Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.

T4T
B
(unblocked state) the particle susceptibility is
equal to w
0
. For ToT
B
(blocked state), it is given
by w
1
¼ m
0
M
2
s
=3K. Thus, for a broad volume distri-
bution, the susceptibility will be
wðT; t
m
Þ¼
m
0
M
2
s
3kTZ
Z
V
c
0
V
2
f ðVÞdV
þ
m
0
M
2
s
3KZ
Z
N
V
c
Vf ðVÞdV ð16Þ
with V
c
such that T
B
ðV
c
Þ¼T. The fraction of un-
blocked particles decreases with temperature so that
the w versus T curve has a maximum. The
temperature T
max
at maximum is proportional to the
average blocking temperature according to the type
of volume distribution function.
The thermal variation of the initial susceptibility is
commonly studied by measurements at very low field
of the in-phase a.c. susceptibility w
0
ac
ðnÞ¼dM=dH
with n the frequency, and d.c. susceptibility w ¼M/H
(Fig. 2(a)). The d.c. susceptibility measured after
cooling the sample in zero field (ZFC susceptibility)
shows a maximum, like w
0
ac
. The d.c. susceptibility
measured after cooling the sample under field (FC
susceptibility) grows continuously with decreasing
temperature, splitting from the ZFC curve at
T
sp
4T
max
, and finally saturating below some tem-
perature T
sat
. T
sp
and T
sat
correspond to the largest
and smallest T
B
value, respectively. For T4T
sp
, the
system is at equilibrium. For ToT
sp
, it is in a non-
equilibrium state.
In the blocked state, any change of the magnetic
field is followed by a time variation of the magnet-
ization, e.g., both the ZFC and remanent (M
r
) mag-
netizations are time dependent. For a single particle
with relaxation time t
N
(ac1), an exponential time
decay
M
r
ðtÞ¼M
r
ð0Þexp
t
t
N
ð17Þ
was predicted by Ne
´
el. For a volume distribution,
M
r
ðtÞ¼ð1=ZÞ
R
N
0
M
r
ðt; VÞVf ðVÞdV. A decay of the
form M
r
ðtÞ¼A SlnðtÞ is usually found, but devi-
ations can be observed depending on the experimen-
tal conditions (e.g., recording time of the relaxation)
and sample characteristics. The blocked state gives
rise to bulk-like hysteresis cycles, affected by particle
size and anisotropy, responsible for the difference
in saturation, remanence and coercive force H
c
. For
uniaxial anisotropy, when all the moments are
blocked, M
r
¼ M
s
=2 and H
c
¼ H
K
=2 if the easy ax-
es are randomly oriented, and M
r
¼ M
s
and H
c
¼
H
K
if they are aligned parallel to the field. An ori-
entation texture can easily be induced by freezing a
ferrofluid under field.
6. A.c. Susceptibility
The a.c. susceptibility, wðoÞ¼w
0
ðoÞiw
00
ðoÞ,with
o ¼ 2pn the angular frequency, is determined by the
moments able to follow the small a.c. field H ¼ H
0
e
iot
.
Frequencies of interest for ferrofluids range from hertz
to gigahertz, i.e., up to ferromagnetic resonance. For a
uniaxial particle where the moment deviates slightly
from the easy axis, the resonant condition is
o ¼ gm
0
H
K
. Far below resonance, for a random as-
sembly of particles with volume V and relaxation time
t, the particle magnetization is given by
MðtÞ¼H
0
½w
0
ðw
0
w
N
Þe
t=t
ð18Þ
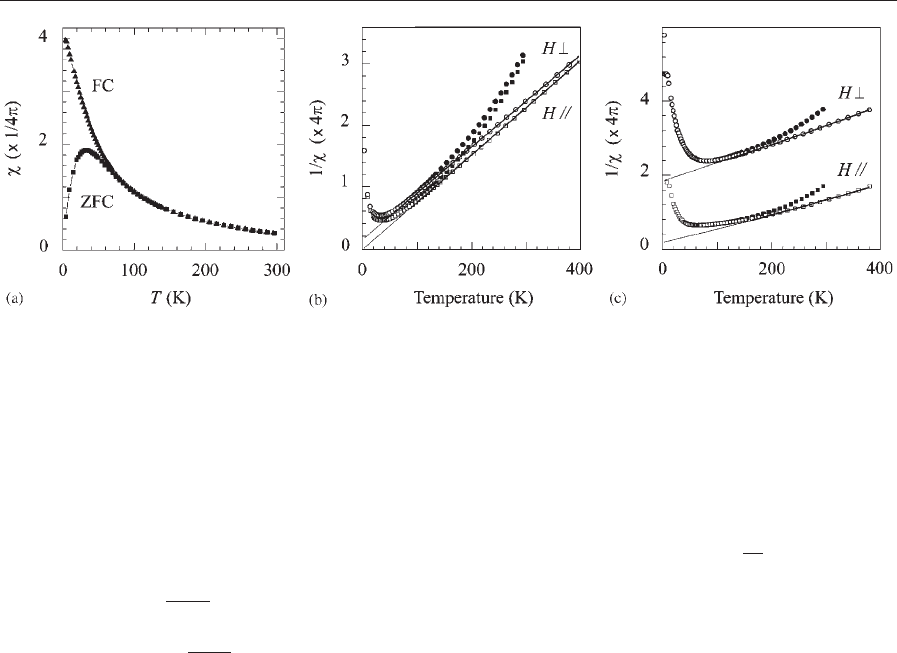
Figure 2
Thermal variation of the initial susceptibility (w) for polymerized g-Fe
2
O
3
ferrofluids. Applied field H ¼800 A m
1
. (a)
Field-cooled (FC) and zero-field-cooled (ZFC) susceptibilities for a dilute sample. (b) Inverse ZFC susceptibility for
parallel or perpendicular geometry; and (c) Concentrated sample. Experimental data (filled symbols); corrected (open
symbols) for the thermal variation of the intrinsic magnetization. Volume fraction of particles (a, b) 0.0075, (c) 0.195.
Average diameter 7.7 nm. (Unpublished data courtesy of M. Nogue
´
s).
