Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.

FLUIDS, ELECTROLYTES, & ACID-BASE
67
Therapy of these disorders should be aimed at identifying
the source of the aldosterone or other mineralocorticoid.
When the condition follows excessive administration of min-
eralocorticoids, their use should be stopped. If the patient
has excessive aldosterone secretion from an adrenal ade-
noma, medical management with an inhibitor of aldosterone
(eg, spironolactone) may play a role until more definitive
therapy can be planned.
Galla JH: Metabolic alkalosis. J Am Soc Nephrol 2000;11:369–75.
[PMID: 10665945]
Khanna A, Kurtzman NA: Metabolic alkalosis. J Nephrol 2006;19:
S86–96. [PMID: 16736446]
Respiratory Acidosis
ESSENTIALS OF DIAGNOSIS
Acidemia with increased Pa
CO
2
and near-normal (acute)
or appropriately elevated [HCO
3
–
] (chronic).
Fatigue, weakness, confusion, and headaches.
If severe, lethargy, stupor, and coma.
Decreased cardiac contractility, pulmonary artery hyper-
tension, and splanchnic vasodilation.
General Considerations
Elevated Pa
CO
2
(hypercapnia) with resulting acidemia is
termed respiratory acidosis. After going into solution, dis-
solved CO
2
turns into hydrogen ion and bicarbonate. The
major problem in acute hypercapnia is that dissolved CO
2
can rapidly produce tissue acidosis because CO
2
diffuses eas-
ily into tissues and cells. This is particularly important at the
blood-brain barrier, such that the pH of CSF falls rapidly
after an acute increase in Pa
CO
2
.
Hypercapnia is usually attributed to lung disease, such as
COPD. However, hypercapnia can be produced either by an
increase in the production of CO
2
without compensatory
elimination or by constant production with decreased elimi-
nation. The second mechanism is usually seen in patients with
COPD or restrictive pulmonary disease, in those with a
severely deformed chest wall or neuromuscular weakness, after
trauma, and following anesthesia, where either the respiratory
mechanics or the drive for CO
2
elimination are compromised.
Increased CO
2
production is not uncommon because
CO
2
output follows metabolic rate, and patients in the ICU
are frequently hypermetabolic. What is unusual, however, is
failure of the ventilatory control mechanisms to respond to
the increase in CO
2
production by stimulating ventilation
and maintaining Pa
CO
2
at a constant value.
Common causes of respiratory acidosis among criti-
cally ill patients are listed in Table 2–19, and there is
additional discussion of hypercapnic respiratory failure in
Chapter 12.
An acute change in Pa
CO
2
produces a blood pH change
within minutes. There is a small rise in plasma bicarbonate
concentration owing to acute “mass action” shifts. The pre-
dicted response of [HCO
3
–
] is an increase of approximately
0.25 meq/L for each 1 mm Hg increase in Pa
CO
2
.More
marked changes in plasma bicarbonate concentration sug-
gest that a mixed acid-base disturbance is present.
Hypercapnia stimulates renal ammonia production and
increases urinary ammonium excretion because of an increase
in local Pa
CO
2
and because of the fall in pH. Urine pH decreases
appropriately as newly generated bicarbonate is added to the
blood in exchange for acidifying the urine. An increase in bicar-
bonate absorptive capacity also occurs so that increased quanti-
ties of filtered bicarbonate can be reabsorbed completely. Once
equilibrium has been reached after several days, the plasma
bicarbonate concentration should increase by about 0.5 meq/L
for each 1 mm Hg increase in Pa
CO
2
. Arterial pH actually may
become slightly alkalemic because of the avid retention of bicar-
bonate; this is one situation in which “complete”correction may
occur and may not represent a mixed acid-base disturbance.
Clinical Features
A. Symptoms and Signs—There are no symptoms or signs
specific to mild respiratory acidosis. Findings are usually
Acute Chronic
Airway obstruction
Emesis with aspiration
Bronchospasm
Laryngospasm
Airway obstruction
Chronic obstructive pulmonary
disease
Respiratory center depression
General anesthesia
Sedative or narcotic over-dose
Head injury
Respiratory center depression
Chronic sedative overdose
Obesity (Picwickian syndrome)
Brain tumor
Circulatory collapse
Cardiac arrest
Pulmonary edema
Neurogenic causes
Cervical spine injury
Guillain-Barré syndrome
Myasthenic crisis
Drugs (paralytic agents,
organophosphates)
Neurogenic causes
Multiple sclerosis
Muscular dystrophy
Amyotrophic lateral sclerosis
Myxedema
Posttraumatic diaphragmatic
paralysis
Phrenic nerve injury
Restrictive defects
Hemothorax or pneumothorax
Flail chest
ARDS
Restrictive defects
Hydrothorax or fibrothorax
Ascites
Obesity
Table 2–19. Causes of respiratory acidosis.
CHAPTER 2
68
related to the underlying cause, such as COPD, obesity-
hypoventilation syndrome, CNS disease, or severe hypothy-
roidism. When airway obstruction is the cause, patients may
present with shortness of breath and labored breathing. If
respiratory center depression is the cause, slow and shallow
or even apneustic breathing may be noted. As discussed in
Chapter 12, patients may have tachypnea or hyperpnea
(increased minute ventilation) despite hypercapnia (alveolar
hypoventilation).
In cases of marked respiratory acidosis, fatigue, weakness,
and confusion are present. In milder cases, patients may
complain of headache. Physical findings are nonspecific and
include tremor, asterixis, weakness, incoordination, cranial
nerve signs, papilledema, retinal hemorrhages, and pyram-
idal tract findings. The syndrome of pseudotumor cerebri
(increased CSF pressure and papilledema) may be simu-
lated by respiratory acidosis. Coma begins at levels of CO
2
that vary from 70–100 mm Hg depending on arterial pH
(pH <7.25) and the rate of increase of Pa
CO
2
. It is critical to
remember that almost all patients with hypercapnia will have
concomitant hypoxemia unless they are receiving supple-
mental oxygen.
