (b) Structure of the
M
1
C
60
intercalated compounds showing the metal
intercalated between the C
60
spheres. Note that the size
of the intercalated atoms has been chosen arbitrarily.
235
Fullerene Formation

of carbon, Euler’s law can be written:
N
5
N
7
2N
8
¼ 6w ¼ 12 ð1Þ
where N
n
is the number of polygons with n (5, 7, and
8) sides. As Eqn. (1) shows, N
5
, N
7
, and N
8
are de-
fects in a ‘‘graphitic’’ structure which contribute to
the curvature and topology of the arrangement (Terr-
ones and Mackay 1992). N
6
is absent since hexagons
do not contribute to curvature of the structure. In
addition, Eqn. (1) demonstrates that it is possible to
introduce heptagons and octagons in a closed graphi-
tic cage if their proportion is maintained. Therefore,
if pentagons are present in C
60
, one cannot rule out
the presence of higher-membered rings (e.g., hepta-
gons) in larger fullerenes.
It turns out that there are 1812 possible ways of
arranging 12 pentagons and 20 hexagons in C
60
,so
what is special in buckminsterfullerene? It is the
smallest cage in which each pentagon is surrounded
by five hexagons, thus leading to icosahedral (I
h
)
symmetry (the highest symmetry than can be ob-
tained by a polyhedron). This suggests that curved
graphite follows an isolated pentagon rule to form
fullerenes, and explains why smaller fullerenes (less
than 60 atoms) are difficult to produce.
4. Fullerene Gro wth
Although the precise growth mechanism leading to
C
60
and other fullerenes is not well understood, it is
worth reviewing ways in which they might be formed.
First, the mass spectrometry data obtained by laser
vaporization and arc discharge experiments indicate
that there are three main groups of structures, de-
pending upon the size: chains, rings, and fullerenes. In
other words, for a small number of atoms (o10) car-
bon chains are the most stable (one dimension); sub-
sequently rings start to appear (410 atoms), thus
avoiding dangling bonds at the ends (two dimensions);
and finally fullerenes emerge in order to avoid dan-
gling bonds in larger, three-dimensional structures.
According to Euler’s law, the smallest fullerene that
can possibly exist is the dodecahedron with 20 atoms
or 12 pentagons (C
20
; Fig. 5). Although this structure
is quite regular (one of the five Platonic solids), with
icosahedral symmetry, building it from sp
2
carbons
introduces considerable strain. This is because the
internal angle in a regular pentagon is 1081, which
differs substantially from the internal (hexagon) 1201
angles in graphite. Therefore, in order to relieve
this strain, hexagons are required to stabilize larger
fullerenes. At the same time, with 20 atoms, it is
also possible to obtain a ring (cyclic polyyne) or a
Table 1
Physical constants for C
60
.
Quantity Value
Average C–C distance 1.44 A
˚
C–C bond length on a pentagon 1.46 A
˚
C–C bond length on a hexagon 1.40 A
˚
C
60
mean ball diameter 7.10 A
˚
C
60
ball outer diameter 10.34 A
˚
Moment of inertia 1.0 10
43
Kg m
2
Volume per C
60
1.87 10
22
cm
3
Number of distinct carbon sites 1
Number of distinct C–C bonds 2
Binding energy per atom 7.40 eV
Heat of formation (per g 10.16 Kcal
carbon atom)
Electron affinity 2.6570.05 eV
Cohesive energy per atom 1.4 eV
Spin–orbit splitting of carbon (2p) 0.000 22 eV
First ionization potential 7.58 eV
Second ionization potential 11.5 eV
Optical absorption edge 1.65 eV
Source: Dresselhaus et al. (1995).
Table 2
Physical constants for crystalline C
60
in the solid state.
Quantity Value
F.c.c. lattice constant 14.17 A
˚
C
60
–C
60
distance 10.02 A
˚
C
60
–C
60
cohesive energy 1.6 eV
Tetrahedral interstitial site
radius
1.12 A
˚
Octahedral interstitial site
radius
2.07 A
˚
Mass density 1.72 g cm
3
Molecular density 1.44 10
21
cm
3
Compressibility (dlnV/dP) 6.9 10
12
cm
2
dvn
1
Bulk modulus 6.8–8.8 GPa
Young’s modulus 15.9 GPa
Transition temperature (T
01
) 261 K
dT
01
/dp 11 K bar
1
Volume coefficient of thermal
expansion
6.1 10
5
K
1
Optical absorption edge 1.7 eV
Work function 4.770.1 eV
Velocity of sound, v
t
2.1 10
5
cm s
1
Velocity of sound, v
l
3.6–4.3 10
5
cm s
1
Debye temperature 185 K
Thermal conductivity (300 K) 0.4 W m
1
K
1
Electrical conductivity 1.7 10
7
Scm
1
Phonon mean free path 50 A
˚
Static dielectric constant 4.0–4.5
Melting temperature 1180 1C
Sublimation temperature 434 1C
Heat of sublimation 40.1 kcal mol
1
Latent heat per C
60
unit 1.65 eV
Source: Dresselhaus et al. (1995).
