Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.

DERMATOLOGIC PROBLEMS IN THE INTENSIVE CARE UNIT
627
myocarditis, encephalitis, and DIC also may occur along with
extensive hemorrhagic skin lesions.
In the normal host, herpes zoster is an acute, self-limited,
usually painful unilateral eruption in a dermatomal distribu-
tion. Characteristically, clusters of vesicles occur on a back-
ground of erythema. Occasionally—especially in persons
with compromised immune systems—herpes zoster may
become severe and necrotic. Wide dissemination of multiple,
small, varicella-like vesicles may develop. In such patients,
secondary bacterial infection is an added risk.
B. Laboratory Findings—A Tzanck smear demonstrates
diagnostic multinucleated epithelial giant cells. This test is
performed by scraping the base of a vesicular lesion with a
scalpel blade, transferring the material to a glass slide, and
staining with Giemsa’s or Wright’s stain. The Tzanck smear is
also positive in HSV infections. Culture of a lesion may con-
firm the diagnosis but requires approximately 10 days of
growth. An immunofluorescent antibody test using materials
from a lesion is also available. Acute and convalescent sera
may be helpful.
Differential Diagnosis
Varicella may be confused with widespread impetigo, dis-
seminated herpes zoster, disseminated herpes simplex,
eczema herpeticum, and smallpox. Eczema herpeticum is a
widespread cutaneous infection with HSV occurring in
patients with preexisting skin disorders such as atopic der-
matitis. The lesions in smallpox begin as red macules and
evolve in synchrony through vesicular and pustular stages,
and they predominately affect the face and extremities.
Treatment
Systemic acyclovir in the doses listed is recommended in the
following situations: (1) All VZV infections in immunocom-
promised individuals (10 mg/kg intravenously every 8 hours
for 7–10 days), (2) varicella in teenage and adult patients
(800 mg orally five times a day for 5–7 days), and (3) elderly
patients and patients with severe, painful, or destructive
zoster when seen within 48–72 hours after onset (800 mg
orally five times a day for 7 days). Alternative drugs for acute
herpes zoster are famciclovir, 500 mg three time daily for
7 days, and valacyclovir, 1 g three times daily for 7 days.
Secondary bacterial infection should be treated aggres-
sively. Cool compresses and antihistamines may help to
remove crusts and alleviate pruritus. Analgesics should be
provided as necessary.
Dworkin RH et al: Recommendations for the management of her-
pes zoster. Clin Infect Dis 2007;44:S1–26. [PMID: 17143845]
Gardella C, Brown ZA: Managing varicella zoster infection in preg-
nancy. Cleve Clin J Med 2007;74:290–6. [PMID: 17438678]
Heininger U, Seward JF: Varicella. Lancet 2006;368:1365–76.
[PMID: 17046469]
Rubeola (Measles)
ESSENTIALS OF DIAGNOSIS
Incubation period of 7–14 days, followed by high
fever, malaise, cough, coryza, and conjunctivitis with
photophobia.
Three to 5 days later, discrete erythematous macules
and thin papules appear on the forehead and behind
the ears, spreading to the trunk and extremities, with
coalescence and increased redness.
Koplik’s spots usually appear on buccal mucosa 1–2
days before exanthem.
Complications include secondary bacterial infection, oti-
tis media, pneumonia, viral myocarditis, liver function
abnormalities, and thrombocytopenia.
General Considerations
Rubeola is an acute epidemic disease characterized by
marked upper respiratory symptoms and a widespread ery-
thematous maculopapular rash. It is caused by a paramyx-
ovirus transmitted by inhalation of infected droplets. The
severity of the illness varies with the age and immunologic
status of the patient.
