Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.


1. Basic Biomo lecule Labeling and Detection with
Magnetic Par ticles
As for many other biomolecular labels, magnetic
labeling can be easily accomplished using specific lig-
and–receptor interactions, as illustrated generically in
Fig. 1. The illustrated ‘‘sandwich’’ configuration pro-
ceeds as follows:
(i) Receptor molecules specific for the target bio-
molecules are attached to the surface of a solid subst-
rate. Often arrays of different probe spots are used to
simultaneously detect multiple targets.
(ii) When target molecules are present in a sample
solution, they are captured by the surface.
(iii) Magnetic particles coated with a second set of
receptor molecules for the target are introduced,
labeling the previously captured targets.
(iv) The label particles are detected by a magnetic
sensor.
Sandwich assays are commonly performed using
antibody–antigen pairs and complementary DNA
strands, and often use the strongly binding biomo-
lecular combination of streptavidin and biotin to
attach the particle labels to the receptor molecules.
For biosensing applications, the magnetic particles
used as labels should be paramagnetic, i.e., only have
a magnetic moment when a magnetic field is applied.
The absence of a permanent magnetic moment pre-
vents agglomeration of the particles in solution which
would render them ineffective as labels. When select-
ing label particles, properties of importance include
the size and size uniformity, the type of magnetic
material and its volume fraction within the particle,
and the particle surface chemistry. For example,
though nanometer-scale particles are matched in size
to biological macromolecules such as DNA, the small
magnetic fields they generate are difficult to detect.
Second, the surface of the particles must be suitable
for chemical attachment of biomolecules. A variety of
paramagnetic beads with biocompatible polymer or
silica shells are commercially available with diameters
that range from 1 mmto5mm.
Because magnetic particles experience a force in the
presence of a magnetic field gradient, they may be
manipulated by magnets. This phenomenon can be
used to apply a controlled force that selectively pulls
off only those labels not bound to the surface by
specific binding (Lee et al. 2000). Such a force dis-
crimination assay increases the sensitivity of detec-
tion by greatly reducing the background signal and
thereby permitting very low signal levels to be de-
tected with confidence.
2. Magnetic Par ticle Detection
The detection of magnetic particles can be accom-
plished by a number of methods, as mentioned above.
Here we will focus on the use of solid-state magnetic
field sensors embedded in the substrate, a method
that illustrates many of the advantages of magnetic
labeling for biomolecule detection. For example, such
an approach has the potential to eliminate the re-
quirement for an external detection system, thereby
reducing the size, cost, and power of the sensor
system.
One of the simplest solid-state magnetic field sen-
sors to incorporate into a microelectronic substrate is
a giant magnetoresistive (GMR) wire (Baselt et al.
1998). GMR materials are multi-component thin
films with alternating magnetic and nonmagnetic lay-
ers (see Giant Magnetoresistance). The relative orien-
tation of the magnetic moments of these layers is
altered in the presence of a magnetic field, changing
the electrical resistance of the wire. When a magnetic
particle is present above a GMR sensor, the resist-
ance of the sensor changes; the larger the number of
particles present, the larger the change. The availa-
bility of suitably sensitive GMR sensors was made
possible by recent advances in magnetic materials for
high-density data storage, including magnetic disk
drive read-out sensors and nonvolatile magnetic
memory elements (Prinz 1998).
When paramagnetic microbeads are used, such as
those commercially available, GMR sensors have
been designed that are only sensitive to a planar
component of the dipolar magnetic field induced in
the particles, as illustrated in Fig. 2. As discussed by
Miller et al. (2001), when an external magnetic field is
applied normal to the plane of the GMR sensor, the
field strength at the sensor is given by
B B m=z
3
ð1Þ
Figure 1
Generic illustration of magnetic labeling of targets
captured onto a solid substrate in a ‘‘sandwich’’
configuration using specific biomolecular ligand–
receptor recognition.
120
DNA Microarrays using Magnetic Labeling and Detection
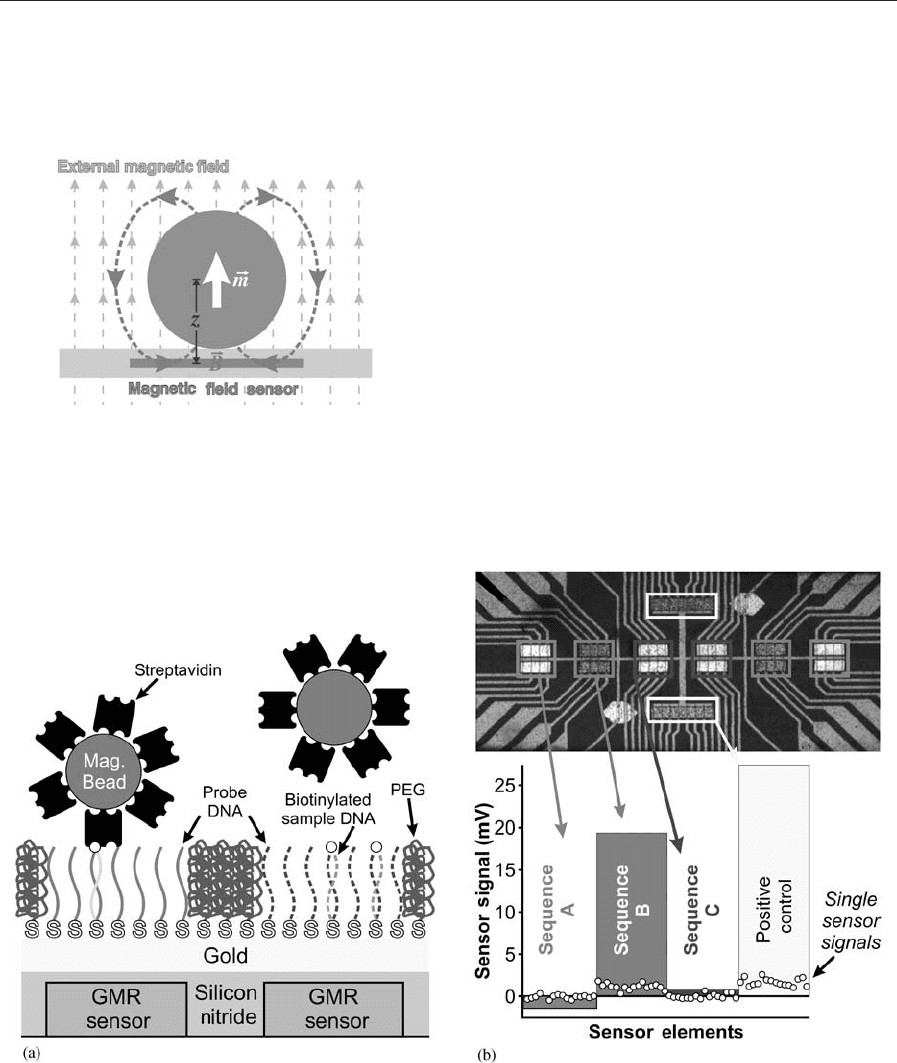
where m is the magnetic moment of the bead and z is
the distance from the center of the bead to the sen-
sor.Therefore, the resistance change in the sensing
wire is roughly proportional to the number of mag-
netic particles present above it, a signal that can be
easily measured. For maximum signal, the particle
should have as large a magnetization as possible and
be as close to the sensor as possible.