200
Ferrofluids: Magnetic Properties
where w
N
is the high-frequency susceptibility. The
Fourier transform gives the susceptibility according to
Debye’s theory
wðoÞ¼
w
0
þ iotw
N
1 þ iot
w
0
ðoÞ¼w
N
þ
w
0
w
N
1 þ o
2
t
2
w
00
ðoÞ¼
ðw
0
w
N
Þot
1 þ o
2
t
2
ð19Þ
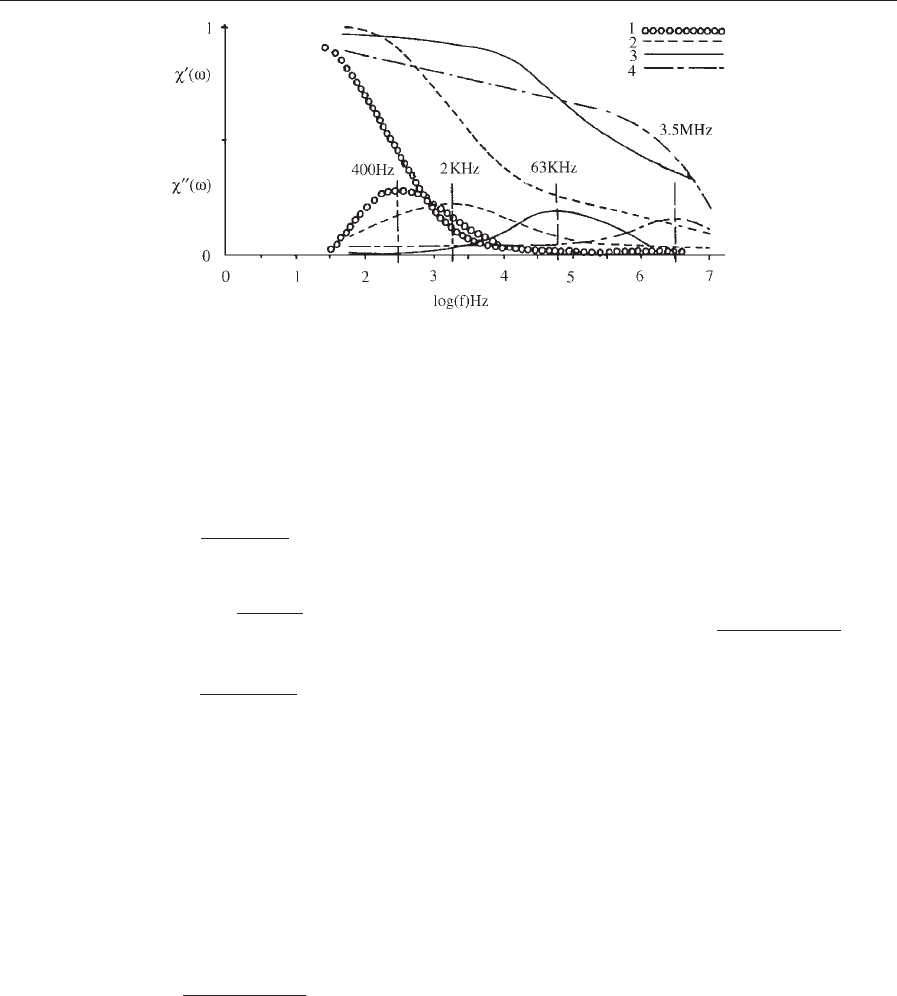
w
0
ðoÞ falls monotonically with increasing o, whereas
w
00
ðoÞ has a maximum at ot ¼ 1(Fig.3).Thisenables
one to determine the effective relaxation time and
particle size in a ferrofluid in a relatively simple way.
A single loss peak will be found if the relaxation
time is distributed continuously, in the range
1–100 MHz when the Ne
´
el mechanism dominates,
and much lower when the Brownian process prevails.
For a random assembly of particles, wðoÞ can be de-
scribed in terms of its parallel, w
8
ðoÞ, and perpen-
dicular, w
>
ðoÞ, components according to
wðoÞ¼
w
8
ðoÞþ2w
>
ðoÞ
3
ð20Þ
w
8
ðoÞ and w
>
ðoÞ refer (Eqn. (19)) to longitudinal and
transverse relaxation times, respectively. With in-
creasing frequency, w
8
ðoÞ remains purely relaxational
in character. w
>
ðoÞ is associated with resonance,
which corresponds to w
0
>
ðoÞ going negative. From
the frequency dependence of wðoÞ in the range
100 MHz10 GHz, parameters such as H
K
, K , and
M
r
ðtÞ can be determined.
7. Interparticle Interaction Effects
The magnetic dipolar interparticle interaction always
exists in a particle assembly. In a ferrofluid, its con-
sequences can range from liquid-like short-range
order to highly anisotropic chain configurations as
observed in large applied fields. The energy of particle
i due to interaction with all other particles is given by
E
i
¼m
0
md
X
iaj
H
ij
; H
ij
¼
3ðm
j
dr
ij
Þr
ij
m
j
4pd
3
ij
ð21Þ
where H
ij
is the magnetic field seen by particle i due
to the interaction with particle j, and r
ij
and d
ij
are
the unit vector and distance defining the vector con-
necting the centers of the two particles with moments
m
i
and m
j
. The interaction energy adds to the indi-
vidual anisotropy energy. The particle energies are
interdependent and the whole system tends to min-
imize its magnetostatic energy. A ferrofluid system,
unconstrained topologically, will partition itself into
an ensemble of subsystems with low magnetostatic
energy.
Relaxation in the particles can be assumed blocked
and the leading parameter is l ¼ m
0
m
2
=4pD
3
h
kT with
D
h
the hydrodynamic particle diameter, neglecting
size distribution. The stability of the suspension of
particles limits the value of l. No spatial correlation
occurs if lt1. Clustering develops with growing l
and when l is large enough, all particles are aggre-
gated at equilibrium, as shown by Monte Carlo
simulations. Short linear chains (dimers, trimers)
predominate at small l values. Bending and branch-
ing appear when l increases. At medium values,
closed magnetic configurations prevail. For large l
values, the cluster structure is more and more iso-
tropic and dense, with the orientation of the mo-
ments tending to be randomized. In large applied
Figure 3
Normalized plots of the real and imaginary parts of the a.c. susceptibility against frequency for cobalt ferrite (1,3) and
magnetite (2,4) ferrofluids. Median particle radius of (1–3) 5 nm, (4) 4.5 nm. (Reprinted with permission from Fannin
1994; r Elsevier Science B.V.)
201
Ferrofluids: Magnetic Properties

fields, the spatial order becomes anisotropic. The
orientation effect of the field strengthens the interac-
tions favoring chain-like configurations, which give
rise to the anisotropic magneto-optical properties of
ferrofluids.
The interactions influence the magnetic properties
especially in low applied fields. They cause deviations
from the Langevin behavior (Figs. 2(b) and (c)). The
equilibrium magnetization is given by the Langevin
law, referring to the effective field, H
eff
, seen by the
particle, which is not equal to the applied field, H
app
,
in the presence of interaction. Since the sample is
magnetized, boundary phenomena give rise to de-
magnetizing field effects. The demagnetizing field is
constant inside the sample if this is an ellipsoid with
an axis parallel to the applied field. The field exterior
to the particles is then given by
H
ext
¼ H
app
N
e
C
v
M ð22Þ
with N
e
(0pN
e
p1) the demagnetizing factor of the
sample. The measured susceptibility w
app
¼ M=H
app
depends on the geometrical experimental parameters,
unlike w
ext
¼ M=H
ext
.
Different models have been proposed for estimat-
ing H
eff
as a function of H
ext
. They all lead to first
order, with a varying range of applicability, to the
same formula for the initial susceptibility
w
ext
¼ w
0
ð1 þ N
i
C
v
w
0
Þð23Þ
where N
i
¼ 1=3. This agrees with experimental re-
sults, after correcting for size distribution and ther-
mal variations of M
s
and C
v
, and with numerical
simulations for w
n
0
¼ C
v
w
0
t3. In the limit w
n
0
{3, the
susceptibility takes a Curie–Weiss form; it is in no
way indicative of a magnetic phase transition. For
w
n
0
\3, correlations cannot be neglected and Eqn. (23)
does not hold; the influence of the microstructure
ðw
n
0
E8lC
v
Þ no longer reduces to a concentration ef-
fect and Monte Carlo simulations are in practice the
only reliable way of modeling. Since the texture varies
with field and temperature (l), the magnetic behavior
of ferrofluids can be extremely complex. In real sys-
tems, clustering of physicochemical origin may bring
extra complexity.