B. Laboratory Findings—Respiratory acidosis is manifested
by acidemia and elevated Pa
CO
2
in the presence of an appro-
priate [HCO
3
–
] (see Figure 2–5). Because renal ammonia
production and hydrogen ion secretion are stimulated, urine
pH falls. In chronic respiratory acidosis, plasma pH may be
very close to normal as bicarbonate concentration rises in
compensation. A mild increase in potassium secretion
occurs, although hypokalemia is not inevitable.
Treatment
The key to management of respiratory acidosis is correction
of its primary cause (see Chapter 12). For some patients, this
will require endotracheal intubation and mechanical ventila-
tion or noninvasive positive-pressure ventilation.
Restoration of pH and Pa
CO
2
should take place over sev-
eral hours if the respiratory acidosis is chronic to prevent
alkalemia. The compensatory increase in plasma bicarbonate
in response to hypercapnia may take hours to days to be
eliminated if the Pa
CO
2
is immediately corrected to normal.
This results in a form of “metabolic alkalosis” that requires
renal elimination of bicarbonate to a normal value. In many
patients, overcorrection of chronic hypercapnia to normal is
not advised because these patients have poor lung or ventila-
tory function. When mechanical ventilation is removed, they
will be unable to maintain sufficient ventilation to keep the
Pa
CO
2
at the lower level, resulting in recurrence of severe
acute respiratory acidosis.
In general, there is no role for respiratory stimulant drugs
except in a few circumstances. Administration of antagonists
to opiates or benzodiazepines may be helpful if respiratory
depression from these agents is suspected.
Current Controversies and Unresolved Issues
Acute hypercapnia and respiratory acidosis almost always
can be reversed effectively by increasing minute ventilation
until the underlying disorder can be treated (eg, COPD, neu-
romuscular weakness, etc.). It is now recognized that exces-
sively high tidal volume may be associated with damage to
the lungs, prolonged hospitalization, and increased mortal-
ity. Therefore, low tidal volume strategies are recommended
(see Chapter 12). The consequence is mild to moderate
hypercapnia in some of these patients. The bulk of the evi-
dence from large studies suggests that such hypercapnia is
well tolerated and rarely associated with complications. In
fact, some studies have suggested that hypercapnia is not
merely a consequence that must be tolerated but that it actu-
ally may be instrumental in improving outcomes of patients
with acute lung injury (eg, ARDS). For example, in ARDS, a
tidal volume of 6 mL/kg was associated with lower mortality
than 12 mL/kg. However, in the patients randomized to
12 mL/kg, the odds ratio for mortality was 0.14 in those who
were hypercapnic compared with those with a normal Pa
CO
2
.
While there have been limited studies of the effects of
ameliorating the fall in pH during so-called permissive
hypercapnia, it remains to be seen if preventing the fall in pH
with bicarbonate or other buffers is beneficial, hazardous, or
neither. Nonbicarbonate buffers may be preferred to avoid
generation of additional CO
2
.
Kallet RH, Liu K, Tang J: Management of acidosis during lung-
protective ventilation in acute respiratory distress syndrome.
Respir Care Clin North Am 2003;9:437–56. [PMID: 14984065]
Kregenow DA et al: Hypercapnic acidosis and mortality in acute
lung injury. Crit Care Med 2006;34:1–7. [PMID: 16374149]
Laffey JG, Engelberts D, Kavanagh BP: Buffering hypercapnic aci-
dosis worsens acute lung injury. Am J Respir Crit Care Med
2000;161:141–6. [PMID: 10619811]
Respiratory Alkalosis
ESSENTIALS OF DIAGNOSIS
Alkalemia with decreased Pa
CO
2
and normal or appro-
priately decreased [HCO
3
–
].
Anxiety, irritability, vertigo, and syncope.
Flattened ST segments or T waves.
Tetany in severe cases.
General Considerations
A primary decrease in arterial P
CO
2
(hypocapnia) indicates
respiratory alkalosis. By definition, alveolar hyperventilation
is synonymous with hypocapnia. The most common causes

FLUIDS, ELECTROLYTES, & ACID-BASE
69
of hyperventilation include hypoxemia, CNS disorders,
pulmonary disease, and excessive mechanical ventilation.
Patients who are anxious, pregnant, have liver failure, or are
toxic from salicylates often will hyperventilate. A few patients
seem to have primary hyperventilation of unknown mecha-
nism. In the ICU, hyperventilation may be an early feature of
sepsis. Hyperventilation resulting in respiratory alkalosis
must be distinguished from the low Pa
CO
2
seen as compen-
sation for metabolic acidosis. In both, Pa
CO
2
is reduced and
plasma HCO
3
–
is low. The difference is that in respiratory
alkalosis, low Pa
CO
2
is primary and pH is above normal,
whereas in metabolic acidosis, pH is in the acidic range and
low HCO
3
–
is the primary disturbance.
The principal compensatory response for respiratory
alkalosis is renal elimination of bicarbonate, which takes sev-
eral hours to days to complete. Hypocapnia itself reduces
bicarbonate reabsorption from the proximal tubule, but
hydrogen ion secretion in the distal nephron is also
decreased, resulting in loss of tubular bicarbonate. Increased
delivery of bicarbonate from the proximal tubule stimulates
a marked kaliuresis. In the steady state, plasma bicarbonate
concentration falls by about 0.5 meq/L for each 1 mm Hg
decrease in Pa
CO
2
during chronic respiratory alkalosis, and
there is a smaller decreased in plasma bicarbonate with acute
respiratory alkalosis. The arterial pH therefore is corrected
toward normal but not to normal.