3. Detection with a GMR Sensor Microarray
A well-developed system that uses a GMR microarray
to detect different DNA sequences in a sample is the
bead array counter (BARC) sensor (Baselt et al. 1998,
Edelstein et al. 2000, Miller et al. 2001). In this system,
the test is performed inside a small, quartz flow cell
mounted over the sensor chip. The chip itself is wire
bonded to a printed circuit board housed in a dis-
posable plastic cartridge that contains the required
liquid reagents. The cartridge plugs into an automated
electronic controller and connects to a miniature
pumping system (Tamanaha et al. 2002).
Before assembly, the surface of the sensor chip is
coated with a biocompatible polymer film (polyeth-
ylene glycol (PEG)) and an array of single-stranded
DNA capture probes (Fig. 3(a)). Note that the GMR
sensors and associated interconnects must be pro-
tected from the reactive and conductive salt solution
used in the biochemical test, in this case by a film of
Figure 2
Schematic of the magnetic field induced around a
paramagnetic microbead on top of a magnetic field
sensor when an external field is applied normal to the
substrate.
Figure 3
(a) An illustration of the BARC biosensor approach. Note that the elements are not to scale; in particular, the beads
and sensors are much larger in proportion to the biomolecules. (b) A micrograph of a BARC chip after a DNA test,
with the eight sensing zones outlined. For each of the probe zones, the graph shows the measured signals from the
individual sensing elements (open circles) and the integrated signal for all 16 elements (colored bar). The assay for
sequence ‘‘B’’ was performed with 10 nM DNA hybridized for 30 min. The total test required about B60 min.
121
DNA Microarrays using Magnetic Labeling and Detection

silicon nitride. The chip is also coated with a thin
gold layer so that the DNA probes and PEG can be
attached to the surface by strong gold–sulfur bonds.
Each sensor zone is covered with strands of DNA
complementary for a different target strand. The
PEG film inhibits undesirable, nonspecific, sticking of
target DNA and magnetic particles in the areas out-
side of the DNA probe spots.
To test for the presence of specific target DNA
sequences, the sample is introduced into the flow cell
and allowed to hybridize with the capture probes. In
the illustrated example, the target strands have been
pre-modified to have biotin molecules attached at one
end. Therefore, the presence of target in the sample
will result in double-stranded, biotin-tagged DNA
bound above a sensor. The captured targets are then
labeled with streptavidin-coated magnetic micro-
beads. Beads just resting on the surface can be re-
moved by a magnetic force, and the remaining beads
detected by the underlying GMR sensors.
The results of an actual experiment performed in
this way are shown in Fig. 3(b) on a chip with eight
sensor zones (Miller et al. 2001). A micrograph shows
the surface of the chip after the test. In this test, each
of the three different DNA sequences and the positive
control (a probe with biotin already on it) were each
applied to two zones. Introducing a sample containing
only sequence ‘‘B’’ results in the complementary zones
and the positive control being covered with beads
(appearing dark). The presence of the beads is accu-
rately detected by the eight GMR sensors in each zone.
4. Summary
Magnetic labeling and detection holds great promise
for sensing biomolecules. As demonstrated by the
BARC system, advances in microelectronics and ma-
terials can be adapted to analytical applications in the
biosciences with great success. Magnetic particles,
magnetic force manipulation, and magnetoelectronic
detection can be combined to create relatively simple,
compact, sensor systems. Such technology promises
to fulfill the need for ever faster, more sensitive, and
more portable systems in fields as diverse as home-
land defense, clinical diagnostics, genomics, pro-
teomics, and forensics.
See also: SQUIDs: Biomedical Applications
Bibliography
Baselt D R, Lee G U, Hansen K M, Chrisey L A, Colton R J
1997 A high-sensitivity micromachined biosensor. Proc.
IEEE 85, 672–80
Baselt D R, Lee G U, Natesan M, Metzger S W, Sheehan P E,
Colton R J 1998 A biosensor based on magnetoresistance
technology. Biosens. Bioelectron. 13, 731–9
Besse P-A, Boero G, Demierre M, Pott V, Popovic R 2002
Detection of a single magnetic microbead using a miniatur-
ized silicon hall sensor. Appl. Phys. Lett. 80, 4199–201
Edelstein R L, Tamanaha C R, Sheehan P E, Miller M M,
Baselt D R, Whitman L J, Colton R J 2000 The BARC
biosensor applied to the detection of biological warfare
agents. Biosens. Bioelectron. 14, 805–13
Ko
¨
titz R, Matz H, Trahms L, Koch H, Weitschies W,
Rheinla
¨
nder T, Semmler W, Bunte T 1997 SQUID based
remanence measurements for immunoassays. IEEE Trans.