The interactions affect the Brownian dynamics
(Fig. 3), especially because a cluster is a rotating
Brownian object, with relaxation time proportional
to the volume. They modify the dynamics in the par-
ticles depending on the topology and relative mag-
nitudes of the anisotropy and interaction energies. In
random solid systems, for weak enough interactions,
the dynamics can still be described in the framework
of the Ne
´
el relaxation. The interactions increase the
energy barrier. In the Dormann et al. (1997) model,
valid when all (most) moments are above or near
their blocking temperature, the increase for particle i
is approximately given by
E
Bint
ðiÞ¼
X
j
q
ij
L
q
ij
kT
; q
ij
¼
m
0
4p
m
i
m
j
3cos
2
x
ij
1
d
3
ij
ð24Þ
where x
ij
is an angle parameter of r
ij
. For strong
enough interactions, the behavior will become co-
operative in type. In any case, typical features (e.g.,
aging effect on the magnetization relaxation) of cor-
related disordered systems, such as spin–glass sys-
tems, will be observed if the experimental conditions
(temperature, time window) are suitably chosen. In
ferrofluids, the dynamics in aggregated particles in-
fluences the lifetime of their association.
8. Summary
The theoretical models of magnetic dynamics in
ferrofluids and main macroscopic experimental fea-
tures have been outlined. The main difference be-
tween Ne
´
el and Brownian relaxations lies in their
timescales. It is the key to the specific properties of
ferrofluids, i.e., a perfectly soft behavior (irrespective
of particle size, concentration, and aggregation), an
orientational texture under field, and a spatial texture
caused by the magnetic interparticle interaction.
See also : Ferrofluids: Applications; Ferrofluids: In-
troduction; Ferrofluids: Neutron Scattering Studies;
Ferrofluids: Preparation and Physical Properties;
Ferrohydrodynamics
Bibliography
Brown F J Jr. 1979 Thermal fluctuations of fine ferromagnetic
particles. IEEE Trans. Magn. Mag-15, 11961–208
Chantrell R W, Walmsley N, Gore J, Maylin M 2000 Calcu-
lations of the susceptibility of interacting superparamagnetic
particles. Phys. Rev. B63, 024410
Coffey W T, Cregg P J, Kalmykov Yu P 1993 On the theory of
Debye and Ne
´
el relaxation of single domain ferromagnetic
particles. In: Prigogine I, Rice S A (eds.) Advances in Chem-
istry and Physics. Wiley, New York, Vol. 83
Coffey W T, Crothers D S F, Dormann J L, Geoghegan L J,
Kennedy E C 1998 Effect of an oblique magnetic field on the
superparamagnetic relaxation time. Phys. Rev. B58, 3249–66
Coverdale G N, Chantrell R W, Martin G A R, Bradbury A,
Hart A, Parker D A 1998 Cluster analysis of the microstruc-
ture of colloidal dispersions using the maximum entropy
technique. J. Magn. Magn. Mater. 188, 41–51
Dormann J L, Fiorani D, Tronc E 1997 Magnetic relaxation in
fine-particle systems. In: Prigogine I, Rice S A (eds.) Ad-
vances in Chemistry and Physics. Wiley, New York, Vol. 98
Dunlop D J, O
¨
zdemir O
¨
1997 Rock Magnetism. Cambridge
University Press, Cambridge
Fannin P C 1998 Wideband measurement and analysis tech-
niques for the determination of the frequency-dependent,
complex susceptibility of magnetic fluids. In: Prigogine I,
Rice S A (eds.) Advances in Chemistry and Physics. Wiley,
New York, Vol. 104
202
Ferrofluids: Magnetic Properties
Kodama R H 1999 Magnetic nanoparticles. J. Magn. Magn.
Mater. 200, 359–72
Pshenichnikov A F, Mekhonoshin V V 2000 Equilibrium mag-
netization and microstructure of the system of super-
paramagnetic interacting particles: numerical simulation. J.
Magn. Magn. Mater. 213, 357–69
Raikher Yu L, Stepanov V I 1997 Linear and cubic dynamic
susceptibilities of superparamagnetic fine particles. Phys.
Rev. B55, 15005–17
E. Tronc
C.N.R.S., Universite
´
Pierre et Marie Curie
Paris, France
D. Fiorani
Institute of Materials Chemistry, C.N.R., Rome, Italy
Ferrofluids: Neutron Scattering Studies
The investigation of the internal structure of mag-
netic fluids is often carried out by indirect techniques
like magnetization measurement or ex situ investiga-
tions, e.g., the electron micrography of specially pre-
pared fluid samples. This causes strict limitations
concerning the interpretation of the fluid microstruc-
ture itself as well as of the dependence of macroscopic
properties of the fluids on microscopic structural
changes.
A possibility to overcome these restrictions is the
use of neutron scattering to explore microstructural
properties of magnetic fluids. In contrast to normal
light, scattering neutrons are able to penetrate ferro-
fluid samples of reasonable thickness and to give ap-
propriate scattering intensities on experimentally
practicable time scales. The intensity of neutrons scat-
tered by a ferrofluid contains an additional anisotrop-
ic magnetic part besides the usual nuclear scattering.
This magnetic part of the scattering intensity I
m
de-
pends on the relative orientation between the scatter-
ing vector q
!
, the neutron polarization p
!
, and the
magnetization of the fluid sample m
!
respectively:
I
m
pfð q
!
p
!
Þðq
!
m
!
Þg
2
ð1Þ
If unpolarized neutrons are used—and thus the
first bracket becomes a constant factor—the scatter-
ing intensity of magnetic scattering is anisotropic de-
pending on the relative orientation between scattering
vector and sample magnetization:
q
!
m
!
2
¼
0if q
!
8m
!
1if q
!
>m
!
8
>
<
>
:
9
>
=
>
;
ð2Þ
For an unmagnetized sample the ð q
!
m
!