236
Fullerene Formation
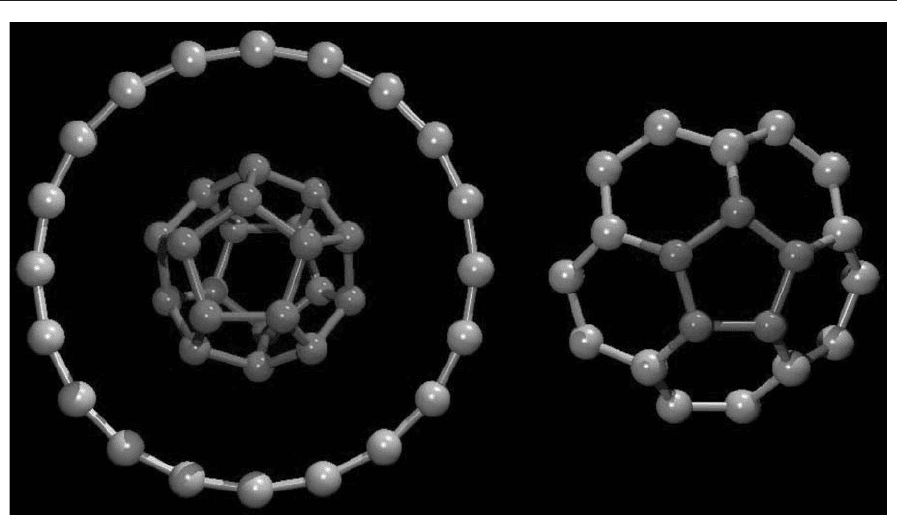
corannulene-like molecule (a bowl-shaped structure
composed of one pentagon surrounded by five hex-
agons; Fig. 5). Various theoretical simulations have
been carried out in order to ascertain which is the
most stable structure composed of 20 atoms. The re-
sults show that, depending upon the computational
method used, the ring, the corannulene-like molecule,
and the dodecahedron are stable and feasible. How-
ever, for fullerene formation (under nonequilibrium
conditions), corannulene and the dodecahedron might
be relevant due to the presence of pentagons in their
structures. Nevertheless, neither of them has ever been
observed as a stable product.
It is worth noting that fullerenes are formed in a
plasma at 3500 1C and under these conditions a wide
variety of carbon clusters are present, e.g., dimers,
trimers, rings, and small clusters. Thus, structures of
the corannulene type or the dodecahedral C
20
may
play a crucial role in the formation of C
60
since C
2
or
bigger units can become attached to the pentagons,
thus stimulating growth.
Two main mechanisms have been proposed to
account for fullerene formation (Dresselhaus et al.
1995). They are based upon: (a) absorption of C
2
units and (b) Stone–Wales (S–W) transformations.
The absorption of a C
2
cluster creates an extra hex-
agon within the structure; therefore, the addition of
several C
2
units into a fullerene would generate a
larger cage. An S–W transformation involves rotating
aC
2
unit by 901, thus changing the symmetry but
maintaining connectivity. A combination of these
two mechanisms is necessary in order to attain a sta-
ble ‘‘graphitic’’ cage. Nevertheless, the amount of
energy involved in an S–W transformation is large
(B7 eV). In this context, it has been shown that au-
tocatalytic effects might overcome the potential bar-
rier involved in this type of transformation (Eggen
et al. 1996). It is also worth noting that fullerenes
smaller than C
60
have been detected only in mass
spectrometers, with one exception being the D
6h
isomer of C
36
, isolation of which has been claimed
(Piskoti et al. 1998).
5. Endohedral Complexes, Intercalated
Compounds and Superconductivity
Kroto et al. (1985) suggested that it should be pos-
sible to encapsulate atoms within the C
60
cage. Short-
ly afterwards a Rice University student, Jim Heath,
succeeded in trapping lanthanum in C
60
by laser va-
porizing a graphite disk impregnated with LaCl
3
(Heath et al. 1985). Following the bulk production
of C
60
, fullerenes containing encapsulated elements,
denoted M@C
n
, have been described, e.g., Ar@C
60
,
He@C
60
, Hf@C
28
, Ti@C
28
, U@C
28
,U
2
@C
28
,
Zr@C
28
, U@C
36
, Co@C
60
, Sc@C
74
, Er@C
82
,
La@C
82
,La
2
@C
82
, Sc@C
82
,Sc
2
@C
82
,Sc
3
@C
82
,
La@C
84
,La
2
@C
84
, etc. (Dresselhaus et al. 1995).
