Buschow K.H.J. (Ed.) Concise Encyclopedia of Magnetic and Superconducting Materials
Подождите немного. Документ загружается.


Liu Z, Richter M, Divis
ˇ
M, Eschrig H 1999 Calcula-
tion of paramagnetic susceptibilities and specific heats by
density-functional-crystal-field theory: PrPd
2
X
3
and
NdPd
2
X
3
(X ¼Al, Ga). Phys. Rev. B 60, 7981–92
Liu Z S, Park J G, Kwon Y S, McEwen K A, Bull M J 2003
Crystal-field excitations and model calculations of CeTe
2
. J.
Magn. Magn. Mater. 256, 151–7
Martinho H, Sanjurjo J A, Rettori C, Canfield P C, Pagliuso P
G 2001 Raman scattering study of crystal field excitations in
ErNi
2
B
2
C. J. Magn. Magn. Mater. 226–230, 978–9
Moze O 1998 Crystal field effects in intermetallic compounds
studied by inelastic neutron scattering. In: Buschow K H J
(ed.). Elsevier, Amsterdam, Vol. 11, pp. 493–624
Moze O, Buschow K H J 1996 Magnetic structure of
ErCo
12
Mo
2
and TbCo
12
Mo
2
determined by time-of-flight
neutron diffraction. Z. Phys. B 101, 521–6
Moze O, Rosenkranz S, Osborn R, Buschow K H J 2000 Mag-
netic excitations in tetragonal HoCr
2
Si
2
. J. Appl. Phys. 87,
6283–5
Rudowicz C 1987 On the derivation of the superposition-model
formulae using the transformation relations for the Stevens
operators. J. Phys. C: Solid State Phys. 20, 6033–6037
Sierks C, Loewenhaupt M, Freudenberger J, Mu
¨
ller K-H,
Schober H 2000 Magnetic excitations in TM
0.05
Y
0.95
Ni
2
11
B
2
C. Physica B 276–278, 630–1–6033
Stra
¨
ssle Th, Altorfer F, Furrer A 2001 Crystal-field interactions
in the pseudo-ternary compound in ErAl
x
Ga
2x
studied by
inelastic neutron scattering. J. Phys.: Condens. Matter 13,
6773–85
Stra
¨
ssle Th, Divis
ˇ
M, Rusz J, Janssen S, Juranji F, Sadykov R,
Furrer A 2003 Crystal-field excitations in PrAl
3
and NdAl
3
at
ambient and elevated pressure. J. Phys.: Condens. Matter 15,
3257–66
Takegahara K 2000 Matrix elements of crystal electric fields in
rare-earth compounds. J. Phys. Soc. Japan 69 (5), 1572–3
Takegahara K, Hisatomo H, Yanase A 2001 Crystal electric
fields for cubic point groups. J. Phys. Soc. Japan 70 (5),
1190–3
Wang Y-L 1971 Crystal-field effects of paramagnetic Curie
temperature. Phys. Lett. A 35, 383–4
O. Moze
Modena University, Italy
100
Crystal Fie ld Effects in Intermetallic Compounds: Inelastic Neutron Scattering Results
Demagnetization: Nuclear and Adiabatic
1. Principles of the M ethod
Adiabatic demagnetization (AD) is one of the cool-
ing methods that have a common principle: the
entropy of the working medium (helium, spins, etc.)
is controlled by some parameter like pressure or
magnetic field. The working medium in AD is a
system of magnetic moments (nuclear spins, electronic
spins, or orbital moments) and the magnetic field is
the control parameter when the magnetocaloric effect
is employed. The magnetization, M, of a paramagnet
or a ferromagnet increases with an applied magne-
tic field, H, and simultaneously both the energy of
magnetic moments in the field and the internal energy
of their exchange interaction decrease. Supposing
negligible pressure and volume effects, the entropy
can be expressed by the following thermodynamic
relation:
dS ¼
1
T
@U
@T
H
dT þ
1
T
@U
@H
T
þM
dH ð1Þ
The internal energy, U, is a function of the
temperature, T, of the sample, and in general it
increases with temperature. Consequently, if the
magnetization of a paramagnetic sample is reduced
adiabatically, the sample temperature should de-
crease. The cooling cycle of the AD method consist
of two steps:
(i) Isothermal magnetization. The sample is in
thermal contact with a constant temperature bath
kept at T
i
. The magnetic field is increased from zero
to H
i
and the heat released to the bath compensates
for the work done on the sample. It leads to an
increase in the order of the magnetic moments and,
consequently, reducing their entropy, S,by
DS ¼
Z
H
0
@M
@T
H
dH ð2Þ
This entropy change is determined by the rate of
change of magnetic energy with respect to the thermal
energy. The efficiency of the process increases with
decreasing temperature.
(ii) Adiabatic demagnetization. The sample is ther-
mally isolated from the bath, and the field is then
reduced from H
i
to H
f
. It is an adiabatic process, i.e.,
dQ ¼TdS ¼0 and the entropy remains constant.
Since the sample performs magnetic work by demag-
netizing at the expense of internal energy, it decreases
in temperature towards T
f
at a rate given by:
dT
ðdS¼0Þ
¼
T
@M
@T
H
C
H
dH ð3Þ
where C
H
is the specific heat.
AD is a one-shut process. After demagnetization
the system will warm up owing to a parasitic heat
load. The energy which can be absorbed, Q ¼
R
Tds,
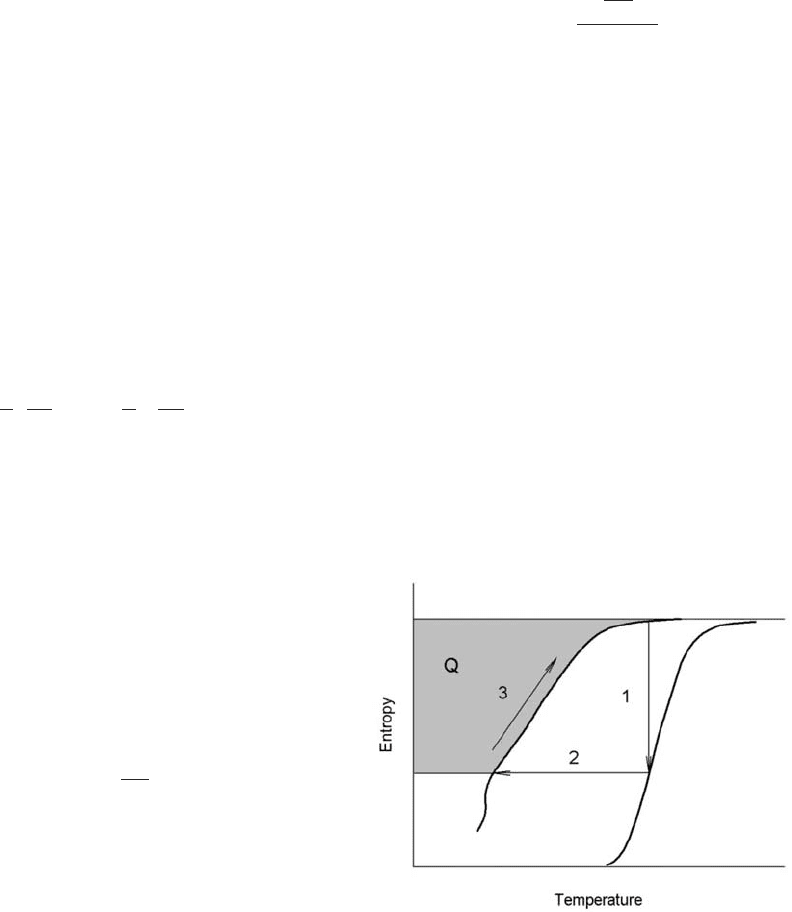
is proportional to the shaded area in Fig. 1.