Þ
2
-term
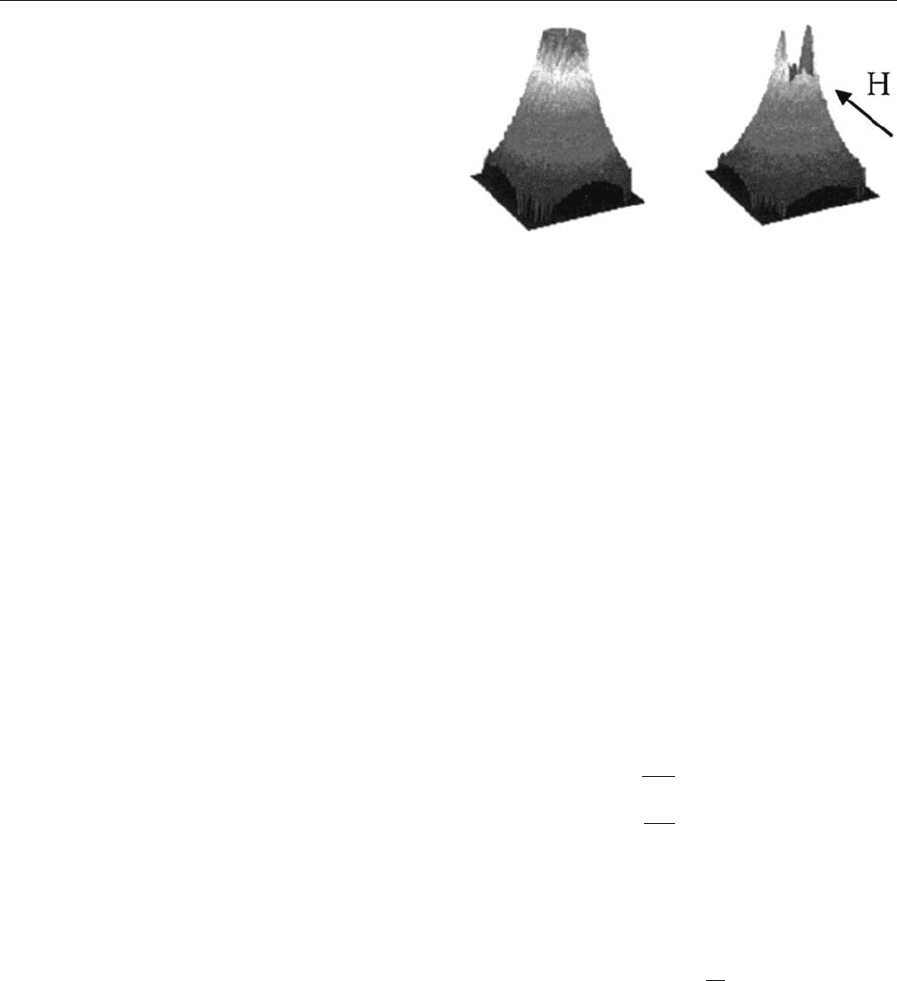
equals 2=3 and the scattering becomes isotropic as it
can be seen from Fig. 1. In particular, magnetic neu-
tron scattering allows direct access to the magnetic
microstructure and thus to the solutions of questions
like magnetic size distribution or cluster and chain
formation of magnetic particles. Details concerning
the possibility to distinguish between nuclear and
magnetic scattering will be discussed in the forth-
coming sections.
1. Selection of Experimental Method
The choice of the particular neutron scattering method
to be used is determined by the size of the scattering
units and the main goal of the investigation itself.
The typical particle size range for the particles sus-
pended in a magnetic fluid—which is usually between
5 nm and 30 nm—requires a range of q-vectors to be
examined between:
q
min
¼
2p
d
max
E0:2
(
A
1
and
q
max
¼
2p
d
min
E1
(
A
1
ð3Þ
For thermal neutrons with a wavelength l of about
6A
˚
to 10A
˚
, which are usually used for the experi-
ments, this corresponds to scattering angles y defined
by
sinðyÞ¼
lq
4p
ð4Þ
in the range between 51 and 251. That means that the
method usually chosen for ferrofluid investigations is
small angle neutron scattering (SANS). For details on
SANS experiments the reader should refer to the basic
textbooks by Guinier (1963) and Egelstaff (1965).
Figure 2 shows the principal set up for a SANS ex-
periment with magnetic fluids. The sample is subjected
to a collimated neutron beam transmitted through
the fluid. The scattered neutrons are usually detected
Figure 1
Typical small angle scattering signal from an
unmagnetized ferrofluid (left) and from a fluid under
influence of a field H in the depicted direction (right)
(after Odenbach 2000).
203
Ferrofluids: Neutron Scattering Studies
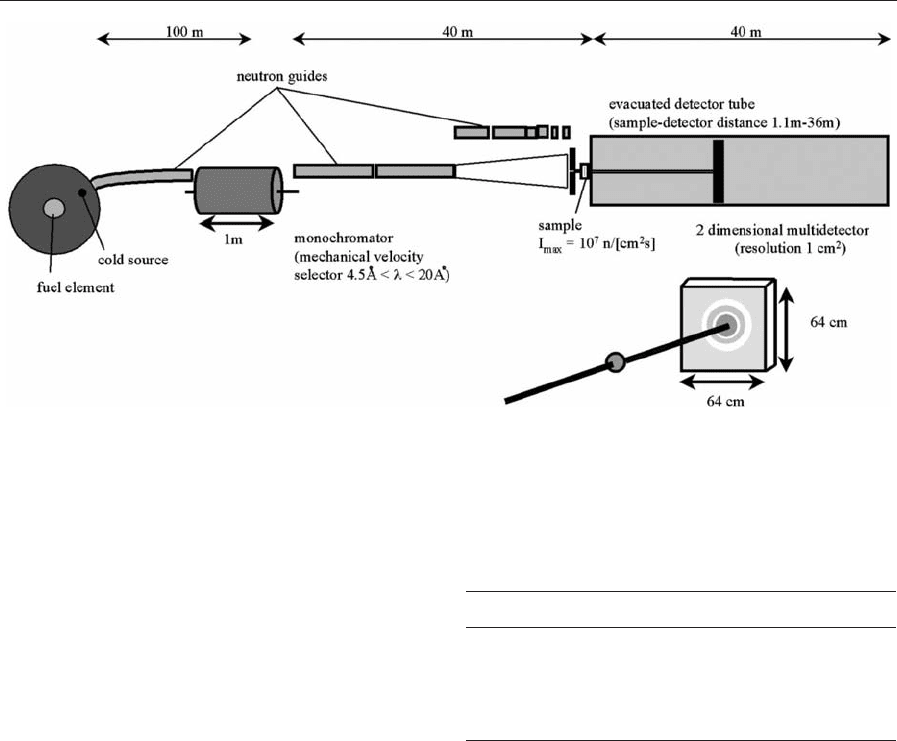
using a two-dimensional array detector. The detec-
tor—sample distance can be altered to vary the max-
imum momentum transfer detectable. To avoid
reduction of scattering intensity, the neutron path to
the sample and from the sample to the detector is
guided through vacuum tubes.
SANS experiments can be carried out using polar-
ized as well as unpolarized neutrons, providing dif-
ferent kind of information, as can be seen from Eqn.
(1). Some basic experimental questions will be dis-
cussed, followed by the experiments with unpolarized
neutrons that represent the major amount of exper-
imental work performed. Finally, experiments with
polarized neutrons and other experimental tech-
niques will be outlined.
2. Nuclear Scattering and Contrast Variation
As was already mentioned, neutron scattering on
magnetizable materials provides an anisotropic part
of scattering intensity as a result of the interaction
between the neutrons magnetic moment and the
magnetization of the sample.
Besides this, the most important part of scattering
intensity will usually be made by conventional nuclear
scattering. The total cross-section for nuclear scatter-
ing is composed from the coherent s
coh
, incoherent
s
inc
, and absorption s
abs
scattering cross-sections of
the particles material, the surfactant, and the solvent.
The scattering cross-sections for the most important
elements contained in a ferrofluid are given in Table 1.