The separation and isolation of these endohedrals is
difficult because the amounts available are minute,
Figure 5
Possible isomers of C
20
: (a) ring, (b) cage, and (c) bowl (corannulene-like molecule).
237
Fullerene Formation
there are solubility problems, and the products may
be oxygen sensitive.
In 1991 Haddon and co-workers succeeded in inter-
calating alkali metals with C
60
in order to produce
materials of stoichiometry M
3
C
60
(M ¼K, Rb, Cs).
These products displayed a relatively high, supercon-
ducting, value of T
c
(18–33 K) (Tanigaki and Prassides
1995). Other stable phases of intercalated C
60
were
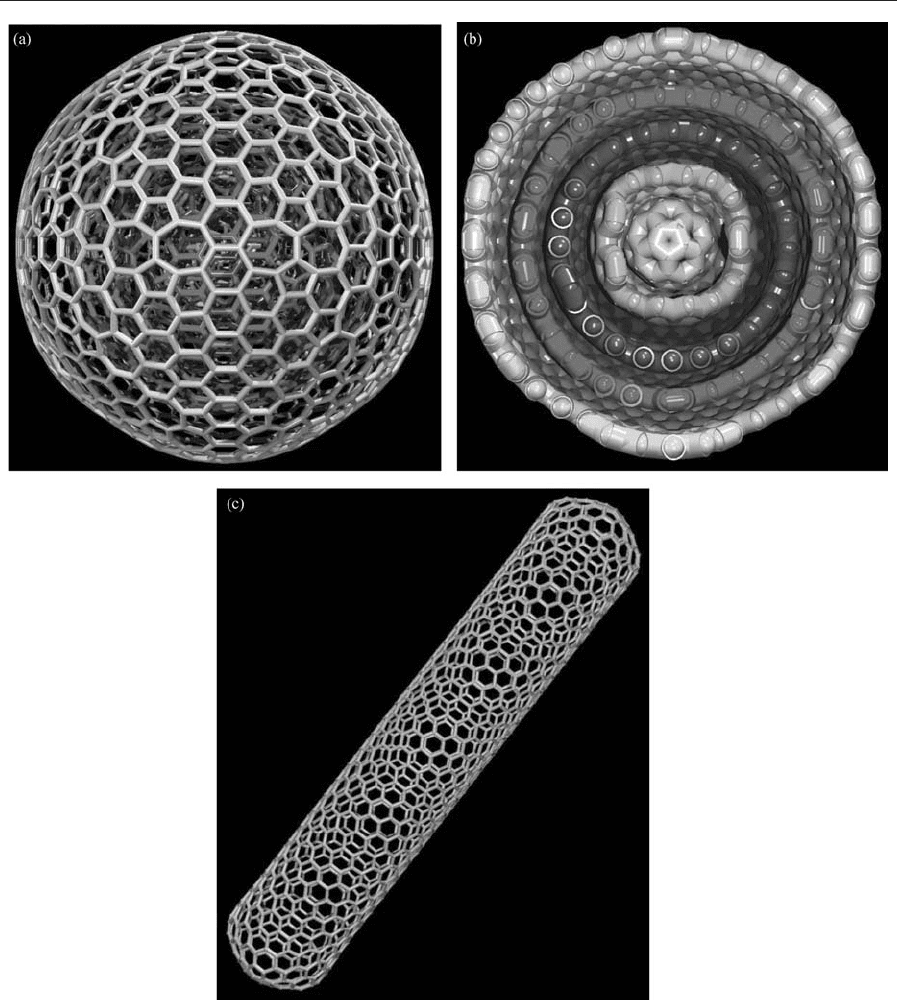
Figure 6
(a) Graphitic onion-like structures: concentric giant fullerenes with heptagons and additional pentagons. (b) Cross-
section of the structure shown in (a) exhibiting concentric shells. (c) Elongated fullerene: a carbon nanotube.
238
Fullerene Formation

prepared (e.g., M
1
C
60
,M
3
C
60
,andM
6
C
60
;Fig.4(b)).
All possess lattice constants greater than that of C
60
(a ¼14.24 A
˚
) due to the introduction of the metal ion
into the crystal phase (Fig. 4(b)). It is important to note
that superconductivity only arises when the tetrahedral
and octahedral sites of C
60
are fully occupied by alkali
atoms, thus creating a M
3
C
60
structure. Other stable
phases and crystal structures with one, two, four, and
six alkali ions per unit cell have been also described.
6. Giant Fullerenes
In 1992 Daniel Ugarte discovered that carbon soot
(generated in plasma arcs), under high-energy elec-
tron irradiation, produced what were effectively nest-
ed giant fullerenes, termed ‘‘graphitic onions’’ (Figs.