Clinical Features
A. Symptoms and Signs—Severe hyperventilation may
result in tetany that is clinically indistinguishable from the
hypocalcemic variety except that total plasma calcium and
the ionized fraction of calcium are normal. Hyperventilation
also may decrease blood pressure and cerebral perfusion,
which can cause increased irritability, anxiety, and inability
to concentrate. Occasionally, awake patients will complain of
vertigo and experience syncope. Other features are those of
the underlying disorder leading to respiratory alkalosis.
Patients with severe damage to the midbrain may have cen-
tral neurogenic hyperventilation. Prolonged respiratory alka-
losis may have adverse effects on patients with head injury
despite transient reduction in intracranial pressure acutely
largely because of decreased oxygen delivery and unloading
in the brain. Interestingly, respiratory but not metabolic
alkalosis may impair fluid resorption from lungs with pul-
monary edema.
B. Laboratory Findings—The hallmark of respiratory alka-
losis is the presence of alkalemia (pH >7.44) and decreased
Pa
CO
2
in the presence of normal or decreased HCO
3
–
.The
extent of plasma bicarbonate reduction depends on the
duration of the respiratory disorder and the effectiveness of
the kidneys. The nomogram in Figure 2–5 may aid in deter-
mining whether the respiratory alkalosis is occurring alone
or is a mixed disorder. Most patients with chronic respira-
tory alkalosis will have a decline in plasma bicarbonate of
0.5 meq/L for each 1 mm Hg decrease in Pa
CO
2
. Mild
hyponatremia and hypochloremia often are present.
Hypophosphatemia owing to excess renal phosphate wasting
seems to be more marked with respiratory alkalosis than in
metabolic alkalosis.
For patients with central neurogenic hyperventilation,
evaluation may include CT scan or MRI of the head. Drug
ingestions (particularly salicylates) can be investigated with a
toxicology screen or blood salicyate determination.
Because hypoxemia appropriately may stimulate respira-
tory drive and cause respiratory alkalosis, the adequacy of
arterial oxygenation must be assessed.
C. Electrocardiography and Electroencephalography—
Electrocardiographic changes may include ST-segment or T-
wave flattening or inversion. Alterations in the QRS complex
also have been reported. Electroencephalographic studies are
usually normal but may show an increase in the number of
slow high-voltage waves.
Differential Diagnosis
The most important differential is metabolic acidosis with
respiratory compensation. As described earlier, in metabolic
acidosis, the blood pH is less than 7.38, whereas respiratory
alkalosis is associated with alkalemia. Mixed or combined dis-
turbances are often seen with respiratory alkalosis—notably
salicylate overdose, which may cause primary metabolic aci-
dosis and primary respiratory alkalosis simultaneously.
Treatment
A. Correction of Underlying Disorder—The key to treat-
ment is identification and management of underlying disor-
ders. If the patient is hypoxemic, the inspired oxygen
concentration may need to be increased. Anemia also may be
contributory and may be helped by blood transfusion. Other
potentially reversible causes include sepsis and liver failure.
Severe CNS disorders may cause respiratory alkalosis.
B. Mechanical Ventilation—Probably the most common
cause of respiratory alkalosis among critically ill patients is
iatrogenic hyperventilation owing to excessive mechanical
ventilation. Strict attention to blood gases and examination
of trends over several days usually will disclose this problem.
If the ventilator has been set to deliver too much minute ven-
tilation and the patient is not triggering, reducing the respi-
ratory rate and tidal volume will cause a marked and
predictable fall in pH. One reasonable goal is to reduce
minute ventilation just until the patient begins to trigger
spontaneous ventilation. At this point, pH is likely to be near
normal.
On the other hand, if the patient is already triggering the
mechanical ventilator, that is, choosing the respiratory rate,
then he or she is generating the primary drive for hyperventi-
lation. In most of these cases, changing the settings on the
CHAPTER 2
70
ventilator will not affect the patient’s spontaneous respiratory
rate. Hyperventilation sometimes can be moderated by
increasing paradoxically the inspiratory flow rate or tidal
volume.
Clinicians often opt for intermittent mandatory ventila-
tion to treat respiratory alkalosis; controlled trials have
shown that this is ineffective. Adding dead space to the ven-
tilator circuit tubing should not be done. In rare circum-
stances, severe respiratory alkalosis that cannot be managed
in any other way may require paralyzing the patient and con-
trolling the Pa
CO
2
and pH.
Laffey JG, Kavanagh BP: Hypocapnia. N Engl J Med
2002;347:43–53. [PMID: 12097540]
Laffey JG, Kavanagh BP: Carbon dioxide and the critically ill: Too
little of a good thing? Lancet 1999;354:1283–6. [PMID:
10520649]
Myrianthefs PM et al: Hypocapnic but not metabolic alkalosis
impairs alveolar fluid reabsorption. Am J Respir Crit Care Med
2005;171:1267–71. [PMID: 15764729]
Wise RA, Polito AJ, Krishnan V: Respiratory physiologic changes in
pregnancy. Immunol Allergy Clin North Am 2006;26:1–12.
[PMID: 16443140]

71
The discovery of the ABO and Rh blood groups and the
development of nontoxic anticoagulant-preservative solu-
tions for blood storage during the first half of the 20th cen-
tury made it possible for human blood to be widely used as
lifesaving therapy in critically ill patients. Subsequent refine-
ments in cross-matching and the development of sophisti-
cated screening tests for transmissible diseases have made
blood transfusion a safe and often lifesaving form of therapy.
Because of the wide range of potential adverse effects of
transfusion therapy, however, the clinician must have a clear
understanding of the indications, efficacy, and complications
of blood component therapy.
BLOOD COMPONENTS
In modern transfusion practice, blood is separated into vari-
ous components (see Table 3–1), and individual components
are selected for transfusion based on the needs of the patient.
Blood component therapy is superior to whole blood
replacement because it concentrates those portions of blood
a patient needs, thereby increasing efficiency and minimizing
volume and subsequent transfusion requirements—as well
as increasing the efficiency of blood banking by putting
donated blood to maximal and optimal use.