Essential for scattering experiments is the relation
between the coherent scattering providing the signal
and incoherent scattering and absorption leading to
unwanted noise and signal reduction. For the metallic
particles the coherent scattering cross-section is dom-
inant compared to the incoherent part and only weak
absorption occurs leading to only small reduction of
the scattered intensity. For surfactant and carrier
liquid, one has to distinguish between deuterated and
nondeuterated material. If nondeuterated substances
are used, the scattering behavior will be dominated by
the incoherent scattering due to the hydrogen atoms.
In this case the scattering from the surfactant and the
carrier liquid will dominate the incoherent scattering
of the whole sample investigated. The scattering from
the particles will be just a small additional part over a
strong incoherent noise and thus detection may be
difficult. Furthermore, the maximum thickness of the
sample in the direction of the beam will be reduced
Figure 2
A typical SANS scattering geometry at the example of the small angle scattering instrument D11 at the Institute Laue
Langevin in Grenoble.
Table 1
Scattering cross-sections for relevant elements in barns.
Element s
coh
s
inc
s
abs
Fe 1.86 0.46 1.4
H 1.76 79.7 0.19
D 5.59 2.01 5 10
4
O 4.23 0.01 1 10
4
C 4.52 0.98 3 10
3
Source: Egelstaff (1965).
204
Ferrofluids: Neutron Scattering Studies
due to the incoherent scattering by hydrogen. Exper-
iments (Odenbach et al. 2000) have shown that the
scattering intensity rises by a factor of approximately
three if deuterated carrier liquids are used and the
necessary counting time for a given sample thickness
and for reasonable statistics can be furthermore re-
duced due to the improvement of the signal/noise
ratio in the case of absence of hydrogen.
Beside this, the partial replacement of hydrogen by
deuterium allows special experimental approaches
providing additional information about the ferrofluid
sample. If one defines the excess scattering from the
particles over the scattering from the carrier liquid as
the scattering contrast (Cebula et al. 1983b), one can
change the scattering intensity by variation of the
scattering from carrier liquid and surfactant. The
optimization of the contrast between particles and
carrier solvent provides optimum statistics of the sig-
nal and ensures that the scattered signal is dominated
by the internal particle structure of the ferrofluid. By
comparison of scattering intensities from a fluid with
deuterated surfactant with that from one having con-
ventional surfactant, information about the thickness
of the surfactant layer can be obtained. The best way
to do that is the use of a Guinier plot, i.e., a plot of
the logarithm of scattered intensity over the square
of q. This plot provides a linear relation for small
q-vectors with a slope proportional to the mean size
of the scattering units. Thus in the first case a Guinier
plot provides the core size of the particles, in the sec-
ond case it reflects the size including the surfactant.
3. SANS Experiments for Determination of Size
Distribution
Size distribution determination for the particles in a
magnetic fluid can be done by electron microscopy or
magnetic measurement. In the first case a sample
must be prepared by a process that may modify the
size distribution, while the second method provides
values that strictly depend on the underlying mag-
netic theory, in particular the question of interaction
between the particles can cause serious problems.
From Eqns. (3) and (4) it is seen that the scattering
angle depends on the size of the scattering structures,
i.e., in the case of a ferrofluid on the size of the
particles
sinðyÞ¼
l
2d
ð5Þ
A monodisperse colloid would provide a SANS
signal which would directly allow the determination
of the particle size from a Guinier plot. For a poly-
disperse system the situation becomes more difficult,
since multiple signal structures are superimposed.
Under these conditions a model is needed to fit the
data and to obtain the size distribution. Most often
the usual log normal distribution is used as base
model for the fits, but newly developed fitting soft-
ware at several neutron sources allows more model-
independent determination of the distribution. The
experimental results obtained in SANS studies show
various deviations from the distributions determined
with conventional methods like magnetization meas-
urement that shed a light on some basic problems in
the understanding of magnetic fluids. In some exper-
iments (e.g., Potton et al. 1983) an expansion of the
size distribution to larger radii was obtained from the
SANS data compared to magnetization measure-
ment, while Upadhyay et al. (1993) observed a nar-
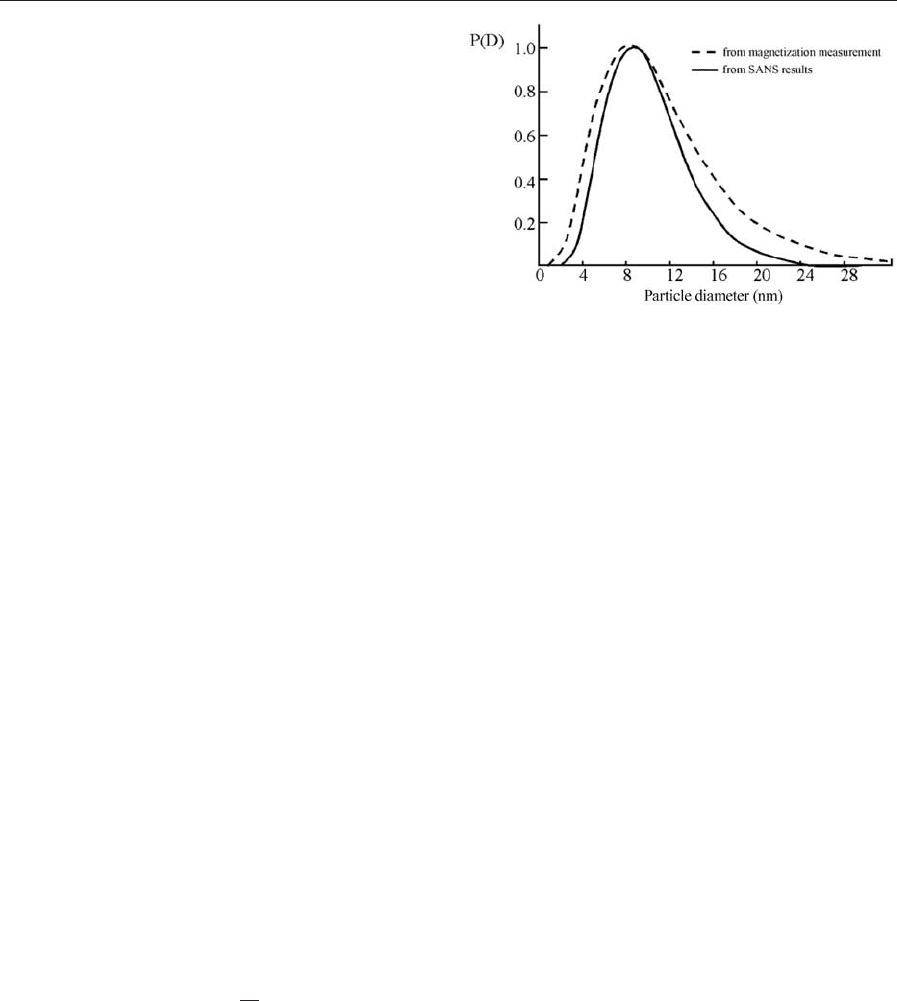
rower distribution in neutron experiments (Fig. 3).