In modeling a system of noninteracting moments,
m, the entropy is simply a function of H/T. For an
isoentropic process T
f
¼T
i
H
f
/H
i
and absolute zero
temperature could be reached for H
f
¼0, in contra-
diction to the third law of thermodynamics. This does
not happen in a real system, however, because the
assumption of noninteracting spins breaks down
and the energy levels in zero applied field are not
degenerate. The lowest temperature achievable by the
AD method is limited by the existence of an internal
interaction in a spin system. This interaction causes
spin ordering at sufficiently low temperatures and a
spontaneous decrease of the entropy of a system. The
detailed nature of the interactions may be compli-
cated. It may be, for example, the direct dipolar
interaction between magnetic moments in the case of
dielectrics or the RKKY interaction in metals. It
simplifies matters to define an effective internal field,
Figure 1
Graphical representation of the processes of isothermal
magnetization (1) and adiabatic demagnetization (2).
The shaded area shows the cooling power during a warm
up (3).
D
101
h, such that it would produce a Zeeman splitting
equal to the splitting, e, due to the interaction, i.e.,
e ¼mm
0
h. Then:
T
f
¼ T
i
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
H
2
f
þ h
2
H
2
i
þ h
2
s
ð4Þ
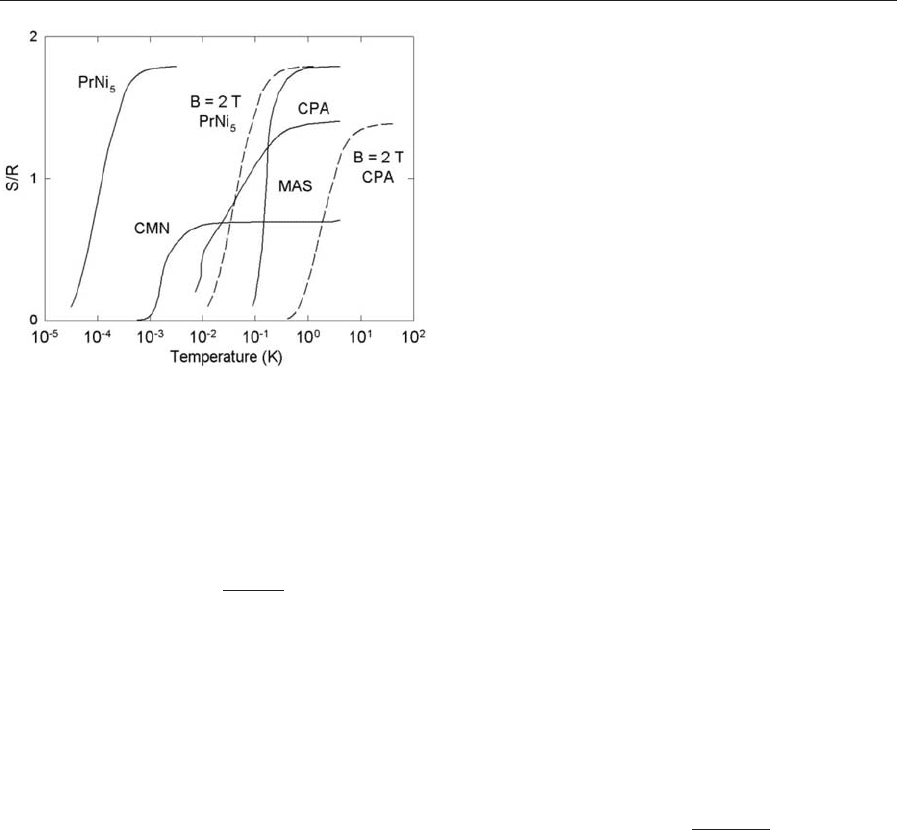
The temperature dependence of the entropy of
some substances used for this cooling method is
shown in Fig. 2.
2. Practical Aspects
The most common substances used for the AD
method are paramagnetic salts such as MAS ¼
Mn
2 þ
SO
4
(NH
4
)
2
SO
4
.6H
2
O(T
c
E0.17 K; bE80 mT),
FAA ¼Fe
3þ
2
(SO
4
)
3
(NH
4
)
2
SO
4
.24H
2
O (0.03 K; 50 mT),
CPA ¼Cr
3þ
2
(SO
4
)
3
K
2
SO
4
.24H
2
O (0.01K; 10 mT),
CMN ¼2Ce
3 þ
(NO
3
)
3
3Mg(NO
3
)
2
.24H
2
O (0.002K;
4mT), Gd
2
(SO
4
)
3
.8H
2
O (0.18 K), van Vleck para-
magnets such as PrNi
5
(0.0004 K; 16 mT) and PrCu
6
(0.0026 K), and pure metals such as copper (58 nK;
0.3 mT) and silver (0.56 nK). The ordering tempera-
ture and the effective internal field are given in
parentheses.
Besides cooling magnetic moments by AD, for any
practical use it is also necessary to cool the lattice of
which the magnetic atoms form a part. The exchange
of energy between the spins and the lattice is
characterized by the spin–lattice relaxation time, t
1
.
For metals the exchange is mediated by the conduction
electrons and t
1
is given by the Korringa relation. In
nonmetals, owing to their poor thermal conductivity,
t
1
can reach hours or days at very low temperatures.
The use of the magnetocaloric effect to produce
temperatures well below 1 K was proposed by
Giauque and Debye in 1926. A practical realization
of AD cooling was announced in 1933 by Giauque
and MacDougall, reaching 0.53 K using Gd
2
(SO
4
)
3
.
8H
2
O at starting conditions of 3.4 K and 0.8 T. On
the other hand, de Haas, Wiersma, Kramers used
CeF
2
, and after demagnetization from 3 T to 0.1 T
decreased the temperature from 1.26 K to 0.27 K.
According to present knowledge, 0.001 K is the
estimated lower limit achievable by the AD technique
employing electronic paramagnetism.
In 1934 Gorter proposed nuclear magnetic refrig-
eration but owing to severe experimental problems it
was only in 1956 that Kurti succeeded in reducing the
nuclear spin temperature in copper wires to about
20 mK. Osgood and Goodking in 1966 were the first
to achieve considerable electron and lattice refrigera-
tion. The progress made in the AD technique enabled
the Helsinki group, in 1982, to observe spontaneous
nuclear order of copper below the Ne
´
el temperature
T
N
¼58 nK (Oja and Lounasmaa 1997). The nuclear
order in silver with T
N
¼560 pK has been investi-
gated and the long spin–spin relaxation time of silver
made it possible to produce a negative nuclear spin
temperature and to find ferromagnetic order in silver
above T
C
¼1.9 nK (Oja and Lounasmaa 1997). The
lowest temperature achieved is 280 pK and 750 pK
is the highest temperature obtained (Oja and
Lounasmaa 1997).