Red Blood Cells
The products available for replacement of red blood cells are
listed in Table 3–1. Homologous packed red blood cells from
volunteer donors are transfused most often. Leukocyte-poor
red blood cells are prepared by a variety of techniques to
remove at least 70% of leukocytes. Washing red blood cells
in saline removes most plasma proteins and some leukocytes
and platelets. Red blood cells frozen in liquid nitrogen with
glycerol as a cryoprotective agent can be stored for up to
10 years. Extensive washing after thawing removes most
plasma proteins and cellular debris. Neocytes (young red blood
cells) can be prepared by differential centrifugation or cell
separators and have a longer circulating life span than stan-
dard red cells, but they are rarely used. Directed donations of
red blood cells from ABO- and Rh-compatible individuals
who are appropriately screened may be substituted for
homologous red blood cells at the patient’s request.
Autologous red blood cells may be collected preoperatively,
by perioperative blood salvage, or by acute normovolemic
hemodilution to decrease homologous red blood cell use.
Indications
Red blood cell transfusions are indicated to promote oxygen
delivery in patients who are actively bleeding, for sympto-
matic anemia unresponsive to conservative management, or
when time does not permit alternative treatment. Red blood
cell transfusions also may be useful for improving the bleed-
ing tendency of a severely anemic patient with platelet dys-
function (eg, uremia) or severe thrombocytopenia.
The decision to transfuse red blood cells should be made
only after consideration of several factors. The age and gen-
eral condition of the patient and the presence of coexisting
cardiac, pulmonary, or vascular conditions will influence the
patient’s ability to tolerate acute blood loss or chronic ane-
mia. The degree and chronicity of the anemia are also impor-
tant determinants of the physiologic responses to anemia.
Finally, the cause of the anemia must be considered because
alternative therapy (eg, iron sulfate, vitamin B
12
, folate, or
epoetin alfa [erythropoietin]) may eliminate the need for
transfusions altogether.
A. Chronic Hypoproliferative Anemia—Chronic anemia is
accompanied by several physiologic adaptations that enhance
oxygen delivery despite a reduced red blood cell oxygen-
carrying capacity. Increased cardiac output, increased
intravascular volume, and redistribution of blood flow to vital
organs maintain organ function. Tissue extraction of oxygen
occurs over a wide range of hemoglobin concentrations and is
3
Transfusion Therapy
Elizabeth D. Simmons, MD
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.
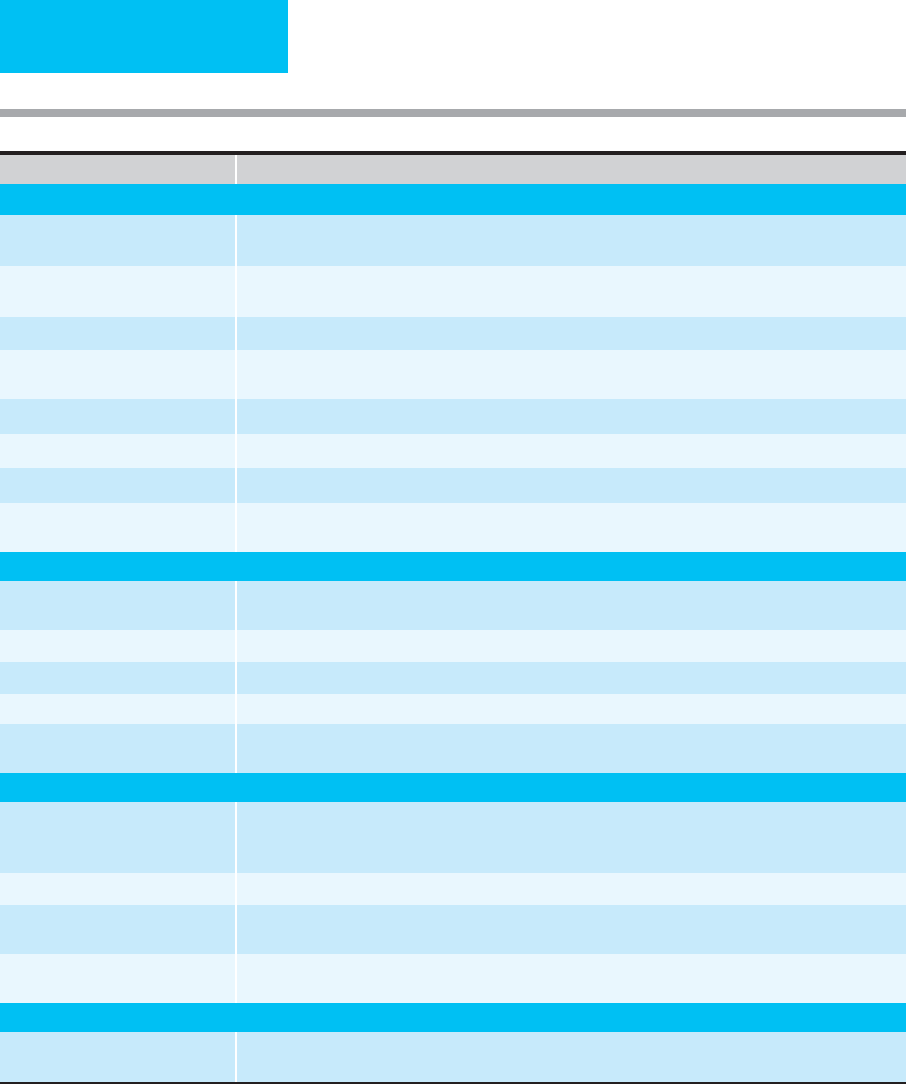
Table 3–1. Blood component therapy.
enhanced by a rightward shift in the oxyhemoglobin dissoci-
ation curve (owing to increased erythrocyte 2,3-DPG produc-
tion and the Bohr effect). Additional responses to anemia
include increased erythropoietin production and early release
of young red blood cells into the circulation. These adaptive
responses allow most individuals to tolerate severe decreases
in oxygen-carrying capacity. Therefore, red blood cell transfu-
sions are rarely necessary for patients with chronic anemia
who have hemoglobin concentrations above 7 g/dL unless
significant cardiopulmonary disease is present, and transfu-
sions may result in circulatory overload if given rapidly or in
excessive quantity.