The explanations for these discrepancies are usu-
ally taken from the lack of knowledge about basic
data of the fluids themselves. While the narrower
distribution is explained by field-induced magnetic
interaction between the particles in magnetization
measurement, not accounted for in the data evalua-
tion of these measurements, the expansion to larger
radii could be explained by the difference between the
bulk saturation magnetization and that of small par-
ticles (So
¨
ffge et al. 1981). Furthermore, the assump-
tion of spherical particles which is usually used when
fitting the neutron data together with the consider-
ably large errors of neutron scattering data in general
could also lead to discrepancies. Thus it is seen that
the underlying model for the data evaluation and the
assumptions within as well as the accuracy of the data
obtained are of significant influence on the results
obtained. While the models used can only be im-
proved on the basis of new experimental results, the
quality of the scattering data must be optimized
Figure 3
The comparison between particle size distributions
measured with conventional magnetization
measurement and by SANS. The discrepancy is
explained by the neglection of field-induced interaction
of the particles in magnetization measurement analysis
(after Upadhyay et al. 1993).
205
Ferrofluids: Neutron Scattering Studies

(e.g., by appropriate choice of deuterated carrier liq-
uids) to avoid additional uncertainties.
4. SANS Experiments for Observation of Cluster
Formation
While SANS methods are only an alternative in de-
termining particle size distribution, they provide a
unique tool if the formation and development of
clusters and cluster like structures of magnetic par-
ticles are considered. Aggregation of particles forms
structures—determining the scattering process—
which have sizes in the order of several hundred
nm. Thus the intensity scattered by these structures
will be found at even smaller scattering angles of the
order of 11 and less. Even the best small angle scat-
tering experiments available nowadays reach with
such requirements the edge of their technological
possibilities. Nevertheless the influence of the scat-
tering from large structures changes the line shape of
the small angle scattering signal even at higher angles
easily available in experiments. Such changes can
then be taken to obtain information on formation of
multiparticle aggregates in magnetic fluids.
In this way, Cebula et al. (1983a) investigated the
appearance of cluster formation in ferrofluids con-
taining cobalt particles. They found a strong depend-
ence of the scattering signal on the volume fraction
of particles at zero field indicating the appearance
of cluster formation due to magnetic dipole—dipole
interaction. Furthermore, they found interference
peaks when a magnetic field was applied, indicating
the appearance of ordered structures like chains
aligned with the magnetic field direction. Finally re-
duction of temperature, resulting in a reduction of
thermal motion, lead to an anisotropy at H ¼0, also
indicating formation of aggregates and chains. Thus
these experiments showed clearly that SANS is a
powerful tool for the in situ observation of aggrega-
tion phenomena in ferrofluids without disturbing ef-
fects due to drying in sample preparation for electron
micrography.
Extended work on aggregation formation in mag-
netite-based ferrofluids was carried out by Rosman
et al. (1990). They combined the SANS measure-
ment in variable magnetic fields with the already
discussed contrast variation method providing them
with the particle size distribution and the structure
of the surfactant layer of the fluids under investiga-
tion. Here again the mentioned differences between
conventional and neutron scattering methods ap-
peared. Even at low volume fraction and in compa-
rably weak magnetic fields of about 100 mT they
found a formation of thin chains of particles in the
direction of the applied field. Higher volume con-
centration enabled formation of three-dimensional
rods in field direction. The interparticle distance
obtained from the scattering data gave rise to the
assumption that the surfactant layers are in contact
in the aggregates.
Even larger structures can be observed in ferrofluid
mixtures (Hayter et al. 1989) when macromolecules
are dispersed in a ferrofluid. These nonmagnetic mol-
ecules act as magnetic voids in the liquid and arrange
to macroscopic structures in presence of magnetic
fields. The neutron diffraction patterns obtained
show an anisotropy that corresponds to these mac-
rostructures. In this case the neutron diffraction ex-
periments were used to prove the possibility of
alignment of nonmagnetic macromolecules by means
of magnetic fluids.
5. SANS Experiments with Polarized Neutrons
During the discussion of neutron scattering data ob-
tained from ferrofluids with unpolarized neutrons, it
became clear that anisotropies in the scattering pat-
tern can be due to magnetic effects (see Eqn. (1)) as
well as due to formation of structures. The use of
polarized neutrons can be a powerful help to distin-
guish between these two kind of anisotropies and
thus to eliminate the nuclear scattering background
completely. To do so the fluid sample is subjected to a
beam of polarized neutrons and a polarization anal-
ysis—distinguishing between neutrons scattered with
spin flip and those scattered without—is carried out
after the scattering process. Coherent nuclear scat-
tering, giving information about the structural phe-
nomena like aggregation by measuring the particle
number density, does not flip the neutrons spin. In
contrast, the magnetic scattering—which is caused by
the component of magnetization perpendicular to
q
!
—causes spin flip for those components of mag-
netization which are perpendicular to the field, while
the parallel one gives nonflip scattering. Therefore
measurement of scattered intensity with q
!
parallel
and perpendicular to the applied field makes it pos-
sible to distinguish between nuclear and magnetic
scattering signals. This technique has been used by
Pynn et al. (1983) to analyze the interparticle struc-
ture in a cobalt ferrofluid.
The previously discussed problem of the influence
of polydispersity on the SANS signal can also be re-
duced by the use of polarized neutrons (Nunes 1988).
Furthermore, a detailed analysis of nuclear and mag-
netic scattering intensities and of the respective size
distributions obtained can shed a light on the exist-
ence of magnetically dead layers.
6. Other Experimental Approaches
Up to now we have outlined the use of neutron scat-
tering for determination of structure and agglomera-
tion processes in magnetic fluids using SANS
techniques. Beside this, there are a couple of experi-
ments taking neutron scattering for other experimental
206
Ferrofluids: Neutron Scattering Studies

goals or using alternative scattering techniques. For
example SANS experiments have been used to inves-
tigate diffusion phenomena in magnetic fluids (Oden-
bach et al. 1995) by determination of the spatial
variation of magnetic scattering anisotropy. Since the
anisotropy is proportional to magnetization of the
fluid and since this is a measure for the concentration
of particles at given magnetic field strength, one can
determine the spatial variation and time dependence of
concentration in a ferrofluid subjected to a magnetic
field gradient. Other SANS experiments have shown
that it is possible to use the anisotropy as a measure
for local variations of flow velocity in a ferrofluid
(Odenbach et al. 1999). The shear in the fluid changes
the mean alignment of the particles with the magnetic
field direction and thus reduces the apparent magnet-
ization in a certain sample volume leading to a change
of anisotropy.
In the characterization of a temperature-sensitive
magnetic fluid the disappearance of magnetic scat-
tering for temperatures above the Curie point has
been used to distinguish between magnetic and nu-
clear scattering, avoiding the loss of intensity con-
nected with the spin flip method discussed above
(Upadhyay et al. 1997).
Finally, powder diffraction with polarized neu-
trons was used as an alternative method to determine
the size distribution of the particles and to get in-
formation about magnetically-disordered layers
(Lin et al. 1995).
7. Outlook
It has been discussed that neutron scattering can be a
helpful tool to investigate the microstructure of mag-
netic fluids, the structure of particles and surfactants
and the formation of clusters, aggregates, and chains.