Using the known potential of van Vleck para-
magnets for nuclear refrigeration, Andres and
Lounasmaa (1982) tested many of them. The
electronic ground states of van Vleck paramagnets
are nonmagnetic singlets. Owing to the nuclear
hyperfine interaction of nuclear and electronic spins
a magnetic moment is induced into the ground state.
This can be formally described by assuming that the
nuclear magnetic moment is enhanced by a factor
1 þ K 1 þ
Aw
VV
g
J
m
B
g
N
m
N
ð5Þ
where A is the hyperfine interaction constant and w
VV
the van Vleck temperature-independent susceptibility.
For Pr or Tm compounds, K is typically 10–100. PrNi
5
(hyperfine interaction-enhanced nuclear ferromagnet,
T
C
¼0.4 mK) or PrCu
6
(T
N
¼2.6 mK) serve in a lower
stage in many nuclear refrigerators. The starting con-
ditions for demagnetization, B/TE500–1000 TK
1
,
are fulfilled by available dilution refrigerators and
superconducting magnets. Advantages of these mate-
rials are a huge cooling power and a short t
1
.
3. Outlook
AD is mostly used in the temperature range below
1 mK but its ability to work in zero gravity has led
to the development of prototypes for satellite
Figure 2
Entropies per mole of substance, S /R, of four salts and
PrNi
5
in zero field (solid curves) and in a 2 T field
(dashed curves).
102
Demagnetization: Nuclear and Adiabatic

applications. It is a prospective method as AD can
reach higher efficiency than traditional vapor com-
pression technology. To apply this method to a wide
temperature range up to room temperature, con-
siderable progress in materials research is needed.
Materials with a large magnetocaloric effect are
fundamental for AD but they are also suitable for
regenerators in other types of refrigerators. For
devices suitable for cooling at room temperature
(see Magnetocaloric Effect: From Theory to Practice).
See also: Magnetic Refrigeration at Room Tempera-
ture
Bibliography
Andres K, Lounasmaa O V 1982 Recent progress in nuclear
cooling. In: Brewer D F (ed.) Progress in Low-Temperature
Physics. North-Holland, Amsterdam, Vol. 8, pp. 222–87
Betts D S 1976 Refrigeration and Thermometry Below One
Kelvin. Sussex University Press, Brighton, UK
Hagmann C, Richards P L 1995 Adiabatic demagnetization
refrigerators for small laboratory experiments and space
astronomy. Cryogenics 35, 303–9
Hudson R P 1972 Principles and Application of Magnetic
Cooling North-Holland, Amsterdam
Oja A S, Lounasmaa O V 1997 Nuclear magnetic ordering in
simple metals at positive and negative nanokelvin tempera-
tures. Rev. Mod. Phys. 69, 1–136
Pecharsky V K, Gschneidner K A Jr. 1999 Magnetocaloric
effect and magnetic refrigeration. J. Magn. Magn. Mater.
200, 44–56
Pobell F 1992 Matter and Methods at Low Temperatures.
Springer, Berlin
J. Sebek
Institute of Physics ASCR, Prague, Czech Republic
Density Functional Theory: Magnetism
Spin density functional theory (SDFT) is the basis of
most of the modern electronic structure calculations.
It has been reviewed in detail by Jones and Gun-
narsson (1989), Trickey (1990), Eschrig (1996), and
many others, and is only briefly outlined here.
1. Spin Density Functional Theory
1.1 General Theory
At the quantum mechanical level, magnetic properties
of materials are usually well described within the Born–
Oppenheimer (adiabatic) approximation: the electrons,
moving much faster than the heavier atomic nuclei,
are considered in the Coulomb potential v(r) ¼
P
s
Z
s
/
7rR
s
7 created by the nuclei of charge Z
s
in their
instantaneous positions R
s
. In most cases, the values of
R
s
are approximated by the rest positions of the atoms
in a regular structure.
For each set of {R
s
} there is a state of the electrons
with the lowest energy, the ground state 7c
0
S. Its
energy can be written as a functional of the potential,
E
0
[v], i.e., the ground state energy depends on the
Coulomb potential of the nuclei at each point r of
space. The exact functional dependence E
0
[v]isin
general unknown, but reasonable approximations
will be discussed below. They allow for a determina-
tion of the atomic rest positions in molecules and
solids with an accuracy of a few percent, by minimi-
zation of the energy [E
0
þ
P
ss
0
Z
s
Z
s
0
/7R
s
R
s
0
7] with
respect to {R
s
} (Moruzzi et al. 1978).
The calculation of observable quantities from the
true wave function c
0
is not feasible in the case of
many, strongly interacting electrons, since c
0
de-
pends on the coordinates r
i
and on the spin quantum
numbers s
i
of all N electrons, although the total en-
ergy may be expressed in terms of the spin and pair
densities. Hohenberg and Kohn (1964) went further
and proved that the ground state density function
n
0
(r) alone determines v(r) and hence c
0,
together
with all ground state properties uniquely. Since the
ground state spin density matrix n
0
(r)
n
0
ðrÞ¼n
0;ss
0
ðrÞ
¼ N
X
s
2
Z
d
3
r
2
y
X
s
N
Z
d
3
r
N
c
0
ðr; s; r
2
; s
2
; y; s
N
Þ
c
0
ðr; s
0
; r
2
; s
2
; y; s
N
Þð1Þ
comprises the information on the electron density,
n ¼trn, the unique determination of the ground state
by the spin density follows as a corollary. It allows, as
an important generalization, the inclusion of the ac-
tion of an external magnetic field B on the spin, by
the definition of the spin-dependent external poten-
tial v ¼v þm
B
rB. Here, r ¼(s
x
, s
y
, s
z
) denote the
Pauli spin matrices. The vector spin density is given
as R ¼tr(rn). Further, the total spin in a given vol-
ume V is S ¼
R
V
d
3
r R/2, and the related spin magnetic
moment is defined by l
s
¼m
B
2S.
The calculation of E
0
and n
0
becomes possible
with the help of the Hohenberg–Kohn variational
principle:
E
0
½v¼min
c
/c7
#
H7cS
¼ min
n
Z
d
3
rtrðvnÞþmin
c
n
/c
n
7
#
T þ
#
U7c
n
S
R
d
3
rn¼N
ð2Þ
where c
n
means the class of all normalized fermionic
wave functions with the spin density n. The electronic
103
Density Functional Theory: Magnetism

Hamiltonian H
ˆ
consists of the potential v plus the
operators of the kinetic energy, T
ˆ
, and of the elec-
tron–electron interaction energy, U
ˆ
. The latter terms
are further subdivided (Kohn and Sham 1965):
min
c
n
/c
n
7
#
T þ
#
U7c
n
S ¼ T
s
½nþE
H
½nþE
xc
½nð3Þ
Here, T
s
is the ground state kinetic energy of a
model system of electrons that are assumed to have
no mutual Coulomb interaction and to possess the
spin density matrix n:
T
s
½n¼
X
N
i
X
s
/f
i
ðsÞ7 D=27f
i
ðsÞS;
d
ij
¼
X
s
/f
i
ðsÞ7f
j
ðsÞS ð4Þ
n
ss
0
¼
X
N
i
f
i
ðsÞf
i
ðs
0
Þð5Þ
The f
i
denote the N lowest single-particle eigenstates
in a corresponding, yet unknown, effective potential.