CHAPTER 3
72
Products Available Indications for Transfusion
Red Blood Cells (RBC)
Homologous packed RBC Promote oxygen delivery for patients with active bleeding or severe anemia; improve bleeding tendency in
severely anemic patients with platelet dysfunction; replace sickle RBC with normal RBC by exchange transfusion.
Leukocyte-poor RBC Reduce febrile reactions; prevent HLA alloimmunization and CMV infection in potential transplant recipients or
those requiring chronic platelet transfusions.
Irradiated RBC Reduce graft-versus-host disease.
Washed RBC Substitute for homologous RBC in patients sensitive to a plasma component; avoid transfusion of anti-A and
anti-B antibodies when O-negative blood is used in patients who are type A, B, or AB.
Frozen RBC Preserve autologous RBC; maintain store of rare blood types.
Neocytes Increase efficacy of individual transfusion for patients with transfusion-dependent anemia.
Directed donor RBC After screening and informed consent, may be substituted for volunteer RBC at patient request.
Autologous RBC Decrease or eliminate need for homologous RBC in patients undergoing elective surgical procedures or obstetric
delivery.
Platelets
Random donor platelets Treat or prevent bleeding associated with severe thrombocytopenia or platelet dysfunction; replace platelets
lost with massive bleeding; treat excessive bleeding associated with cardiopulmonary bypass.
Platelet pheresis Decrease exposure to infectious agents.
Leukocyte-poor platelets Reduce febrile reactions; reduce HLA alloimmunization for patients requiring chronic platelet transfusions.
Irradiated platelets Reduce graft-versus-host disease.
HLA-matched platelets Treat bleeding associated with thrombocytopenia in patients who are refractory to platelet transfusions due to
HLA sensitization.
Plasma and Derivatives
Fresh frozen plasma (FFP) Correct coagulation factor deficiencies in bleeding patients or those who require invasive procedures if
concentrated or recombinant product not available; treat TTP/HUS, protein-losing enteropathy in infants;
antithrombin III deficiency; C-1 esterase inhibitor deficiency.
Fresh plasma (liquid plasma) Same as FFP, except does not contain factors V and VIII.
Cryoprecipitate-poor plasma Correct coagulation factor deficiencies other than VIII, XIII, fibrinogen, vWF; may be indicated for treatment of
refractory TTP.
Cryoprecipitate Correct severe hypofibrinogenemia; may be useful for treatment of bleeding associated with uremia; provides
factors VIII and XIII, fibrinogen, and vWF.
Granulocytes
Stimulated leukapheresis Treat severe bacterial infections unresponsive to antibiotics in patients with prolonged, severe neutropenia or
congenital neutrophil dysfunction; may be indicated in the management of neonatal sepsis.

TRANSFUSION THERAPY
73
B. Acute Blood Loss—In contrast, physiologic responses
may be inadequate to maintain organ function and hemody-
namic stability following acute blood loss, even with appar-
ently normal hemoglobin concentration, because it takes time
for mobilization of extracellular fluid into the intravascular
space and for increased production of erythrocyte 2,3-DPG.
However, a healthy young person generally tolerates
500–1000 mL of acute blood loss without red blood cell
transfusion, and intravascular volume can be repleted with
crystalloid solutions. Acute blood loss of 1000–2000 mL usu-
ally can be managed with volume replacement alone, but red
blood cell transfusions are necessary occasionally. More than
2 L of acute blood loss usually will require red blood cell
transfusion. Other clinical factors are important. For exam-
ple, because of the vasodilatory effects of anesthesia, intraop-
erative blood loss of more than 500 mL may require red blood
cell transfusion to maintain hemodynamic stability, and burn
patients often require vigorous blood product support
because of volume depletion through denuded body surfaces.
C. High-Risk Patients—Any condition that impairs the
patient’s ability to increase intravascular volume, heart rate,
stroke volume, or blood flow can result in poor tolerance of
chronic anemia or acute blood loss (eg, patients with cardio-
vascular disease, volume depletion from diuretics or gas-
trointestinal losses, vascular fluid redistribution, and elderly
patients). In these circumstances, transfusion may be neces-
sary in patients with physiologic signs of inadequate oxy-
genation despite higher hemoglobin concentrations than in
normal individuals with adequate physiologic reserves. The
use of objective scoring systems (such as the APACHE II
[Acute Physiology and Chronic Health Evaluation II] and the
multiorgan dysfunction scores) to stratify patients according
to severity of illness may be useful for determining which
critically ill patients may benefit from a restrictive transfu-
sion approach.
Studies have demonstrated that patients younger than
55 years of age with less severe illness may have improved
outcomes using a lower hemoglobin threshold (<7 g/dL) for
transfusion compared with more liberal use of transfusion
(<10 g/dL). This lower threshold for transfusion appears to
be at least as safe in older patients and those with more severe
disease as well.
Exceptions include those with acute bleeding (discussed
earlier) or myocardial ischemia. Although baseline anemia in
patients with acute myocardial infarction is associated with
adverse outcomes, including increased mortality, there is no
clear evidence that a liberal blood transfusion strategy (ie,
hemoglobin <10 g/dL as a trigger for transfusion) improves
outcomes. Available clinical data are inadequate to make a
firm recommendation regarding the hemoglobin threshold
for transfusion for these patients; therefore, decisions about
red blood cell transfusions must be individualized.