Appl. Supercond. 7, 3678–81
Kriz C B, Ra
˚
devik K, Kriz D 1996 Magnetic permeability
measurements in bioanalysis and biosensors. Anal. Chem. 68,
1966–70
Lee G U, Metzger S, Natesan M, Yanavich C, Dufreˆ ne Y F
2000 Implementation of force differentiation in the immuno-
assay. Anal. Biochem. 287, 261–71
Miller M M, Sheehan P E, Edelstein R L, Tamanaha C R,
Zhong L, Bounnak S, Whitman L J, Colton R J 2001 A
DNA array sensor utilizing magnetic microbeads and
magnetoelectronic detection. J. Magn. Magn. Mater. 225,
138–44
Pearson J E, Gill A, Vadgama P 2000 Analytical aspects of
biosensors. Ann. Clin. Biochem. 37, 119–45
Prinz G 1998 Magnetoelectronics. Science 282, 1660–3
Richardson J, Hawkins P, Luxton R 2001 The use of coated
paramagnetic particles as a physical label in a magneto-
immunoassay. Biosens. Bioelectron. 16, 989–93
Tamanaha C R, Whitman L J, Colton R J 2002 Hybrid macro–
micro fluidics system for a chip-based biosensor. J. Micro-
mech. Microeng. 12, N7–N17
Vo-Dinh T, Cullum B 2000 Biosensors and biochips: advances
in biological and medical diagnostics. Fresenius J. Anal.
Chem. 366, 540–51
Cy. R. Tamanaha
Geo-Centers, Washington, DC, USA
Lloyd J. Whitman
Naval Research Laboratory, Washington, DC, USA
122
DNA Microarrays using Magnetic Labeling and Detection
Electrodynamics of Superconductors:
Flux Properties
The ‘‘mixed state’’ of type II superconductors occurs
when magnetic flux penetrates the material in the
form of flux lines (vortices) without destroying the
superconducting ground state (Tinkham 1996).
Above the field of first flux penetration, H
p
, the re-
pelling flux lines spontaneously order into a triangu-
lar (Abrikosov) lattice with sixfold symmetry. The
state of zero resistivity is destroyed by vortex motion,
which, however, is retained up to a critical current
density, J
c
, when the flux lines are pinned by crystal-
line defects. Vortex motion can also be driven by
thermal fluctuations. When they are sufficiently large,
the vortex lattice melts to a vortex liquid and the
superconducting ground state is destroyed.
1. Flux Line Lattice
Thermodynamically, a flux line is stable above the
lower critical field, H
c1
(Abrikosov 1957). The flux
line (or vortex) consists of a ‘‘normal’’ core in which
the density of Cooper pairs decreases to zero over a
length scale x(T), the Ginzburg–Landau (GL) coher-
ence length. Around the core superconducting screen-
ing currents circulate, which generate a local
magnetic field of about 2 m
0
H
c1
, where m
0
is the mag-
netic permeability of vacuum. Away from the core
both the field and the screening currents decay over a
length scale l(T), the GL penetration depth. The line
energy of a flux line per unit length is given by
e ¼ e
0
ln k ðF
2
0
=4pm
0
l
2
Þ ln k ð1Þ
where F
0
is the flux quantum, which is also the mag-
netic flux carried by a flux line. The GL parameter k
is the ratio l/x.
Usually, the first penetration of flux occurs at a
field H
p
, which is considerably different from H
c1
,
because the flux line has to enter through the surface
or across the edges of the superconductor. This
means that H
p
is determined by surface and geomet-
rical barriers which depend on sample shape and field
orientation.
Well above H
p
the flux lines form a triangular
Abrikosov lattice with lattice parameter a
D
¼(2/
ffiffiffi
3
p
)
1/2
a
0
, where a
0
¼(F
0
/B) and B is the flux density. Be-
cause of the electromagnetic origin, the range of the
vortex interactions is l, which is generally much larg-
er than a
0
. Elastic deformations of the Abrikosov
lattice are described by elasticity moduli for uniaxial
compression (c
11
), for tilt deformations (c
44
), and for
shear deformations (c
66
). Both c
11
and c
44
strongly
depend on the wavelength of the deformation fields.
Especially for small wavelengths the effect of the de-
formations is considerably reduced by averaging over
the vortex interaction range l, i.e., the flux line lattice
is very soft on scales smaller than l.
For very anisotropic materials, such as high-tem-
perature superconductors, the flux lines caused by a
magnetic field perpendicular to the CuO
2
planes
break up into pancake vortices located in the super-
conducting layers (Blatter et al. 1994). The tilt mod-
ulus is now reduced by a factor equal to the
anisotropy parameter, G, which can be as large as
10
5
. Above a crossover field B
cr
¼F
0
/Gs
2
(s is the in-
terlayer distance) the tilt deformations become irrel-
evant and a quasi two-dimensional interaction results
in which only compression and shear modes between
pancake vortices in the same layer survive. When the
magnetic field is parallel to the CuO
2
plane it pen-
etrates in the form of Josephson vortices (Bulaevskii
1973), which possess no normal core. For arbitrary
field directions crossing lattices of Abrikosov pan-
cake vortex stacks and Josephson vortices may
coexist (Koshelev 1999).
2. Thermodynamics
Usually the mixed state is represented by the mag-
netization curve M(H) which in principle consists of
five regimes: up to H
c1
in the Meissner state M ¼H,
just above H
c1
it drops exponentially, for H
c1
o
HoH
c2
it is characterized by Mpln(H
c2
/H), near
H
c2
it is linearly proportional to H
c2
H, and above
H
c2
M ¼0. Because of surface and geometrical effects
there may occur deviations from this ideal behavior.
In this picture it was believed that the phase transi-
tion to the superconducting state takes place at the
upper critical field, H
c2
(T). It is now generally ac-
cepted that the real thermodynamic phase transition
occurs at the melting line, H
m
(T). In pure single
crystals of high-temperature superconductors the
melting transition is of first order. It manifests itself
by a jump in B, the flux liquid is slightly denser than
the solid (Zeldov et al. 1995). In the presence of
quenched disorder the transition becomes of second
order.
Melting occurs when thermal fluctuations generate
elastic modes in the lattice that are strong enough to
destroy its shear strength (Nelson 1988). In a three-
dimensional lattice both shear and tilt deformations
determine the mean square displacement /u
2
S
1/2
.
According to the Lindemann criterion the lattice melts
when this quantity is a significant fraction of the lattice
parameter, i.e., /u
2
S
1/2
¼c
L
a
0
,wherec
L
is the Linde-
mann number (c
L
¼0.1–0.3). This criterion gives a
good description of the melting line. For thin films in a
perpendicular field the tilt modes of the flux lines do
E
123

not contribute to the thermal disorder and the system
should be considered as a two-dimensional solid con-
sisting of rigid flux bars. Thermal fluctuations in two
dimensions give rise to the formation of edge disloca-
tion pairs, which unbind at a second-order melting line
in accord with the Kosterlitz–Thouless theory for two-
dimensional melting.