Further, E
H
is the spin-independent Hartree energy:
E
H
¼
1
2
ZZ
d
3
rd
3
r
0
nðrÞnðr
0
Þ
7r r
0
7
ð6Þ
and E
xc
[n], the so-called exchange-correlation (xc)
energy, is an unknown functional of the spin density
matrix, containing all contributions beyond the mean
field approximation from electron–electron interac-
tions in the ground state.
The essence of the Kohn–Sham procedure is to
subdivide the total energy into large contributions
that are either well-known functionals (
R
d
3
r tr(vn)
and E
H
), or at least computable quantities (T
s
), and a
much smaller remaining part (E
xc
). Then, approxi-
mations applied to the latter part are expected to in-
troduce only reasonably small errors. However, all
known approximations to E
xc
are unfortunately on
a heuristic level and cannot be systematically im-
proved. Thus, the spin density functional theory
(SDFT) described here and in the following has to be
considered as a model theory, in the sense that the
applicability of any chosen approximation is justified
a posteriori by experience from comparison with ex-
periment. On the other hand, within a given approx-
imation, the calculations are usually carried out
without adjustable parameters. In this way, after val-
idating the approximation for a certain class of ma-
terials, reliable forecasts of experimental results on
this class can be expected from theory.
For this aim, a numerically tractable formulation is
needed to allow for the calculation of n
0
and E
0
. Such
a formulation is given by the Kohn–Sham equations
that are obtained by: (i) inserting Eqn. (3) in Eqn. (2),
(ii) using the Ansatz Eqn. (5) also in the case with
electron–electron interaction, and (iii) varying with
respect to the f
*
i
:
X
s
0
D
2
d
ss
0
þ v
eff;ss
0
f
i
ðs
0
Þ¼e
i
f
i
ðsÞð7Þ
The Kohn–Sham equations (Eqn. (7)) contain the
above-mentioned effective potential:
v
eff;ss
0
¼ v
ss
0
ðrÞþ
Z
d
3
r
0
nðr
0
Þ
7r r
0
7
d
ss
0
þ v
xc;ss
0
ð8Þ
consisting of external, Hartree, and xc potentials. The
latter is defined by: v
xc;ss
0
¼ dE
xc
½n=dn
ss
0
.
Together with Eqn. (5), these equations form a
nonlinear system of integro-differential equations
which have to be solved by iteration (self-consistent
solution). Due to the complexity of the Kohn–Sham
equations (reflecting part of the complexity of na-
ture), more than one stationary solution may exist.
The further solutions can be related to physically
relevant metastable magnetic states (for instance,
low-spin/high-spin states, see Sect. 3.3, or different
magnetic moment arrangements). This problem is in-
vestigated in some detail by Moruzzi and Marcus
(1993).
1.2 Approximations
The most widely used approximation for E
xc
[n] is the
local spin density approximation (LSDA):
E
xc
½nEE
LSDA
xc
½n¼
Z
d
3
rnðrÞe
hom
xc
n
m
ðrÞ; n
k
ðrÞ
ð9Þ
Here, e
hom
xc
means the exchange-correlation energy per
electron of the spin-polarized homogeneous electron
gas, a model system frequently used in electronic
structure theory. The quantities n
m
and n
k
denote
spin-up and spin-down densities. They are obtained
by rotating the spin quantization axis at each point in
space in the direction that yields a diagonal spin
density matrix.
Another, more recent, approximation is called the
generalized gradient approximation (GGA). At var-
iance with the LSDA, it takes into account not only
the local value of n, but also its spatial derivatives.
If electrons in one of the open shells do not con-
tribute to the chemical bonding, like 4f states in most
rare-earth systems, they should be distinguished from
the valence electrons in the theoretical treatment as
well. Then, the so-called open-core approximation, or
the self-interaction-corrected (SIC) LSDA, are mod-
ern approximations to be used (Richter 1998). The
latter approximation is also relevant to finite systems
like atoms and small molecules.
The energy e
hom
xc
can be subdivided into e
hom
xc
¼
e
hom
x
þe
hom
c
. Here, e
hom
x
describes the effect of ex-
change interaction: according to the Pauli exclusion
104
Density Functional Theory: Magnetism

principle, electrons with the same spin quantum
number should have a zero probability density of
being at the same point in space. For this reason, the
Coulomb energy between pairs of electrons with the
same spin is lower than between pairs with different
spin, and e
hom
x
o0. The energy e
hom
c
describes the ef-
fect of Coulomb correlation: electrons with arbitrary
spin do not move in a stochastic way either, but tend
to avoid each other due to their mutual Coulomb
repulsion. This correlated motion yields a smaller to-
tal energy than estimated by a mean field treatment,
and e
hom
c
o0 holds as well.
1.3 Hund’s Rules
In atoms, exchange interaction is the reason for in-
completely filled shells to maximize their spin polar-
ization (SP) in the ground state. This behavior is
known as Hund’s First Rule (see Magnetism in Sol-
ids: General Introduction). It is driven by an energy
gain E
SP
BS(S1/2) in comparison to an unpolarized
shell (Melsen et al. 1994), where the total spin S is
zero. If LSDA is applied to an atom, then
E
SP
EDE
xc
¼E
LSDA
xc
(S)E
LSDA
xc
(0)EIS
2
, yielding a
good approximation for the relation above in the case
of large total spins. This fact indicates that LSDA is
particularly suited for extended systems which are
closer to the idealized homogeneous electron gas
than atoms are. The prefactor I is called the Stoner
parameter.
While free atoms in their ground state always carry
a maximum spin moment, this is not the case in con-
densed matter. The reason for this difference is that
band splitting lifts the degeneracy of the atomic
states. As a consequence, energy is gained when the
spin polarization is reduced, since then states at the
majority spin Fermi surface with a higher Fermi mo-
mentum are emptied in favor of states at the minority
spin Fermi surface with a lower Fermi momentum.
The energy gain DT
s
is the bigger the broader the
band is, and vanishes in the atomic limit.
This consideration is the essence of Stoner theory
(see Itinerant Electron Systems: Magnetism (Ferro-
magnetism)), which evaluates the counterbalance of
DE
xc
and DT
s
to give a criterion for the onset of long-
range spin order when starting from a spin-compen-
sated state. The condition for the instability of the
Pauli paramagnetic state is that:
INðE
F
Þ41 ð10Þ
where N(E
F
) denotes the density of states at the Fer-
mi energy for one spin direction. Detailed discussion
of the first-principles investigation of the magnetic
ordering is given in Sect. 2.
Orbital magnetism also plays a big role in solids,
e.g., for the phenomenon of magnetocrystalline an-
isotropy. It arises if the other two of Hund’s rules are
considered. Hund’s Second Rule states that of all
atomic terms with maximum spin, the term with the
largest total angular momentum L is the lowest in
energy. The related energy gain is called orbital po-
larization (OP) energy, E
OP
, which vanishes for s- and
p-shells. Coulomb correlation is the reason behind
OP. Though the LSDA accounts for a part of
Coulomb correlation contributions, OP cannot be
obtained in a local approximation, since any defini-
tion of an angular momentum density is ambiguous,
due to the gauge freedom of this quantity. Reason-
able heuristic approximations, called OP corrections,
(Eriksson et al. 1989) use the local gauge where the
angular momentum density vanishes in the interstitial
regions. The angular momenta of the individual
atomic shells are assumed to give contributions to
the xc energy of the form E
OP
EE
xc
(L)E
xc
(0) ¼
const.