D. Hemolytic Anemia—Red blood cell transfusions are
indicated in the management of some patients with a variety
of severe and symptomatic hemolytic anemias. Patients with
markedly symptomatic antibody-mediated hemolytic ane-
mias may require red blood cell transfusion until definitive
therapy is effective. Autoantibodies are often reactive with all
donor red blood cells in vitro such that cross-matching is
impossible. Transfusion of ABO- and Rh-compatible red
blood cells is usually safe in these patients; the blood bank
can perform an extended cross-match to identify units with
the least degree of in vitro hemolysis. Patients with cold-
reacting antibodies (usually IgM) should receive blood
through a blood warmer if transfusion is necessary.
E. Sickle Cell Anemia—Patients with sickle cell anemia may
require red blood cell transfusion (and, in selected cases, par-
tial or complete exchange transfusion) for management of
specific complications, including splenic sequestration and
aplastic crises (with rapidly falling hemoglobin concentra-
tion), recurrent priapism, chronic unremitting osteomyelitis,
severe leg ulcers, pneumonia, or pulmonary sequestration
crises. Red blood cell transfusion is also indicated for such
patients undergoing major surgery, particularly those under-
going orthopedic procedures. Simple preoperative transfu-
sion to achieve hematocrit levels of about 30% appears to be
as effective as regimens aimed at reducing the fraction of
hemoglobin S to 30% of total hemoglobin (by exchange
transfusion or multiple transfusions over time) and is associ-
ated with fewer transfusion-related complications. Patients
with sickle cell anemia are not candidates for autologous
donation and transfusion.
Exchange transfusion is also indicated in the manage-
ment of acute central nervous system infarction or hemor-
rhage (followed by chronic transfusion therapy to prevent
recurrent strokes). Chronic prophylactic transfusion reduces
the risk of initial stroke in children with sickle cell disease
who have abnormal cerebrovascular blood flow on Doppler
ultrasonography; however, alloimmunization (even with
phenotypically matched, leukocyte-depleted red blood cells),
iron overload, and infections complicating chronic transfu-
sion programs have limited the acceptance of this approach.
Furthermore, the duration of transfusion required to prevent
stroke is unclear. Recent studies demonstrate that the risk of
stroke increases once chronic transfusions are stopped.
Routine transfusion during pregnancy should be avoided.
Patients with severe, symptomatic sickle cell anemia or those
suffering recurrent painful crises may require periodic trans-
fusion during pregnancy. Likewise, routine transfusion is not
indicated in the management of painful vaso-occlusive sickle
cell crises and should be reserved for patients with sympto-
matic anemia. Patients with sickle cell anemia appear to be
unusually susceptible to the development of alloantibodies
(see “Complications of Transfusion”), which limits the utility
of chronic transfusion programs. The use of blood from
racially matched donors that has been screened for selected
minor blood group antigens may prevent alloimmunization
in patients requiring chronic transfusion therapy, but this
approach awaits confirmation.

CHAPTER 3
74
F. Perioperative Transfusion—Transfusion is rarely indi-
cated for patients undergoing noncardiac surgery who have
hemoglobin values greater than 7–8 g/dL and no risk factors
for myocardial ischemia. However, elderly patients with hema-
tocrits less than 28% (hemoglobin of approximately 9 g/dL)
may be at risk for myocardial ischemia during surgery, espe-
cially if tachycardia is present. In these patients—and others at
risk for myocardial ischemia—a hemoglobin value of less than
10 g/dL probably warrants transfusion. The threshold for
intraoperative transfusion depends on many factors, such as
the presence of hemorrhage or coagulopathy, hemodynamic
instability, and ischemic electrocardiographic changes.
G. Unacceptable Indications—Red blood cell transfusions
should not be used to enhance a patient’s general sense of
well-being, to promote wound healing, or to expand vascular
volume when oxygen-carrying capacity is adequate.
Red Blood Cell Transfusion Requirements
There is no single hemoglobin threshold that is universally
appropriate for determining transfusion requirements. The
amount of red blood cells to be transfused should be deter-
mined by the clinical status of the patient rather than by the
hemoglobin concentration. In patients who are actively
bleeding, crystalloid volume repletion is essential.
Hemodynamic instability, symptoms and signs of impaired
organ function, rate of blood loss, and response to transfu-
sion should be used to determine how much blood should be
transfused. Patients with chronic anemia should receive only
the amount of red blood cells necessary to reverse symptoms
and signs. Patients with self-limited anemia (eg, transient
blood loss, hemolysis, or marrow suppression) or those for
whom alternative therapy is available (eg, nutritional defi-
ciencies, anemia of renal failure) should receive red blood
cells only when an immediate need for increased oxygen-
carrying capacity is present, such as during myocardial
ischemia, heart failure, impaired central nervous system oxy-
genation, hypotension, or other evidence of tissue hypoxia.
The patient should be reevaluated after each unit of red
blood cells is transfused rather than giving an arbitrary or
predetermined number of units. Volume overload following
red blood cell transfusion in patients with chronic severe
anemia may eliminate any benefit of increasing the oxygen-
carrying capacity and must be monitored carefully.
When untreatable chronic anemia is present (eg, bone
marrow failure or chronic severe hemolytic anemia), red
blood cell transfusions must be given conservatively to delay
long-term treatment complications such as alloimmuniza-
tion, infections, and iron overload. Red blood cell transfu-
sions may be administered more liberally in the treatment of
anemia associated with severe thrombocytopenia or platelet
dysfunction (eg, acute leukemia or uremic bleeding
episodes) because the salutary effect of increased hematocrit
on platelet function may decrease platelet transfusion
requirements and lessen clinical bleeding.