In very anisotropic materials the low-temperature
phase consists of a solid of flux lines (stacked pancake
vortices) which at the first-order transition line decays
to a plasma of decoupled pancake vortices. In all
situations the state of true superconductivity is
destroyed at the melting line and ohmic behavior is
recovered.
3. Flux Pinning and the Critical Current
A moving flux line lattice (FLL) dissipates energy.
When this motion is driven by a transport current
with density J, a steady state with velocity v is
reached when the driving force, JF
0
, on each vortex is
compensated by a frictional force Zv. An illustrative
model for the friction coefficient, Z, is that of Bardeen
and Stephen in which the vortex core is treated as a
moving cylinder of normal metal. The ratio between
flux flow resistivity, r
f
, and normal state resistivity,
r
n
, is then simply represented by the normal fraction,
i.e., B/B
c2
, which leads to Z ¼F
2
0
r
1
n
/2px
2
.
A moving FLL should thus be considered as a
normal metal with a (much) smaller resistivity r
f
.
Obviously, for applications, the flux flow state should
be suppressed. In practice, most materials can be
made with a high density of inhomogeneities acting
as pinning centers which trap the flux lines up to a
critical force J
c
F
0
, where J
c
is the critical current
density. Defects deviate from the background mate-
rial by a different local density, elasticity, electron–
phonon coupling, electron mean free path P, etc. The
first three properties give rise to a local change in T
c
,
whereas the latter leads to a variation in P. Conse-
quently, one may distinguish between dT
c
pinning
and dP pinning, each with its characteristic temper-
ature and field dependence (Kes 1991).
The collective action of the pinning centers breaks
up the FLL in domains of average size L
c
parallel to
the field and transverse size R
c
perpendicular to the
field. Its volume is V
c
ER
c
2
L
c
. The critical current
density follows from the statistical average over dis-
tributions of pinning centers with pinning strength
W ¼n
p
/f
2
S, where n
p
is the concentration of pins
and /f
2
S the squared average of the pinning force of
a single defect. This leads to the well-known collective
pinning relationship (Larkin and Ovchinnikov 1979)
J
c
BEðW=V
c
Þ
1=2
ð2Þ
In the original theory the domain sizes were deter-
mined by the balance of pinning energy and the
energy of the elastic deformations in the FLL. How-
ever, when the pinning is strong, the local strains in
the FLL may grow beyond the elastic limit so that
topological defects such as edge dislocations are cre-
ated. These now determine the positional disorder in
the FLL and the domain size V
c
. Optimal pinning
occurs when the transverse order is totally destroyed,
i.e., R
c
Ea
0
, and individual flux line segments of
length L
c
make up the average domain size. In an-
isotropic high-temperature superconductors this
length can be as small as the height, s, of a single
pancake vortex. The transition from elastic to plastic
disorder may be seen as a peak effect in J
c
. It usually
happens near H
c2
or near the melting line. Interesting
finite size effects may occur in thin films in a perpen-
dicular field leading to two-dimensional collective
pinning.
When the pinning is weak, as is the case in amor-
phous thin films, L
c
can be larger than the film thick-
ness. Now only shear deformations are relevant,
which makes the analysis conveniently transparent
(Kes and Tsuei 1983).
4. Thermal Effects, Depinning, and Creep
The FLL in the presence of pinning centers may be
seen as an elastic medium in a disorder potential with
each domain sitting in a local potential minimum.
With increasing temperature thermal fluctuations will
give rise to positional fluctuations of the domains and
thermal smearing of the potential roughness. When
the mean square displacement is of the order of the
range, r
f
, of the disorder potential, this effect (called
thermal depinning) is large enough to cause a con-
siderable decrease of the pinning force and of J
c
. The
corresponding depinning or irreversibility line in the
temperature–field diagram separates a strong pinning
regime at low temperatures from a weak pinning re-
gime at high temperatures.
Thermal fluctuations also make the FLL domains
jump over energy barriers between different potential
minimums of almost equal energy. In the presence of
a driving force this process leads to a unidirectional
creep of the FLL with an average velocity Bexp
(U
p
/k
B
T), where U
p
is the typical height of the
energy barrier and k
B
is the Boltzmann constant. For
relatively small values, U
p
/k
B
Tp10, one thus gets
linear resistivity at small J, called thermally assisted
flux flow (TAFF):
r
TAFF
¼ r
f
expðU
p
=k
B
TÞð3Þ
Although r
TAFF
may be extremely small, TAFF
does not describe a true superconducting state with
zero resistance at small J, but rather a normal metal
with ohmic resistance. The reason is that U
p
is sup-
posed to be finite and independent of J. It has been
shown, however, that for collective creep of an elastic
124
Electrodynamics of Superconductors: Flux Properties

medium in random disorder the domain size, the dis-
tance between available metastable states, and the
corresponding energy barriers should grow when J
decreases in order to maintain the balance between
deformation energy and work done by the driving
force. In fact, U
p
B(J
c
/J)
m
with m of order one, which
leads to true zero resistance for J-0, according to
rBr
f
exp[c(J
c
/J)
m
]. A similar result is obtained in
the vortex glass model where the flux flow is supposed
to be triggered by the motion of topological defects in
the FLL. It shows once more that the mixed state
with strong pinning not only is a very practical phe-
nomenon, but that it is a rich area of research for
solid-state and statistical physics.
See also: Electrodynamics of Superconductors:
Weakly Coupled
Bibliography
Abrikosov A A 1957 On the magnetic properties of supercon-
ductors of the second group. Sov. Phys. JETP 5, 1174
Blatter G, Feigelman V M, Geshkenbein V B, Larkin A I,
Vinokur V M 1994 Vortices in high-temperature super-
conductors. Rev. Mod. Phys. 66, 1125–388
Bulaevskii L N 1973 Magnetic properties of layered super-
conductors with weak interaction. Sov. Phys. JETP 37, 1133
Kes P H 1991 In: Cahn R W, Haasen P, Kramer E J (eds.)