.
L
2
o0, resembling the form of the SP term.
Finally, Hund’s Third Rule determines the cou-
pling between spin and orbital degrees of freedom to
be antiparallel or parallel in the ground state of less
or more than half-filled atomic shells, respectively.
This behavior is a relativistic effect: the fast-moving
electrons experience a magnetic field generated by the
relative motion of the nuclear charges with respect to
the rest system of the electrons. This field interacts
with the individual electron spins and yields an ad-
ditional contribution to the kinetic energy of the
form: E
SO
¼
P
i
x
i
/s
i
l
i
S
i
, where x
i
are radial inte-
grals called spin-orbit coupling (SOC) constants, and
l
i
are single-particle angular momentum operators.
The important role of SOC in solid state magnetism
is discussed in more detail in Sects. 2 and 3.
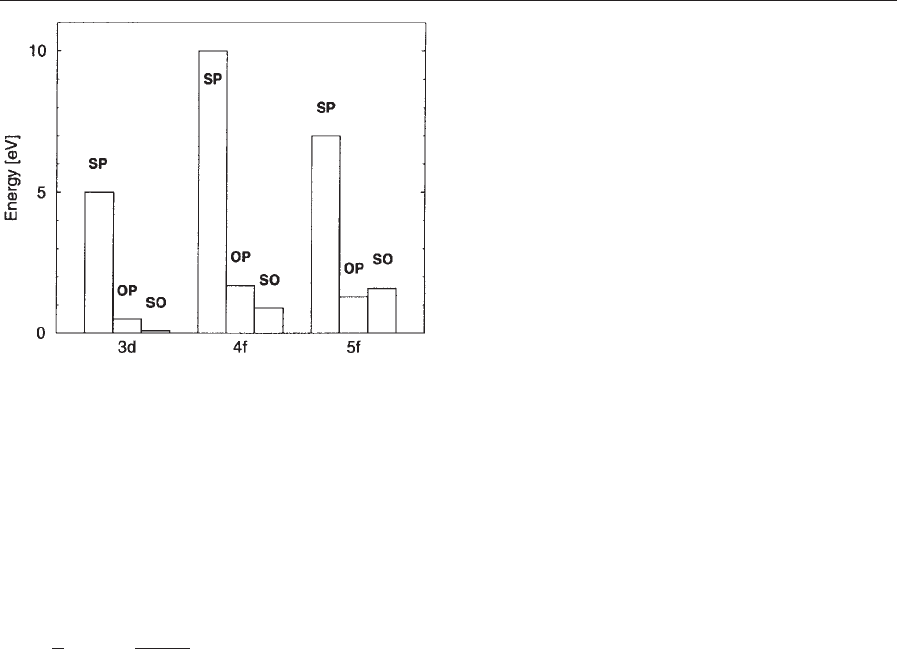
Figure 1 shows the range of energies related to
Hund’s rules couplings in those atomic shells that are
most important in solid-state magnetism: transition
metal 3d, rare-earth 4f, and actinide 5f shells. The
spin polarization energy, E
SP
, is maximum for half-
filled shells, while the orbital polarization energy,
E
OP
, and the spin–orbit coupling energy, E
SO
, are
large for shell fillings close to 1/4 and 3/4. In metals
and compounds, spin and orbital polarization energy
can be much smaller, or even zero, since losses of T
s
and E
H
due to polarization compensate at least part
of the polarization gains: for instance, E
SP
E0.4 eV
in iron metal. In general, the atomic values given in
Fig. 1 put an upper boundary on the energy scales
observed in solids.
1.4 Single Particle Excitations
The Kohn–Sham orbitals f
i
, with related single-
particle energies e
i
, have been introduced above as
auxiliary quantities needed to compute the spin den-
sity matrix. In a hypothetical system without elec-
tron–electron interaction, these Kohn–Sham states
would coincide with the quasi-particle excitations of
the system, which can be probed by optical, x-ray or
electron spectroscopies (Sections 3.4 and 3.5). To
105
Density Functional Theory: Magnetism
evaluate single-electron or single-hole excitations c
i
close to the Fermi level of a realistic system, the
Dyson equation:
D
2
þ
Z
d
3
r
0
nðr
0
Þ
7r r
0
7
c
i
ðs; rÞ
þ
X
s
0
v
ss
0
þ
Z
d
3
r
0
M
ss
0
ðr; r
0
; E
i
Þ
c
i
ðs
0
; r
0
Þ
¼ E
i
c
i
ðs; rÞð11Þ
has to be solved. However, full knowledge of the self-
energy operator kernel M is even further away than
full knowledge of v
xc
; both quantities must rather
be approximated on the basis of physical intuition.
As a matter of fact, the replacement of M
ss
0
by
d(rr
0
)v
xc,ss
0
(n), i.e., the LSDA, turns out to work
fairly well for many systems (weakly correlated sys-
tems). In particular, the spectra related to s and p
valence states of simple and transition metals, as
well as rare-earth 5d and actinide 6d states, are well
described.
These states form broad bands; the quasi-particle is
extended over many atoms due to the large hopping
rate, and thus correlation effects are small. If the
bands become narrower, the hopping rate is lower,
and hence a stronger intra-atomic Coulomb repul-
sion/attraction is felt by additional electrons/holes.
Strong on-site correlations result in a jump of M,asa
function of energy, at the Fermi level E
F
by an
amount U
eff
. This quantity is related to the on-site
Coulomb matrix element U, but is strongly screened
in metals. A simple approximation for this situation
is called LSDA þU, where the energy dependence of
M is reduced to the jump at E
F
and otherwise the
LSDA is used. A certain problem in this approach is
that the screening depends much on chemical com-
position and on local structure, such that the value of
U
eff
is not just an atomic property as U is; it has to be
estimated for each individual situation.
Finally, the relationship between U
eff
and the band
width W determines if the related states contribute to
the chemical bonding or not. Accordingly, these
states may exhibit itinerant or localized magnetic
behavior. The rare-earth 4f shells where U
eff
bW
show local moment behavior in most cases. On the
other hand, elemental 3d metals (U
eff
oW) are itine-
rant magnets. Actinide systems as well as transition
metal compounds may show both types of behavior.
1.5 Model Parameters
Model parameters like U
eff
may be obtained by
means of constrained SDFT calculations (Dederichs
et al. 1984). The general idea behind this method is to
impose a symmetry restriction on the considered sys-
tem that models, in a static way, a certain quasi-
particle excitation. If the time-scales of the excitation
and of the remaining degrees of freedom are different
enough, the response of the latter, treated in a self-
consistent calculation, provides a fairly realistic de-
scription of the screening process (Gunnarsson 1990).
Prominent examples are so-called frozen magnon
calculations, where the atomic spin directions are
fixed to form a periodic wave. Then, the ground-state
energy is calculated for both the relaxed and the
constrained system, and the energy difference is
equated with the related excitation energy. Heisenb-
erg model parameters describing the interatomic ex-
change coupling may be fitted to frozen magnon
spectra or to other constrained magnetic configura-
tions. They are used to describe thermodynamic
properties of magnetic substances.
Finally, crystal field model parameters should be
mentioned. They enter model theories for the de-
scription of magnetism related to localized 4f states
(see Localized 4f and 5f Moments: Magnetism). The
parameters may be obtained by applying shape re-
strictions on the atomic 4f charge density (Brooks
et al. 1997, Richter 1998).