From this it is astonishing that the amount of work
done in this field is not as high as one would expect
considering the potential of the method. A possible
reason for this could be based on the fact that shortly
after the first efforts in this field the two major neu-
tron sources in Europe, providing sufficient intensity
for ferrofluid experiments—the high flux research re-
actors at ILL in Grenoble and at KFA in Ju
¨
lich, were
out of order for approximately five years for main-
tenance reasons. Now, after restart of both sources,
the activities on neutron scattering experiments with
ferrofluids have been reactivated and new results are
expected in the future. In particular the investigation
of structure formation in the presence of magnetic
field and shear flow—which is the microscopic reason
for the appearance of non-Newtonian effects in ferro-
fluids—is expected to become a vivid field of interest.
See also: Ferrofluids: Introduction; Ferrofluids: Pre-
paration and Physical Properties; Ferrofluids: Mag-
netic Properties; Ferrohydrodynamics
Bibliography
Cebula D J, Charles S W, Popplewell J 1983a Aggregation in
ferrofluids studied by neutron small angle scattering. J. Phys.
44, 207–13
Cebula D J, Charles S W, Popplewell J 1983b Neutron scat-
tering studies of ferrofluids. J. Magn. Magn. Mater. 39, 67–70
Egelstaff P A 1965 Thermal Neutron Scattering. Academic
Press, London
Guinier A 1963 X-ray Diffraction in Crystals. Freeman, San
Francisco, pp. 319–50
Hayter J B, Pynn R, Charles S W, Skjeltorp A T, Trewhella J,
Stubbs G, Timmins P 1989 Ordered macromolecular struc-
tures in ferrofluid mixtures. Phys. Rev. Lett. 62, 1667–70
Lin D, Nunes A C, Majkrzak C F, Berkowitz A E 1995
Polarized neutron study of the magnetization density distri-
bution within a CoFe
2
O
4
colloidal particle. J. Magn. Magn.
Mater. 145, 343–8
Nunes A C 1988 Effect of magnetic polydispersion in super-
paramagnetic colloids on neutron scattering line shapes. J.
Appl. Cryst. 21, 129–35
Odenbach S, Gilly H, Lindner P 1999 The use of magnetic small
angle neutron scattering for the detection of flow profiles in
magnetic fluids. J. Magn. Magn. Mater. 201, 353–6
Odenbach S, Schwahn D, Stierstadt K 1995 Evidence for dif-
fusion-induced convection in ferrofluids from small angle
neutron scattering. Z. Phys. B 96, 567–9
Potton J A, Daniell G J, Eastop A D, Kitching M, Melville D,
Poslad S, Rainford B D, Stanley H 1983 Ferrofluid particle
size distributions from magnetization and small angle neu-
tron scattering data. J. Magn. Magn. Mater. 39, 95–8
Pynn R, Hayter J B, Charles S W 1983 Determination of
ferrofluid structure by neutron polarization analysis. Phys.
Rev. Lett. 51, 710–3
Rosman R, Janssen J J M, Rekveldt M Th 1990 Interparticle
correlations in Fe
3
O
4
ferrofluids, studied by the small angle
neutron scattering technique. J. Appl. Phys. 67, 3072–80
So
¨
ffge F, Schmidbauer E 1981 AC susceptibility and static
magnetic properties of an Fe
3
O
4
ferofluid. J. Magn. Magn.
Mater. 24, 54–8
Upadhyay R V, Sutariya G M, Mehta R V 1993 Particle size
distribution of a laboratory synthesized magnetic fluid. J.
Magn. Magn. Mater. 123, 262–6
Upadhyay T, Upadhyay R V, Mehta R V, Aswal V K, Goyal P
S 1997 Characterization of a temperature-sensitive magnetic
fluid. Phys. Rev. B 55, 5585–8
S. Odenbach
Universita
¨
t Bremen, Germany
Ferrofluids: Preparation and Physical
Properties
A ferrofluid, or magnetic fluid as it is also commonly
referred to, consists of a stable colloidal suspension of
single-domain nano-sized particles of ferri- or ferro-
magnetic materials in a carrier liquid. A wide range of
carrier liquids have been employed, and many ferro-
fluids are commercially available to satisfy particular
207
Ferrofluids: Preparation and Physical Properties

applications, e.g., in rotary vacuum lead-throughs, it
is essential that the carrier liquid has a very low vapor
pressure. In other applications, temperature—either
high, low, or both—may be a critical consideration.
Theoretically it should be possible to produce dis-
persions in any liquid thereby being able to tailor the
requirements of viscosity, surface tension, tempera-
ture and oxidative stability, vapor pressure, stability
in hostile environments, etc., to the particular appli-
cation in mind, whether it be technological or bio-
medical (see Ferrofluids: Applications).
In order to achieve a stable colloidal suspension,
and in this context stability refers to situations where
the ferrofluid is subjected to a magnetic field, mag-
netic field gradient, and/or gravitational field, the
magnetic particles generally have to be of approxi-
mately 10 nm in diameter. Particles of this size,
whether they are ferrite or metal, possess a single
magnetic domain only, i.e., the individual particles
are in a permanent state of saturation magnetization.
As a result, a strong long-range magnetostatic at-
traction exists between individual particles which
would inevitably lead to agglomeration of the parti-
cles and subsequent sedimentation unless a means of
achieving a repulsive interaction can be incorporated.
A repulsive mechanism (see Ferrofluids: Introduction)
can be achieved by coating the particles with a sur-
face-active material (surfactant) to produce an en-
tropic repulsion or by incorporating a charge on the
surface of the particles thereby producing an electro-
static repulsion. In the case of liquid–metal carriers,
no such interaction has yet been achieved to produce
a stable suspension.
The various methods by which small magnetic
particles can be prepared and subsequently colloidal-
ly dispersed is described in this article. The prepara-
tion of particles and ferrofluids has been the subject
of numerous patents and research publications. A
comprehensive bibliography of this information can
be found updated in each of Proceedings of the In-
ternational Conference on Magnetic Fluids (ICMF),
under the following authors Zahn and Shenton
(1980), Charles and Rosensweig (1983), Kamiyama
and Rosensweig (1987), Blums et al. (1990), Cabuil
et al. (1993), Bhatnagar and Rosensweig (1995). Re-
views of the subject have been given by Rosensweig
(1979, 1985, 1988), Charles and Popplewell (1980),
Martinet (1983) and Scholten (1978).
1. Preparation of Nano-sized Magnetic Particles
The preparation of nano-sized magnetic particles can
be conveniently considered under two headings; one
dealing with metal particles and the other with ferrites.
1.1 Metal Particles
There are a number of methods of producing small
magnetic metallic particles, the commonest of which
for ferrofluid preparation being the decomposition of
organometallic compounds. In addition, reduction of
solutions of metal salts and other miscellaneous
methods are available. The decomposition of organo-
metallic compounds will be discussed in most detail
as it is the easiest and most versatile method of pro-
ducing metal particles suitable for ferrofluids. In most
of these methods, the presence of a surfactant is cru-
cial. By selecting an appropriate surfactant, it has
been shown (Charles and Wells 1990) to be an effec-
tive method of controlling particle size.