Materials Science and Technology. VCH, New York, Vol. 3A,
Part I
Kes P H, Tsuei C C 1983 Two-dimensional collective flux pin-
ning, defects and structural relaxation in amorphous super-
conducting films. Phys. Rev. B 28, 5126–39
Koshelev A E 1999 Crossing lattices, vortex chains, and angular
dependence of melting line in layered superconductors. Phys.
Rev. Lett. 83, 187–90
Larkin A I, Ovchinnikov Yu N 1979 Pinning in type II super-
conductors. J. Low Temp. Phys. 34, 409
Nelson D 1988 Vortex entanglement in high-T
c
superconduc-
tors. Phys. Rev. Lett. 60, 1973–6
Tinkham M 1996 Introduction to Superconductivity. McGraw-
Hill, New York, 2nd edn
Zeldov E, Majer D, Konczykowski M, Geshkenbein V B,
Vinokur V M, Shtrikman H 1995 Thermodynamic observa-
tion of first-order vortex lattice melting in Bi
2
Sr
2
CaCu
2
O
8
.
Nature 375, 373–6
P. H. Kes
Leiden University, The Netherlands
Electrodynamics of Superconductors:
Weakly Coupled
Systems consisting of two superconducting samples
connected by a weak electrical contact possess several
unusual properties. First, the net current flowing
across the contact—the Josephson junction (see
Josephson Junctions: Low-T
c
; Josephson Junctions:
High-T
c
)—contains a supercurrent I
S
.IfI
S
is smaller
than the critical current of a junction, I
C
, then its flow
does not lead to a voltage drop across the structure.
The supercurrent depends on the phase difference
j ¼w
1
w
2
of the superconductor order parameters
D
1
exp(iw
1
) and D
2
exp(iw
2
) of the samples (stationary
Josephson effect). This function is exactly 2p periodic
and, in the simplest cases, is sinusoidal:
I
S
ðjÞ¼I
C
sinðjÞð1Þ
The critical current, I
C
, is a constant determined by
the transport parameters of the contact material and
the shape of the Josephson junction. At I
S
4I
C
there
is a voltage drop, V, across the junction, which is
related to j by the fundamental law (nonstationary
Josephson effect)
dj
dt
¼
2e
_
V ð2Þ
where e is the electron charge and _ is Planck’s
constant.
The physical origin of the stationary Josephson ef-
fect lies in the specific form of electron reflection from
a superconducting interface. Electrons with energy e
smaller than the superconducting gap, O, of the elec-
trodes are trapped in a contact material. They cannot
penetrate into an electrode since there is a gap in the
density of states near the Fermi level of a supercon-
ductor. However, if the energy e of an electron is in
the interval 0pepO, its interaction with an electrode
results in the creation, with a probability equal to
one, of an extra pair of correlated electrons (Cooper
pair) in the superconductor and a hole with energy e
in the contact material. The latter moves in the
direction opposite to that of the initial electron (so-
called Andreev reflection). This hole, in turn, inter-
acts with another electrode and causes annihilation of
a pair of correlated electrons and the creation of an
electron with energy e, which moves in the same
direction as that of the initial particle. As a result of
this cycle, a pair of correlated electrons transfers from
one superconductor to another leading to a super-
current flow across a junction, i.e., to the stationary
Josephson effect.
The nonstationary Josephson effect is a direct con-
sequence of the Schro
¨
dinger equation. In the station-
ary state the wave function of correlated electrons has
the form c(r, t ) ¼c(r)exp(iEt/_), and the Schro
¨
dinger
equation for the phase of the wave function reduces
to _@w
1,2
/@t ¼E
1,2
, where E
1,2
is the energy of the
superconducting condensate in the samples. Thus
_@j/@t ¼E
2
E
1
and this latter difference should be
equal to the difference E
F2
E
F1
of the electrochem-
ical potentials E
F2
and E
F1
across the junction, which
is exactly equal to 2 eV.
125
Electrodynamics of Superconductors: Weakly Coupled

1. Current Components
In the general case, the net current, I, across a Joseph-
son junction has several components in addition to
I
S
(j):
I ¼ I
S
ðjÞþI
N
ðVÞþI
D
ðVÞþI
F
ðtÞð3Þ
where I
N
is the normal (quasiparticle) current, I
D
the
displacement current, and I
F
the fluctuation current.
1.1 Supercurrent
In accordance with Eqn. (1) a d.c. supercurrent in the
range
I
C
pI
S
ðjÞpI
C
ð4Þ
can flow across the junction as a result of a constant
phase difference j. An external system supporting
this phase difference represents work on the super-
current and, thus, stores potential energy in the junc-
tion given by
Uðj Þ¼E
C
½1 cosðjÞ þ const; E
C
¼
_I
C
2e
ð5Þ
Since the energy is conserved in the form of persistent
current, the Josephson junction can be considered as
having a nonlinear inductance
L
1
S
¼ L
1
C
cosðjÞ; L
C
¼
_
2eI
C
ð6Þ
This means that for weak signals the supercurrent is
equivalent to an inductance L
S
dependent on j(t) and
on the critical current of the junction.
The most impressive property of a supercurrent is
its ability to oscillate (Josephson oscillations) with a
frequency
f
J
¼
o
J
2p
¼
2e
h
%
V;
2e
h
E483 MHzmV
1
ð7Þ
if a nonzero d.c. voltage,
%
V, is fixed across the junc-
tion. At typical voltages (10
6
–10
2
V) the oscillation
frequency, f
S
, is of the order of 10
9
–10
13
Hz.
1.2 Normal Current
The scale of the normal current is given by the nor-
mal conductance of a junction G
N
. Independent of
transport properties of the contact material at volt-
ages larger than (D
1
þD
2
)/e, the I
N
(V) dependence of
the junction is close to Ohmic at all temperatures:
I
N
¼ G
S
V þ dI ð8Þ
The voltage-independent factor dI in Eqn. (8) is
the so-called ‘‘excess’’ (dIp(D
1
þD
2
)) or ‘‘deficit’’
(dIp(D
1
þD
2
)) current observed if the contact
material has a metallic type of conductivity (SNS
weak links). In structures with transparent interfaces
between the contact material and superconducting
samples there is excess current on I
N
(V). Andreev
reflection of electrons is responsible for this effect.