2. Magnetic Orde ring
One of the major tasks of the SDFT is the first-prin-
ciples description of the ground-state magnetic order.
There is a great variety of possible magnetic ground
states, for example: Pauli paramagnetism in vanadi-
um, ferromagnetism in nickel, antiferromagnetism in
chromium, spiral magnetic structures in g-iron and
rare-earth metals, complex noncollinear magnetic
Figure 1
Range of energies related to Hund’s rules in atoms:
transition metal 3d shells, rare-earth 4f shells, and
actinide 5f shells. The labels SP, OP, and SO denote the
maximum values, within the related shell, of the energies
E
SP
, E
OP
, and E
SO
(Hund’s First, Second, and Third
Rule coupling energies, respectively).
106
Density Functional Theory: Magnetism
structures in compounds of the transition metals and
actinides, and disordered magnetic state in amor-
phous iron. In principle, the machinery of the SDFT
allows one to start with an arbitrary magnetization
and, by calculating to self-consistency, to determine
the ground state magnetization. However, in this
general approach the study of even the simplest mag-
netic system becomes a complex computational prob-
lem. This kind of calculation is unavoidable for the
systems with no regular magnetic structure, but oth-
erwise available experimental and theoretical infor-
mation about the magnetic ground state is usually
used to simplify calculations.
According to its basic theorems (Sect. 1.1), the
SDFT supplies physical information about the state
of the system which realizes the minimum of the en-
ergy-functional. The applications of the theory can,
however, be extended by the possibility of the con-
strained calculations, where the process of minimiza-
tion takes place under certain restricting conditions
(Dederichs et al. 1984) imposed upon, for example,
the symmetry of the magnetic density and effective
potential.
An important example of the constraint is the con-
straint of zero magnetisation: m(r) ¼0 for any r. Even
for magnetic systems, it is possible to calculate the
lowest energy nonmagnetic state and to study the
physical reasons for the instability of this state with
respect to the formation of the magnetic structure
(see Magnetism in Solids: General Introduction).
2.1 Ferromagnetic Structure
The best-studied magnetic crystals are the elementary
3d metals with ferromagnetic magnetic ordering: a-
iron (iron with the body-centered cubic lattice), co-
balt, and nickel. The instability of the nonmagnetic
state of a 3d metal with respect to the formation of a
ferromagnetic structure can be studied with the help
of the Stoner criterion, Eqn. (10). The density of
states (DOS) of the nonmagnetic a-iron is shown in
Itinerant Electron Systems: Magnetism (Ferromag-
netism) (Fig. 3). The DOS has a peak at the position
of the Fermi level that indicates the instability of the
nonmagnetic state. Indeed, if the constraint of zero
magnetization is removed by placing a small mag-
netic moment at each iron site, the calculation results
in the ferromagnetic state with the atomic magnetic
moment which is close to the observed value (e.g.,
2.15 m
B
in the calculation of Moruzzi et al. (1978)).
One obtains a similar situation in cobalt and nickel
(Moruzzi et al. 1978).
2.2 Antiferromagnetic Structure
For chromium, the Stoner product is less than 1. Still
the nonmagnetic state of chromium is unstable, but
in this case with respect to the formation of an
antiferromagnetic structure. The antiferromagnetic
structure can be treated as a magnetic structure with
the spatial variation of the atomic magnetic moments
defined by the wave vector q ¼
1
2
K, where K is a
vector of the reciprocal lattice. The Stoner criterion
generalized to the case of the magnetic structures
characterized by an arbitrary value of q takes the
form:
IwðqÞ41 ð12Þ
where w(q) is the unenhanced magnetic susceptibility
of the nonmagnetic state of the system in a non-
uniform static magnetic field, with spatial variation
defined by vector q. For a ferromagnet, q ¼0 and the
susceptibility is equal to the density of states at the
Fermi level.
For a finite q, the susceptibility is given by the
formula:
wðqÞ¼
X
knm
f ðe
nk
Þf ðe
mkq
Þ
e
mkq
e
nk
þ id
/kn e
iqr
k qmS
ð13Þ
and cannot be expressed through the DOS. Here, n
and m are band indices, d is an infinitesimal, and f(e)is
the Fermi–Dirac distribution function.
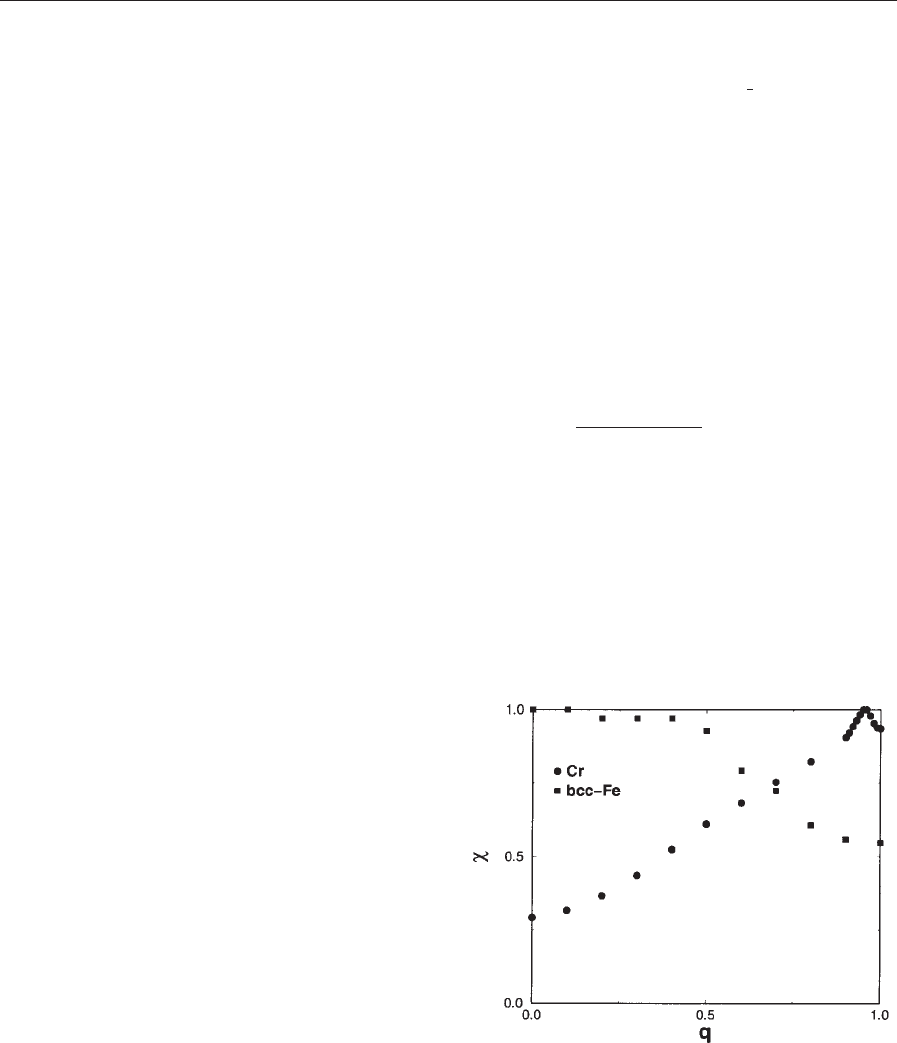
In Fig. 2, we compare the calculated q-dependences
of the susceptibility of a-iron and chromium. The two
curves are essentially different. In the case of a-iron,
the susceptibility is maximum for q ¼0 and decreases
monotonically with increasing q, clearly revealing the
origin of the ferromagnetic instability. In the case of
Figure 2
q-dependence of the unenhanced magnetic susceptibility
of a-Fe (lattice parameter a ¼5.27 atomic units) and Cr
(a ¼5.45 atomic units); q is given in units of 2p/a. The
susceptibilities are scaled to assume a unity value at the
point of maximum. Calculation was carried out with the
scheme of Sandratskii and Ku
¨
bler (1992).