6(a) and 6(b)). It is interesting to note that in 1980
Iijima observed similar round structures in amor-
phous carbon films prepared by vacuum deposition
(the morphology was proposed by Kroto and McKay
in 1988). These closed polyhedrons possess millions
of carbon atoms. It has been shown theoretically
(Terrones et al. 1995) that the high sphericity of the
onions is due to the presence of heptagons and oc-
tagons (Figs. 6(a) and 6(b); Eqn. (1)).
7. Uses of Fullerenes
Current fullerene research is developing rapidly and
due to their exceptional properties (Tables 1 and 2),
various applications can be envisaged. In particular,
the optical properties have facilitated the fabrication
of photodiodes and photovoltaic and photorefractive
devices, etc. Fullerenes, in conjunction with conduct-
ing polymers, could give rise to C
60
-based transistors
and rectifying diodes. However, it should be pointed
out that most of these C
60
devices are unstable in air
due to the diffusion and photodiffusion of dioxygen
into the large interball sites present in the solid.
From the catalysis point of view, fullerenes can be
useful in the hydrocarbon refining industry, where an
active catalyst is needed to enhance the efficiency with
which hydrogen is used. Fullerenes can be used as a
source of pure carbon in order to generate high-pu-
rity carbon allotropes such as diamond or nanotubes
(see Sect. 8). In the nanotechnology area, C
60
and
other ‘‘graphitic’’ particles may prove useful as scan-
ning tunneling microscopy (STM) tips, lubricants,
sensors, and other nanoscale devices.
Finally, C
60
has proved to be useful as an efficient
inhibitor of HIV enzymes and research related to
cancer treatments is underway. Therefore, this excit-
ing area of research is likely to give birth to a new
technology in the twenty-first century.
8. Elongated Fullerenes (Nanotubes)
The fullerenes reviewed above consist of closed,
round cages. However, it is possible to generate
elongated fullerenes, known as nanotubes (Fig. 6(c)).
Iijima (1991) reported the existence of these struc-
tures, consisting of concentric graphite tubes, pro-
duced in the Kra
¨
tschmer–Huffman fullerene reactor
(operating at low direct current). A few months after
Iijima’s paper appeared, the first report on the bulk
synthesis of nanotubes, by Ebbesen and Ajayan, was
published. These authors collected the material from
the inner deposit generated by arcing graphite elec-
trodes in inert atmospheres (a similar procedure to
that used for fullerenes).
Nanotubes can be produced by several methods
(e.g., arc discharge, pyrolysis of hydrocarbons over
catalysts, laser vaporization, electrolysis, etc.), with
the products exhibiting various morphologies (e.g.,
straight, curled, hemitoroidal, branched, spiral, hel-
ical, etc.).
It has been predicted that carbon nanotubes may
behave as metallic, semiconducting, or insulating
nanowires depending upon their helicity and diame-
ter. Conductivity measurements on bulk nanotubes,
individual multilayered tubes, and ropes of single-
walled tubules have revealed that their conducting
properties depend strongly on the degree of graph-
itization, helicity, and diameter. Young’s modulus
measurements also show that multilayered nanotubes
are mechanically much stronger than conventional
carbon fibers (Terrones et al. 1999).
The advances typified by fullerenes and related struc-
tures herald a new age in nanoscale materials engineer-
ing. However, this may be only the ‘‘tip of the iceberg’’
since other layered structures (e.g., MoS
2
,WS
2
,VS
2
,
etc.) are also capable of creating closed cages.
See also: Fulleride Superconductors
Bibliography
Dresselhaus M S, Dresselhaus G, Eklund P C 1995 Science
of Fullerenes and Carbon Nanotubes. Academic Press, San
Diego, CA
Eggen B R, Heggie M I, Jungnickel G, Latham C D, Jones R,
Briddon P R 1996 Autocatalysis during fullerene growth.
Science 272, 87–9
Heath J R, O’Brien S C, Zhang Q, Liu Y, Curl R F, Kroto H
W, Tittel F K, Smalley R E 1985 Lanthanum complexes of
spheroidal carbon shells. J. Am. Chem. Soc. 107, 7779–80
Iijima S 1991 Helical microtubules of graphitic carbon. Nature
354, 56–8
Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E
1985 C
60
: buckminsterfullerene. Nature 318, 162–3
Kroto H W 1992 C
60
: buckminsterfullerene, the celestial sphere
that fell to earth. Angew. Chem., Int. Ed. Engl. 31, 111–29
Piskoti C, Yarger J, Zettl A 1998 C
36
: a new carbon solid.
Nature 393, 771–4
Rohlfing E A, Cox D M, Kaldor A J 1984 Production and
characterization of supersonic carbon cluster beams. J.
Chem. Phys. 81, 3322–30
Tanigaki K, Prassides K 1995 Conducting and superconducting
properties of alkali-metal C-60 fullerides. J. Mater. Chem. 5,
1515–27
239
Fullerene Formation