Owing to a gap in the density of states, an electron
with energy e in the interval
E
F2
þ eV O
2
pepE
F2
þ eV þ O
2
ð9Þ
cannot penetrate into the superconductor as an elec-
tron, as in the normal case. Instead, in accordance
with the Andreev process, it creates a pair of corre-
lated electrons and a hole in the contact material.
Thus, in this energy interval, (as in the normal case)
two electrons instead of one are injected into the
electrode leading to an ‘‘excess’’ current.
In structures with small transparent interfaces be-
tween the contact material and superconducting sam-
ples (SINIS double barrier structures) there is a
current ‘‘deficit’’ on I
N
(V). The probability of And-
reev reflection for an electron with energy defined by
Eqn. (9) is proportional to D
2
, where D51 holds for
the transparency of an interface. Thus, in contrast to
the normal case, there is no electron injection into the
superconductor if the electron energy is in the range
given by Eqn. (9) and, therefore, a current deficit
should be observed on I
N
(V).
In junctions with tunneling-type conductivity of
the contact region (SIS tunnel Josephson junction),
dI ¼0.
1.3 Displacement Current
For most practical Josephson junctions the displace-
ment current is defined by
I
D
¼ CV ¼ C
dV
dt
ð10Þ
where the junction capacitance, C, has the same value
as in the normal state and depends on both the junc-
tion type and its size. The effect of the capacitance on
the processes in the junction is characterized by the
dimensionless parameter
b ¼
2e
_
I
C
R
2
N
C ð11Þ
introduced by McCamber (1968) and Steward (1968).
It compares the normal and displacement currents.
Junctions with b51 have small capacitance or high
damping (I
N
bI
D
), and junctions with bb1 have large
capacitance or low damping (I
N
5I
D
).
1.4 Fluctuation Current
The necessity of taking fluctuations (‘‘noise’’) into
account often arises in an analysis of the sensitivity of
126
Electrodynamics of Superconductors: Weakly Coupled
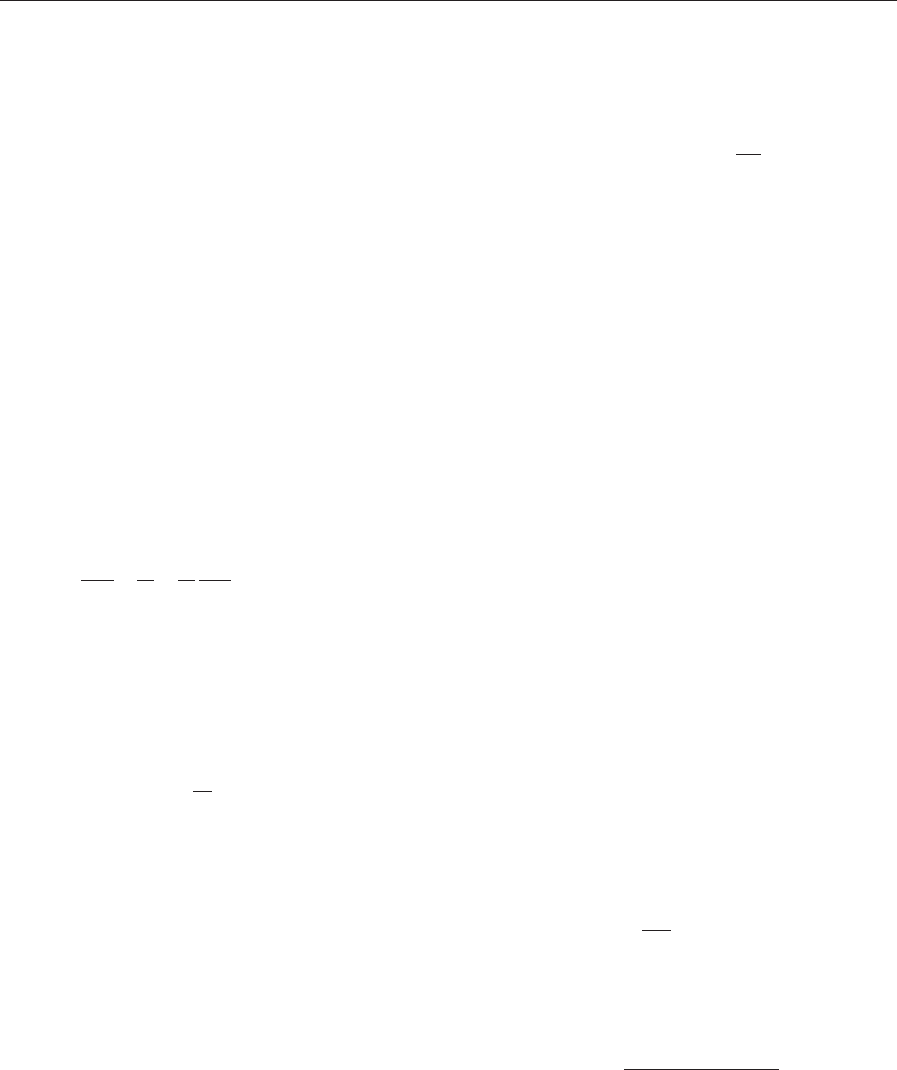
superconducting devices. In most cases this is carried
out by including some fluctuation current, I
F
(t), in
the whole current across the junction. There are sev-
eral sources of current fluctuation. These are external
noise sources, 1/f noise, thermal noise, and shot noise.
Usually, in experimental setups the intensity of an
external noise is effectively suppressed to a level that
is negligibly small compared to that provided by
other sources.
The cutoff frequency of 1/f noise is relatively low.
In a low-temperature Josephson junction it scales
from 1 Hz to 10 Hz, while in high-temperature junc-
tions it is in the range 100–1000 Hz. This means that
at typical Josephson frequencies oEo
J
the net effect
of this noise is very small. Nevertheless, in some
practical devices (e.g., see SQUIDs: Biomedical Appli-
cations) the useful signal is contaminated with 1/f
noise and special techniques such as signal or bias
modulation are used for suppression of this noise
component.
Thermal noise and shot noise are two types of
classical fluctuations which exist since there is a di-
ssipative current, I
N
, across a junction. The relative
intensity of the thermal fluctuations can be charac-
terized by the ratio of the thermal energy, k
B
T, to the
Josephson energy, E
C
:
g ¼
k
B
T
E
C
¼
I
T
I
C
¼
2e
_
k
B
T
I
C
; I
T
½mAE0:0417½Kð12Þ
Thus, if the critical current of the junction is much
larger than I
T
the influence of thermal fluctuations
should be small.