107
Density Functional Theory: Magnetism
chromium, the behavior is the opposite: the suscep-
tibility for q ¼ 1ðin units of
1
2
4KÞ is more than three
times that for q ¼0. The difference between these two
cases reflects the tendency for systems with half-filled
d-bands to be antiferromagnetic, in contrast to the
systems at the end of the 3d series, which order
ferromagnetically (Heine and Samson 1983).
From Eqn. (13), it is seen that the largest contri-
bution to the susceptibility is given by the pairs of
states which are separated in reciprocal space by the
vector q and are close in energy. Since the energy
difference contains one occupied and one unoccupied
state, these states lie close to the Fermi level. Indeed,
the Fermi surface of chromium possesses two similar
sheets shifted in the reciprocal space by the vector q,
which is close to
1
2
K (see Fawcett (1988) for details).
This nesting of the sheets of the Fermi surface is im-
portant for the formation of the antiferromagnetic
instability.
2.3 Spiral Magnetic Structure
The spatial variation of the directions of the atomic
magnetic moments in the simplest magnetic struc-
tures—ferromagnetic and two-sublattice antiferro-
magnetic—can be described by the wave vectors
equal, respectively, to 0 or
1
2
K. Also, the magnetic
structures described by an intermediate value of vec-
tor q were found experimentally.
The spiral magnetic structure can be defined by the
formula:
m
n
¼ mðcosðq R
n
Þsiny; sinðq R
n
Þsiny; cosyÞð14Þ
where m
n
is the magnetic moment of the nth atom
and (q R
n
), y are polar coordinates. For vector q
incommensurate with the vectors of the reciprocal
lattice, the magnetic unit cell representing a transla-
tionally invariant part of the magnetic crystal is in-
finite. The spiral structure possesses, however, a
special symmetry which, for an arbitrary q, allows
reduction of the consideration to the unit cell of the
chemical lattice.
The spiral structure, Eqn. (14), is invariant with
respect to the translation of the crystal by an arbi-
trary lattice vector R
n
, accompanied by the rotation
of all atomic moments by an angle qR
n
about the z
axis. Such operations, {(qR
n
)7R
n
}, are called gener-
alized translations. The Kohn–Sham Hamiltonian
of the magnetic moments of the spiral structure
(Sandratskii 1998) commutes with the generalized
translations {(qR
n
)7R
n
}. As a consequence of the
generalized translational symmetry, a generalized
Bloch theorem can be formulated:
fðqR
n
Þ7R
n
gc
k
ðrÞ¼expðikR
n
Þc
k
ðrÞð15Þ
where the vectors k lie in the first Brillouin zone
of the chemical lattice. The theorem, Eqn. (15), is
sufficient for the reduction of the problem, for an
arbitrary value of q, to the consideration of the
chemical unit cell of the crystal.
The formation of spiral structure leads to the hy-
bridization of the states of the nonmagnetic crystal
which are separated in the reciprocal space by vector
q. The importance of this hybridization is reflected in
the formula for the susceptibility, Eqn. (13), and is
closely related to the phenomenon of the Fermi sur-
face nesting discussed in the case of chromium.
Figure 3 shows the calculated q-dependence of
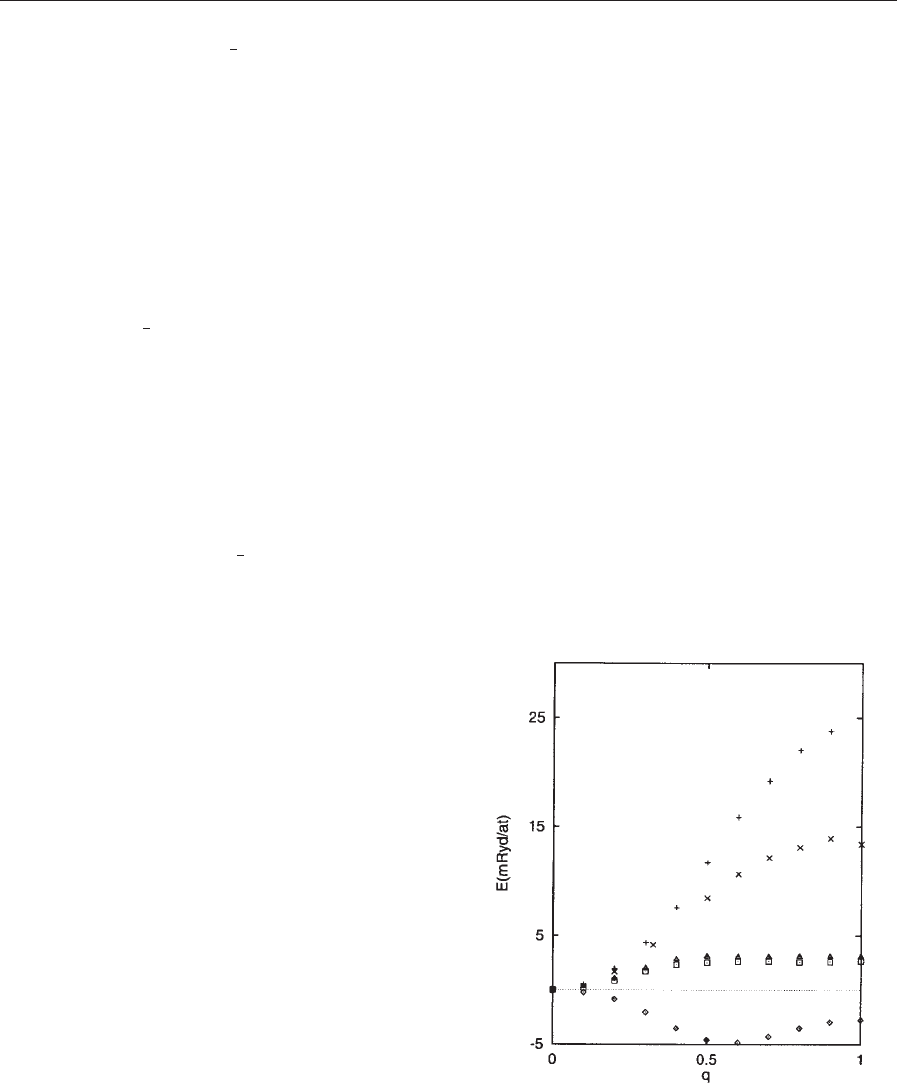
the total energy for a number of transition metals
(Sandratskii and Ku
¨
bler (1992); see also the paper by
Mryasov et al. (1991) for the first, first-principles
calculation of g-iron). In agreement with experiment,
the ground state was found to be ferromagnetic for
all metals, except for the case of g-iron. In g-iron, the
minimum of the total energy occurs at a finite value
of q, i.e., the ground state is spiral.