There are two major advantages of using ferroflu-
ids based on metallic particles, such as cobalt and
iron particles. First, these metals have high saturation
magnetizations compared with ferrites and second,
they can be produced easily with very narrow size
distributions. However, there is also a major draw-
back which has restricted their use in most commer-
cial applications and that is their poor resistance to
oxidation and subsequent loss of magnetic properties.
Only by maintaining these fluids in an inert atmos-
phere can these fluids possess an extended lifetime.
(a) Decomposition of organometallic compounds
In the 1960s, several groups of workers (Thomas
1966, Hess and Parker 1966) reported the production
of colloidal-sized cobalt particles by the thermolysis
of dicobalt octa-carbonyl in hydrocarbon solutions
containing polymeric materials. The process is very
simple in that it only involves refluxing a solution of
the carbonyl, usually in toluene, until the reaction is
complete, with the formation of cobalt metal parti-
cles. Thomas (1966) showed that the particles possess
a very narrow size distribution ideal for the produc-
tion of stable colloids. The particles also possess an
f.c.c. structure instead of the b.c.c. structure of bulk
cobalt. The absence of a polymer in the thermolysis
leads to the formation of large particles (4100 nm).
A detailed investigation by Hess and Parker (1966) of
the presence of different copolymers of acrylonitrile-
styrene in the reaction solution showed that the
greater the concentration of polar groups along the
polymer chain, the smaller the size of the particles
formed.
It was Smith (1981) who proposed that the reaction
is polymer-catalyzed, which results in the formation
of a polymer–carbonyl complex which then decom-
poses to form elemental cobalt. Papirer et al. (1983)
showed that the decomposition could also be cataly-
zed by the presence of surfactants. They investigated
the evolution of carbon monoxide during the decom-
position in the presence of diethyl-2-hexyl sodium
sulphosuccinate (Manoxol-OT) and in its absence,
both experiments carried out as a function of tem-
perature. The results were consistent with the pres-
ence of ‘‘micro-reactors’’ and a diffusion mechanism
to control the growth. The higher the temperature
used, the smaller the particles produced. During the
208
Ferrofluids: Preparation and Physical Properties

process of thermolysis in toluene, the presence of
Co
4
(CO)
12
was identified as an intermediate.
However, the overall reaction mechanism is com-
plex but it seems likely that a carbonyl complex and/
or microreactors are formed which subsequently de-
compose to produce cobalt particles. Charles and
Wells (1990) investigated methods to control the size
of the cobalt particles formed by the use of a variety
of surfactants and combinations of surfactants. By
taking aliquots of the reaction mixture as the reaction
proceeded and making observations using electron
microscopy, they observed the presence of clusters or
microreactors for the first half hour of a four-hour
reaction but no particles. Subsequently, observations
showed the presence of particles only. They were
able to consistently produce particles with median
diameters in the range 5 to 15 nm. The median di-
ameter refers to a lognormal volume distribution (see
Sect. 1.3).
In many of the papers quoted so far, little infor-
mation has been presented concerning the saturation
magnetization of the particles compared to that of
the bulk. Charles and Wells (1990) found the satu-
ration magnetization of the particles to be consist-
ently lower (75%) than that of the bulk value of
17900 Gauss (1.79 T). This result is in agreement with
the work of Hess and Parker (1966). There are several
possible reasons for this, namely oxidation, spin-pin-
ning/canting, or poor crystallization. Experiments
where care had been taken to avoid oxidation still
showed the reduced saturation magnetization. How-
ever, evidence from x-ray diffraction indicated that
the particles were poorly crystallized or amorphous
with similar results being obtained for iron particles
produced by a similar process (Wonterghem et al.
1986). X-ray diffraction and Mo
¨
ssbauer studies indi-
cated the presence of carbon in the particles which is
thought responsible for the poorly crystallized state.
The particles, when annealed, showed an x-ray
pattern consistent with a-Fe and w-Fe
5
C
2
. Some re-
searchers, using transmission electron diffraction,
have reported that the particles produced by therm-
olysis have a crystalline structure. Whether this is a
true indication of the structure of the particles or that
annealing has taken place in the electron beam is not
known. Measurements of the magnetic anisotropy of
cobalt particles have been undertaken by several
groups of workers (Chantrell et al. 1980, Charles and
Wells 1990).
Iron particles of colloidal dimensions can also be
produced by thermolysis (Wonterghem et al. 1986); in
this case of iron pentacarbonyl in decahydronaph-
thalene. Particle size is again controlled by the pres-
ence of surfactants. The actual structure of the
particles is open to question. Some groups have re-
ported a crystalline structure (Kilner et al. 1984),
whilst others (Wonterghem et al. 1986) reported an
amorphous structure which undergoes crystallization
on annealing.
(Hoon et al. 1983) have reported the preparation of
colloidal nickel particles by the irradiation of nickel
carbonyl with ultraviolet light and by the reduction
of nickelocene. The particles were reported to have an
f.c.c. structure from transmission electron diffraction.
Nakatani and Furubayashi (1990) and Nakatani
et al. (1993) have prepared iron nitride particles by
two methods. First, by a plasma CVD reaction of
iron pentacarbonyl and nitrogen gas and second, by
heating a solution of iron pentacarbonyl in kerosene
containing a surfactant through which a constant
stream of ammonia is passed. The crystal structure
observed is e-Fe
3
N.
It is also possible to prepare alloy particles of the
transition metals by the thermolysis of mixed-metal
organometallic compounds in the presence of surfact-
ants. Lambrick et al. (1985, 1987) prepared organo-
metallic compounds from which particles of Fe–Co
and Ni–Fe alloys with median diameters B8 nm were
obtained. The saturation magnetizations of the par-
ticles are 84% of that of the bulk values.
(b) Reduction of metal salts in aqueous solution
Much of the work on the formation of particles by
the reduction of metal salts in aqueous solution using
reducing agents such as sodium hypophosphite or
sodium borohydride stemmed from an interest in
producing particles suitable for magnetic recording
media. The particles produced were typically of
micron size, which precluded their use in ferrofluids
because of their large magnetic moments and conse-
quent strong magnetostatic interactions.
However, there have been a few reports of the for-
mation of particles of nanometer size which could be
used in ferrofluids. The solutions used in the prepa-
ration contain the salts of ferromagnetic elements, a
reducing agent, complexing agents, and buffers to
control the pH during reaction. The formation of
particles is induced by the addition of PdCl
2
to
the solution. By the introduction of water-soluble
viscosity-increasing materials, it has been shown
(Akashi and Fujyama 1969) that particle size can
be reduced so that the particle can remain in stable
suspension. There have been a few other reports of
the formation of such particles. Harada et al. (1972)
have reported the formation of Co-P particles, using
sodium hypophosphite as reducing agent, in aqueous
solutions containing protein or synthetic polypep-
tides. Similar studies on Fe-P particles have also been
undertaken.
The at.% of phosphorus or boron in the particles is
very dependent on the conditions used. Above a crit-
ical concentration of boron, phosphorus, and indeed
carbon, the particles possess an amorphous structure
very similar to structures produced by rapid-quench
techniques. The incorporation of phosphorus, boron,
and carbon is a disadvantage in one respect, in that
the saturation magnetization of the particles may be
209
Ferrofluids: Preparation and Physical Properties