Platelets
Platelet products available are listed in Table 3–1. The choice
of platelet product depends on the underlying condition of
the patient (eg, acute reversible thrombocytopenia versus
chronic thrombocytopenia) as well as the local availability of
supplies. Pooled random-donor platelets or single-donor
platelets obtained by apheresis are the usual products trans-
fused for correction of severe thrombocytopenia. Filtration
or irradiation with ultraviolet B depletes donor platelets of
leukocytes, and these are equally effective strategies for pre-
venting alloantibody-mediated refractoriness to platelet
transfusions. Such leukodepletion is appropriate for patients
likely to require repeated platelet transfusions (eg, acute
leukemia, aplastic anemia, and other bone marrow failure
states). Leukocyte depletion performed shortly after collec-
tion of platelets also may decrease the risk of febrile reactions
by preventing in vitro accumulation of cytokines, which are
released during storage. Single-donor platelets decrease the
total number of donor exposures and may reduce the risk of
transfusion-transmitted infections but do not appear to offer
additional benefit over filtration or irradiation for preven-
tion of alloimmunization.
Product availability often will determine whether pooled
platelets or single-donor platelets are transfused. Whenever
possible, ABO type–specific platelets should be used; how-
ever, because platelets have a limited storage period, they are
not always available. A decreased response to platelet trans-
fusion may result from the use of ABO-incompatible
platelets, but the most significant risk occurs when ABO-
incompatible plasma is infused (ie, type O donor, type A or
B recipient), resulting in hemolysis (estimated risk
1:9000–1:6600). Apheresis units may increase this risk by
increasing the dose of incompatible plasma. If type-specific
platelets are not available, pooled platelets are preferable to
single-donor platelets. Washing the platelets to remove
plasma may help to minimize exposure to incompatible
plasma. Although platelets do not carry Rh antigens, platelets
from Rh-negative donors should be used for transfusion in
Rh-negative women of childbearing years to prevent sensiti-
zation from contaminating red blood cells.
Indications
Platelet transfusions are indicated for treatment of bleeding
associated with thrombocytopenia or intrinsic platelet dys-
function. Platelet transfusions are also indicated in the man-
agement of massive bleeding if severe thrombocytopenia
develops. Patients undergoing cardiopulmonary bypass may
require platelet transfusions if excessive bleeding occurs
because of thrombocytopenia and decreased platelet function
induced by the bypass procedure. Other surgical procedures
in thrombocytopenic patients generally require prophylactic
platelet transfusions to maintain adequate perioperative
platelet counts for at least 3 days (>50,000/μL for major pro-
cedures; >30,000/μL for minor procedures). Prophylactic

TRANSFUSION THERAPY
75
platelet transfusions are also indicated for severely thrombo-
cytopenic (eg, <10,000/μL platelets) patients undergoing
intensive chemotherapy for acute leukemia; the threshold for
transfusion may be higher in the presence of fever, infection,
or drugs that cause platelet dysfunction.
Factors that determine the risk of serious bleeding owing
to thrombocytopenia include the cause and severity of
thrombocytopenia, the presence of vascular defects, the
functional status of the patient’s platelets, and the presence of
other hemostatic defects. Severe anemia also may contribute
to bleeding in patients with thrombocytopenia or platelet
dysfunction. Because of the increased functional capacity of
younger platelets in patients with decreased platelet survival,
decreased production of platelets carries a higher risk of seri-
ous bleeding at any given platelet count than thrombocy-
topenia owing to destruction, consumption, or hypersplenism.
Typical bleeding manifestations related to the level of throm-
bocytopenia are shown in Table 17–8. If bleeding is out of
proportion to a given platelet count, other contributing factors
to bleeding should be investigated.
The risk of bleeding in patients with disorders of platelet
function likewise depends on the cause and severity of the dis-
order and whether vascular defects, other hemostatic abnor-
malities, or severe anemia is present. Bleeding time is the most
widely used test of platelet function, and although it is useful in
the diagnosis of certain disorders (eg, von Willebrand’s disease,
hereditary platelet disorders), prolonged bleeding time in the
absence of a history of bleeding is not a reliable predictor of
subsequent bleeding. A prolonged bleeding time in the absence
of thrombocytopenia or severe anemia in a bleeding patient,
however, may indicate the presence of platelet dysfunction.
The efficacy of platelet transfusions can be assessed by
observing a sustained rise in platelet count in a patient who
has stopped bleeding. Patients with thrombocytopenia
owing to decreased production of platelets are most likely to
experience a significant, sustained increase in platelet count
following platelet transfusion. Patients with increased
destruction of platelets and those who have hypersplenism
usually do not achieve a significant increase in platelet count
after transfusion, and any increase that occurs is usually tran-
sient. Similarly, patients with massive platelet consumption
owing to bleeding will have a suboptimal increase in platelet
count following transfusion. Hemorrhage owing to platelet
dysfunction can be controlled with platelet transfusions only
if the defect is intrinsic to the platelet (eg, aspirin ingestion,
cardiopulmonary bypass, inherited platelet disorders) rather
than extrinsic (eg, von Willebrand’s disease or uremia).
Platelet transfusions are minimally useful in the treatment
of thrombocytopenia owing to decreased platelet survival and
should not be given unless severe life-threatening bleeding
occurs. Platelet transfusions may be harmful in patients with
thrombotic thrombocytopenic purpura–hemolytic uremic
syndrome (TTP-HUS) despite the presence of thrombocy-
topenia, presumably owing to accelerated thrombosis in vital
organs. Because platelet survival is short in this disorder,
platelet transfusions usually are ineffective in controlling
hemorrhage. The diagnosis of TTP-HUS should be suspected
in a patient with severe thrombocytopenia and hemolysis with
schistocytes on peripheral blood smear (microangiopathic
hemolytic anemia) with or without associated central nervous
system dysfunction, renal dysfunction, or fever. Patients with
heparin-associated thrombocytopenia also may suffer
increased thrombotic complications if platelets are transfused.
Platelet transfusions should be administered to these patients
only when the risk of death from bleeding outweighs the
potential risk of clinical deterioration from transfusion.