Another example of the spiral structure is that in
the heavy rare-earth metals (REM) (see Localized 4f
and 5f Moments: Magnetism; Magnetism in Solids:
General Introduction). The intra-atomic exchange
interaction between the 4f states and the valence 5d
and 6s states leads to the spin polarization of the
valence states. In the crystal, the valence states of
different atoms hybridize and form energy bands.
The energy of the hybridized valence states depends
on the directions of the 4f moments of different
atoms.
Figure 3
The total energy as a function of q for the (001)
direction. B ¼g-Fe; þ¼a-Fe; & ¼fcc-Co; ¼hcp-
Co; ¼Ni. In all calculations y ¼901. (Sandratskii and
Ku
¨
bler (1992)).
108
Density Functional Theory: Magnetism
Early calculations of the q-dependence of the un-
enhanced susceptibilities (Jensen and Mackintosh
1991) gave the maximum at the q values close to
the experimentally determined wave vectors of the
spiral structures. In Fig. 4, a first-principles calcula-
tion by Nordstro
¨
m and Mavromaras (1999) of the
total energy of thulium as a function of q is shown.
2.4 Role of the Spin–Orbit Coupling
In the calculations of the magnetic structure dis-
cussed up to now, the SOC (see Sect. 1.3) was not
taken into account. With the SOC being neglected, an
arbitrary rotation of all atomic magnetic moments by
the same angle does not change the energy. However,
the dependence of the energy of the system on the
direction of the magnetic moments with respect to the
lattice, that is, the magnetic crystalline anisotropy, is
a property of primary importance in numerous tech-
nical applications (see also Sect. 3).
In bcc iron, the energy of magnetic anisotropy is of
the order of 10
4
mRy per atom, and is five orders of
magnitude smaller than the interatomic exchange in-
teraction. In the case when the SOC energy becomes
comparable with the interatomic exchange interac-
tion, it influences substantially not only the direction
of the atomic moments with respect to the lattice, but
also the relative directions of the moments. Indeed,
experimental determination of the magnetic structure
in heavy REM revealed a distortion of the spiral
structure due to the influence of the magnetic anisot-
ropy (Jensen and Mackintosh 1991).
The influence of the SOC on the formation of the
magnetic structure increases further when one moves
from the 4f systems (see Localized 4f and 5f Moments:
Magnetism) to the 5f systems (see 5f Electron Sys-
tems: Magnetic Properties). The most effort has been
devoted to the magnetic structure of uranium com-
pounds. The hybridization of the 5f states with the
states of other atoms is essential for the physics of the
uranium compounds. On the other hand, the 5f states
experience strong spin–orbit coupling.
The importance of the SOC can be illustrated by
the phenomenon of the symmetry-predetermined
noncollinearity of the magnetic structure. To intro-
duce this effect, let us consider a collinear magnetic
structure, and let y be a parameter which describes
the noncollinear magnetic structures obtained with
the deviation of the magnetic moments from the col-
linear directions. y
0
¼0 corresponds to the collinear
structure. The following criterion of the instability of
the magnetic structure can be formulated: the struc-
ture corresponding to y ¼y
0
can be stable only in the
case that this state is distinguished by symmetry,
compared to the states obtained with infinitesimal
variation of parameter y (Sandratskii (1998) and ref-
erences therein). Indeed, only in this case can the
minimum of the total energy be at y ¼0.
The effect of the symmetry-predetermined noncol-
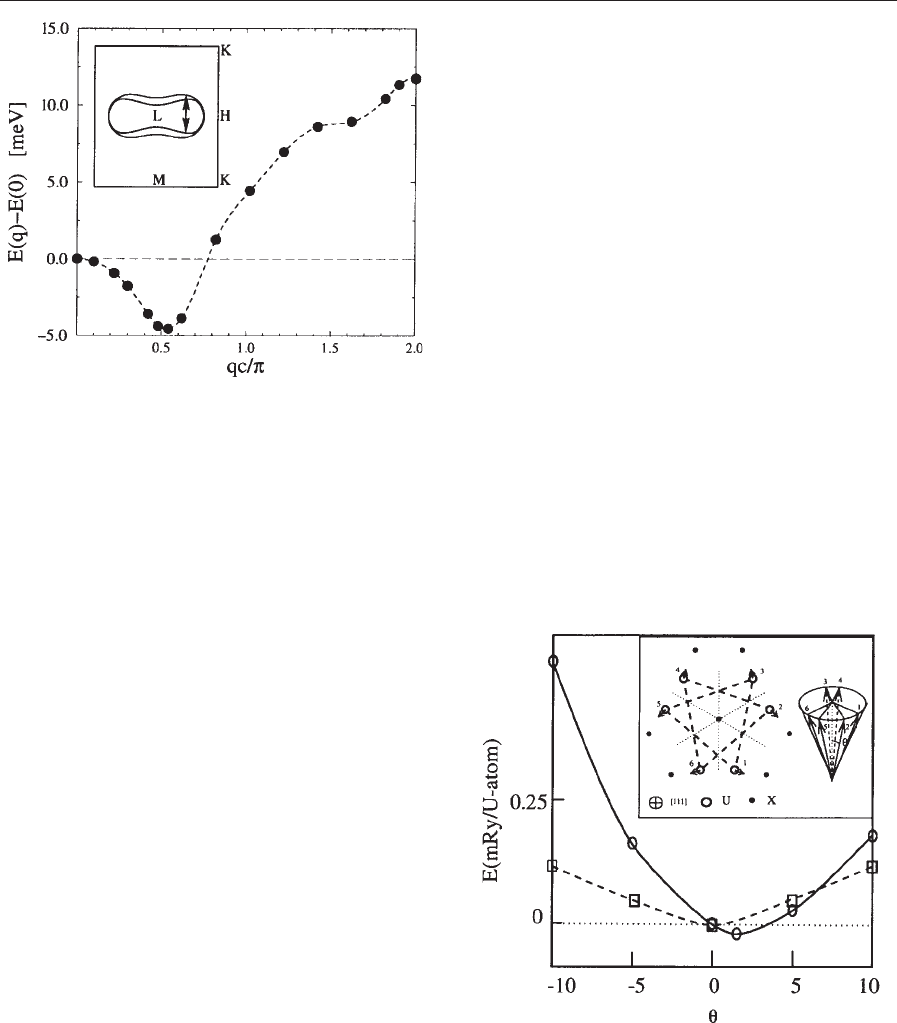
linearity is illustrated in Fig. 5 by the example of
U
3
P
4
. The inset shows the experimentally deter-
mined magnetic structure of U
3
P
4
. An SDFT calcu-
lation starting with the collinear ferromagnetic
Figure 5
The total energy of U
3
P
4
as a function of the deviation
of the magnetic moments from the (111) axis. Solid/
dashed line shows the result of the calculation with/
without the spin–orbit coupling, respectively. The inset
shows the projection of the crystal and magnetic
structure to the (111) plane. The magnetic moments
form a cone structure.
Figure 4
The q-dependence of the total energy of T
m
. The inset
shows the nesting property of the Fermi surface.
(Nordstro
¨
m and Mavromaras (1999))
109
Density Functional Theory: Magnetism