Shot noise is important if the voltage across the
junction is large compared to _o/e and k
B
T/e and can
be characterized by the spectral density
S
I
ðoÞ¼
1
2p
eI
N
¼ const ð13Þ
Equations (2) and (3) form the system of the basic
equations for the Josephson junction. The concrete
expressions for the current components in Eqn. (3)
depend on the type of conductivity of the contact
material and the geometry of the junction. The prob-
lem of finding the relationship between the current
and voltage was solved in solid-state theory (We-
rthamer 1966, Larkin and Ovchinnikov 1966) only
for structures with tunnel-type conductivity of the
contact material. For this reason, even now the ma-
jority of dynamics problems in systems with Joseph-
son junctions are solved in the framework of
phenomenological models, which provide approxi-
mations of the real properties of various junctions.
The resistively shunted junction (RSJ) model intro-
duced by McCamber (1968) and Steward (1968) is the
simplest and the most widely used tool for studying
the processes in junctions.
2. RSJ Model
The RSJ model is based on Eqns. (1) and (8) with
dI ¼0, on Eqn. (10) for the current components in
Eqn. (3)
I ¼ I
C
sinðjÞþG
N
V þ C
dV
dt
þ I
F
ðtÞð14Þ
and on the Josephson relationship (Eqn. (2)).
Quantitatively this model is valid for SIS and SNS
Josephson junctions only in a narrow temperature
interval in the vicinity of the critical temperature of
the superconductor. At lower temperatures G
N
be-
comes a nonlinear function of voltage and often the
I
S
(j) relationship is no longer sinusoidal.
Nevertheless, this model is widely used for describ-
ing the processes in Josephson junctions of any type,
if they are shunted by a conductance G
e
bG
N
.A
shunted junction can be considered as a new Joseph-
son contact with linear normal conductance, G
e
,
which should replace G
N
in Eqn. (14). A real form of
the I
S
(j) relationship can be taken into account by
representing a junction as a junction with
I
S
(j) ¼I
C
sin(j) and effective inductance connected
in series (Zubkov et al. 1981).
The RSJ model is of great importance, since it
provides the possibility of studying the processes in
a variety of devices employing Josephson junctions
without having the exact solutions of solid-state
physics problems for the current components in
Eqn. (3).
There are two experimental tests that are generally
used to verify how close the behavior of real Joseph-
son contacts are to the predictions of the RSJ model.
These are the specific reactions of the structure to
external magnetic and microwave fields.
3. Josephson Junctions in a Magnetic Field
In an external magnetic field, B, the phase difference,
j, across a junction depends on a space coordinate,
x, along the junction and is a solution of the equation
l
2
J
d
2
j
dx
2
¼ Jðx; tÞð15Þ
where J is the local value of a current (Eqn. (3))
through the junction and l
J
is the Josephson pene-
tration depth given by
l
2
J
¼
F
0
2pm
0
ðd þ l
1
þ l
2
ÞJ
C
ð16Þ
where d is the distance between the superconducting
samples, F
0
the magnetic flux quantum, m
0
the vac-
uum magnetic constant, and l
1,2
the London pene-
tration depths of the superconducting samples.
127
Electrodynamics of Superconductors: Weakly Coupled

Solution of Eqns. (14) and (15) essentially depends
on the relationship between the length of the junc-
tion, L, and l
J
.
In a long Josephson junction with Lbl
J
the mag-
netic field penetrates inside the structure in the form
of a Josephson vortex. This is a region with a length
of the order of pl
J
, filled by magnetic flux lines, which
are screened by the supercurrent, J, circulating
around:
B ¼
7F
0
pdl
J
cosh
1
x x
0
l
J
;
J ¼
72J
C
sinh½ðx x
0
Þ=l
J
cosh
2
½ðx x
0
Þ=l
J
In a short junction with L5l
J
the phase difference is
a linear function of B:
jðxÞ¼
Bd
F
0
x þ Z; Z ¼ const ð17Þ
The critical current of a junction
I
C
¼ max
Z
Z
W
0
J
C
sin½jðxÞdx
¼ I
C0
sinðpF=F
0
Þ
ðpF=F
0
Þ
ð18Þ
depends in this case on the magnetic flux, F, inside
the junction and exactly equals zero at F ¼nF
0
. The
form of I
C
(F) dependence is similar to the pattern of
Fraunhofer diffraction of waves passing through a
slit and has been termed the ‘‘Fraunhofer pattern.’’
4. Josephson Junctions in a Microwave Field
In his original paper Josephson (1962) also predicted
the appearance of a set of vertical current steps on the
current–voltage characteristics of short Josephson
junctions under the influence of microwave radiation,
V(t) ¼V
1
cos(ot).
If the normal current component across the junc-
tion is large compared to the supercurrent, then the
solution of Eqns. (2) and (3) has the simple form
j ¼
2e
_
%
Vt þ
2e
_o
V
1
sinðotÞþa ð19Þ
Substituting Eqn. (19) into the expression for I
S
(Eqn. (1)) of the RSJ model gives
I
S
¼ I
C
X
N
m¼N
J
m
2eV
1
_o
sin m þ
o
J
o
ot þ a
hi
ð20Þ
where J
m
(x) are Bessel functions of the first kind. It is
clear from Eqn. (20) that synchronization of the nth
harmonic of the external radiation with the frequency
of the Josephson oscillation (no ¼o
J
) should result in
formation of steps, which look like ‘‘spikes’’ in the
current–voltage characteristic and have amplitude
I
C
J
m
(2eV
1
/_o). According to the well-known prop-
erties of Bessel functions, as the signal amplitude in-
creases the nth step first increases, reaches a
maximum, and then slowly decreases, oscillating with
a period approximately equal to 2p.
These oscillating steps were first observed by
Shapiro (1967) and are referred to as Shapiro steps.
Both effects discussed above (Fraunhofer pattern,
Eqn. (18), and Shapiro steps, Eqn. (20)) are the direct
consequence of the sinusoidal relationship between
supercurrent and phase difference j. It is for this
reason that they are used as a standard experimental
test for the applicability of the RSJ model for the
description of the properties of a Josephson junction
of any type and configuration.