Platelet Transfusion Requirements
The quantity of platelets to be transfused depends on the
source of the platelets, the cause and degree of thrombocytope-
nia, and the observed response to transfusions. The usual ini-
tial amount transfused is 6–8 units of random-donor platelets
or 1 unit of single-donor apheresis product. Platelet packs
should contain a minimum of 5.5 × 10
9
platelets per unit.
The response to platelet transfusions should be deter-
mined by obtaining a platelet count 1 hour after transfusion
and daily thereafter and by observing the effect on control of
bleeding. The 1-hour count should increase by about
5000–10,000 per unit of random-donor platelets or
30,000–50,000 per unit of single-donor platelets. Stored
homologous platelets survive about 3 days in thrombocy-
topenic patients. The 1-hour count and subsequent platelet
survival will be reduced in patients with increased destruc-
tion or hypersplenism. These measurements will help to
determine the magnitude of the benefit to be expected from
subsequent transfusions. If only a minimal response occurs,
or if the platelet rise is short-lived, subsequent prophylactic
transfusions should be withheld. However, in patients with
severe thrombocytopenia owing to destruction or hyper-
splenism who have serious bleeding, platelet transfusions
may be warranted. In any patient, if clinical bleeding does
not improve despite platelet transfusion, other causes of
bleeding should be evaluated and the utility of subsequent
platelet transfusions in such patients reassessed.
The underlying cause of thrombocytopenia or platelet
dysfunction should be determined so that specific therapy
to reverse the process can be given if available. Alternatives
to platelet transfusions in bleeding patients with thrombo-
cytopenia or platelet dysfunction are set forth in Table 3–2.
Plasma
Plasma products available are listed in Table 3–1. Fresh
frozen plasma (FFP) is prepared by separating plasma from
red blood cells (after collection of whole blood or during
plasmapheresis) and freezing it within 6 hours after collec-
tion at –18°C or colder. It can be stored for up to 1 year and
is thawed over 20–30 minutes prior to administration.
Activities of coagulation factors are adequate for 24 hours
after thawing. Fresh plasma and plasma recovered from out-
dated blood products are used for preparation of plasma
derivatives (eg, immunoglobulin, cryoprecipitate, albumin,
coagulation factor concentrates). Fresh plasma may be used
as an alternative to FFP for replacement of coagulation fac-
tors other than factors VIII and V.
Cryoprecipitate-poor plasma is the supernatant plasma
remaining after preparation of cryoprecipitate and contains
adequate quantities of all coagulation factors except fibrino-
gen, factors VIII and XIII, and von Willebrand factor.
Solvent-detergent treatment of plasma (S/D plasma) inac-
tivates lipid-enveloped viruses and has been licensed
recently by the Food and Drug Administration (FDA) to
minimize the risk of transfusion-transmitted infections
and allergic reactions in the management of coagulopathies
and thrombotic thrombocytopenic purpura (TTP). The
highest-molecular-weight von Willebrand factor multimers
are reduced in S/D plasma, enhancing its efficacy in the
treatment of TTP, but protein S and plasmin inhibitor levels
are also variably reduced, potentially causing venous throm-
boembolism (low protein S) or excessive bleeding (low plas-
min inhibitor). Numerous other derivatives of plasma are
now available; Table 3–3 outlines some of these products and
their therapeutic uses.
Alternative Possible Indications
High-dose IgG Life-threatening bleeding in immune-
mediated thrombocytopenia (ITP).
Anti-D immune
globulin
Treatment of bleeding in ITP in Rh-positive
patients.
Desmopressin
(DDAVP)
Bleeding associated with platelet dysfunction,
uremia, von Willebrand’s disease.
Antifibrinolytic agents
(eg, aminocaproic
acid)
Excessive bleeding without evidence of throm-
botic diathesis or hematuria.
Estrogens Bleeding associated with uremic platelet
dysfunction.
Red cell transfusions Severe anemia associated with thrombocy-
topenia or platelet dysfunction.
Erythropoietin Bleeding in anemic, uremic patients.
Corticosteroids ITP, possibly thrombotic thrombocytopenic
purpura-hemolytic uremic syndrome (TTP-HUS).
Splenectomy Refractory ITP, severe hypersplenism,
possibly TTP.
Immunosuppressives,
chemotherapy,
danazol, vinca
alkaloids, interferon
alpha, protein-A
immunoadsorption,
rituximab
Refractory ITP.
Plasma infusion or
exchange
TTP-HUS.
Table 3–2. Alternatives to platelet transfusions.
Table 3–3. Therapeutic products derived from plasma.
Plasma Derivative Therapeutic Use
Fibrin glue (human fibrinogen
combined with bovine
thrombin)
Prevent surgical oozing with
topical use
Albumin (heat-treated) Hypoalbuminemia in nephrotic
syndrome
Plasma-derived factor VIII
concentrate
Hemophilia A*
Humate-P von Willebrand’s disease
Prothrombin complex concentrate Coagulation inhibitors, factor X
and prothrombin deficiencies
Activated factor IX concentrates
(Autoplex, FEIBA)
Factor VIII inhibitors
Plasma-derived factor IX
concentrate
Hemophilia B*
Fibrinogen concentrate Hypofibrinogenemia
Factor VII concentrate Factor VII deficiency
Factor XI concentrate Factor XI deficiency
Factor XIII concentrate Factor XIII deficiency
Antithrombin III concentrate Thrombosis in antithrombin III
deficiency
C1 esterase inhibitor concentrate Angioedema
α
1
-Antitrypsin concentrate Prevent lung damage in
α
1
-antitrypsin deficiency
Protein C and S concentrate Severe protein C or S deficiency
Intravenous immunoglobulin Immunodeficiency states; immune
cytopenias, Kawasaki syndrome,
Guillain-Barré syndrome,
dermatomyositis
Immune serum globulin Passive immunization against hep-
atitis A, measles, poliomyelitis,
varicella, rubella
*Recombinant products are available as an alternative to plasma-
derived product; see Chapter 17.
CHAPTER 3
76