See also: Electrodynamics of Superconductors: Flux
Properties
Bibliography
Barone A, Paterno G 1982 Physics and Application of Josephson
Effects. Wiley, New York
Josephson B D 1962 Possible new effects in superconducting
tunneling. Phys. Lett. 1, 251–4
Kulik I O, Yanson I K 1970 Josephson Effect in Superconduct-
ing Tunnel Structures. Nauka, Moscow (in Russian); 1972,
Keter Press, Jerusalem (in English)
Larkin A I, Ovchinnikov Yu N 1966 Tunnel effect between
superconductors in alternating field. Zh. Eksp. Teor. Phys.
(Sov. Phys. JETP) 51, 1535
Likharev K K 1986, 1991 Dynamics of Josephson Junctions and
Circuits. Gordon and Breach, Reading, UK
McCamber D E 1968 Effect of AC impedance on DC volatage-
current characteristics of superconductor weak-link junc-
tions. J. Appl. Phys. 39, 3113
Shapiro S 1967 Josephson currents in superconducting tunnel-
ing: the effect of microwaves and other observations. Phys.
Rev. Lett. 11,80
Solymar L 1972 Superconducting Tunneling and Application.
Chapman and Hall, London
Steward W C 1968 Current-voltage characteristics of Josephson
junctions. Appl. Phys. Lett. 12, 277
Werthamer N R 1966 Nonlinear self-coupling of Josephson
radiation in tunnel junctions. Phys. Rev. 147, 255
Zubkov A A, Kupriyanov M Yu, Cemenov V K 1981 Station-
ary Properties of Josephson Structure with Ohmic conduc-
tivity. Fiz. Nizk. Temp. (Sov. J. Low Temp. Phys.) 7 (11),
1365–71
M. Kupriyanov
Moscow State University, Russia
5f Electron Systems: Magnetic Properties
5f electron systems are characteristic of actinides,
i.e., elements between actinium, atomic number
Z ¼89, and lawrencium, Z ¼103. These elements
128
5f Electron Systems: Magnetic Properties

are characterized by a gradual filling of the 5f elec-
tronic shell, in analogy to the 4f shell filling in lan-
thanides. The filling tendencies for free-ion states
with increasing Z resemble strongly those in lantha-
nides, but as part of a solid the behavior of the 5f
electrons resembles 3d materials more. The reason is
the spatial extent of the 5f wave functions, being
larger compared with the 4f states in lanthanides.
Therefore the magnetism of most 5f electron systems
is more similar to the d-band magnetism than to the
localized 4f magnetism of lanthanides. This is partic-
ularly true for light actinides (up to plutonium),
whereas for the heavier actinides (from americium
onwards) 5f localization has been documented for the
pure elements. More systematic information on mag-
netism of heavy-actinide compounds is missing.
The specific characteristics of the 5f electron mag-
netism are manifest in systems based on elements
up to plutonium. The higher actinides behave in an
analogous way to the corresponding 4f systems. The
main characteristics of the 5f magnetism comprise
strong spin-orbit interaction, leading to large orbital
moments formed even in the case of band-like states,
exchange interactions mediated or assisted by the
hybridization of the 5 f states with the ligand states,
and enormous magnetic anisotropy arising from the
anisotropy of the hybridization (bonding anisotropy).
Another characteristic is a high sensitivity of mag-
netic properties to external variables, such as pressure
and magnetic field, and to fine details of composition.
1. General Features of Electronic Structure and
Magnetism of Light-actinide Systems
The fundamental difference between the character of
the 4f electron states in lanthanides (see Localized 4f
and 5f Moments: Magnetism) and 5f states in light
actinides can be attributed to a much larger spatial
extent of the 5f wave functions, and thus a much
stronger interaction with the metallic environment,
compared to the 4f case. The 5f electrons are, as
a rule, delocalized due to their participation in
bonding, which leads to a considerable hybridization
of the 5f states with the valence states of neighboring
atoms (5f ligand hybridization). The delocalization of
the 5f electrons has serious consequences. The most
important one is, that the 5f states form a more or
less narrow 5f band intersected by the Fermi energy
E
F
(the bandwidth W
5f
is of the order of several eV)
rather than discrete energy levels. Consequently, the
magnetic moments due to the itinerant 5f electrons
are much smaller than expected for a free ion, and
magnetic moments can disappear in a broad-band
limit leading to weak (Pauli) paramagnetism.
This situation resembles to a certain extent the 3d
transition metals. The strength of magnetic coupling
in cases of existing 5f moments is typically much
larger than for the 4f moments interacting via the
RKKY interaction. The impact on magnetic excita-
tions is even more dramatic, no crystal-field excita-
tions could be observed by inelastic neutron
scattering in a vast majority of systems studied so
far. Instead, one observes typically a rather broad
quasielastic response reflecting the 5f moment’s in-
stability in analogy to, for example, cerium mixed
valence materials. However, the high density of states
at E
F
is projected into high g-values of the low tem-
perature specific heat (which are further renormalized
by strong e–e correlations), and into highly anoma-
lous transport properties, resulting from the hybrid-
ization of such ‘‘heavy’’ electron states with the non-f
states carrying the electrical current.
2. Pure Actini de Elements
The small separation of the ions in the pure elements
leads to large overlap of the 5f wave functions of
nearest neighbors, formation of a broad 5f band and
weakly paramagnetic behavior. With increasing 5f
occupation the value of the Pauli-type susceptibility
increases due to the increase of N(E
F
), the density of
states at the Fermi level, which is corroborated by a
similar increase of the low-temperature electronic
specific heat coefficient g (see Table 1). This tendency
Table 1
Overview of essential properties of elemental actinides, summarizing available data on the g-coefficient of the low-
temperature specific heat, temperature independent susceptibility w
0
,Ne
´
el temperature T
N
and Curie temperature T
C
.
Th Pa U Np Pu Am
g (mJ mol
1
K
2
) 4 6.6 10 14 22 2
w
0
(10
8
m
3
mol
1
) 0.12 0.34 0.48 0.68 0.64 0.85
Cm Bk Cf Es
T
N
(K) 64 34 51(T
C
)
m
eff
(m
B
) 7.55 9.7 9.7 11.3 (?)
129
5f Electron Systems: Magnetic Properties