Clinical Features
A. Symptoms and Signs—After an incubation period of
7–14 days, a prodrome of high fever develops, associated
with malaise, cough, coryza, and conjunctivitis with photo-
phobia. In 3–5 days, discrete erythematous macules and thin
papules appear on the forehead and behind the ears. Over the
next few days, the lesions spread to the trunk and extremities
(including the palms and soles), coalesce on the face and
upper trunk, and intensify in color to a deeper red. The erup-
tion fades after 5–10 days in the order of its appearance, often
with fine desquamation and postinflammatory hyperpig-
mentation. Koplik’s spots—blue-white pinpoint macules
with a red halo—usually appear on the buccal mucosa 1–2
days before the exanthem and remain for several days. Up to
40% of immunocompromised patients with measles have no
rash; the remainder have either typical or unusual skin find-
ings, including urticarial plaques, petechiae, and palpable
purpura. Rubeola must be distinguished from cutaneous
drug reactions as well as other viral exanthems.
Complications may arise from viral dissemination, sec-
ondary bacterial infection, or hypersensitivity phenomena.
Otitis media, sinusitis, pneumonia, and liver function abnor-
malities are common. Viral myocarditis and thrombocy-
topenic purpura (owing to immune-mediated platelet
destruction) may occur. Death, resulting from pneumonitis
or encephalitis, occurs in about 0.1% of patients in the
CHAPTER 28
628
United States. The complication and case-fatality rates for
the very young, the elderly, the malnourished, and patients
with malignancies or HIV infection are significantly higher.
The clinical presentation in immunocompromised patients
is frequently atypical. One-third of such patients present
with no rash.
B. Laboratory Findings—Serologic studies of paired speci-
mens are the most practical method of confirming the diag-
nosis. Measles virus may be isolated from the blood, urine,
nasopharyngeal washings, and throat or from conjunctival
secretions.
Treatment
Therapy is supportive because no proven antiviral agent is
available. Aerosolized ribavirin may be beneficial for the treat-
ment of measles pneumonitis, but its effectiveness has not yet
been proved. Intravenous immunoglobulin and interferon
are other treatment options for measles pneumonitis and
encephalitis. Isolation precautions must be observed.
Garly ML et al: Prophylactic antibiotics to prevent pneumonia and
other complications after measles: Community-based ran-
domised, double-blind, placebo-controlled trial in Guinea-
Bissau. Br Med J 2006;333:1245. [PMID: 17060336]
Greenaway C et al: Susceptibility to measles, mumps, and rubella
in newly arrived adult immigrants and refugees. Ann Intern
Med 2007;146:20–4. [PMID: 17200218]
Perry RT, Halsey NA: The clinical significance of measles: A review.
J Infect Dis 2004;189:S4–16. [PMID: 15106083]
Meningococcemia
ESSENTIALS OF DIAGNOSIS
Petechial (or, less commonly, urticarial or morbilliform)
rash on the trunk and lower extremities; also on the
palms, soles, and mucous membranes; petechiae are
frequently palpable, with gun-metal gray centers and
irregular borders.
If complicated by purpura fulminans, extensive hemor-
rhagic bullae and areas of necrosis.
Other features of meningococcal meningitis or dissemi-
nated meningococcemia, including meningeal signs,
arthritis, myocarditis, pericarditis, and acute adrenal
infarction; hypotension and shock are often present.
Confirmation of Neisseria meningitidis by culture, Gram
stain, or immunologic tests.
General Considerations
N. meningitidis is a gram-negative diplococcus responsible
for a spectrum of illnesses ranging from a mild upper
respiratory infection to fulminant septicemia. Disease occurs
most often in children under age 15, with the attack rate
highest in infants 6–12 months of age. Peak incidence of
infection is in the winter and spring. Asymptomatic colo-
nization of the nasopharynx is common and provides a
source of person-to-person transmission through infected
droplets. People with deficiencies of the terminal compo-
nents of the complement cascade (C5–9) are particularly
susceptible to invasive and recurrent meningococcal disease.
The cutaneous lesions are a consequence of damage to small
dermal blood vessels both by direct bacterial involvement of
skin vessels and by lipopolysaccharide endotoxins.
Clinical Features
A. Symptoms and Signs—Invasive meningococcal disease
usually results in meningitis or meningococcemia. The incu-
bation period varies from 2–10 days. The onset may be
insidious, following a flulike illness, or abrupt, with fever,
chills, malaise, signs of meningeal irritation, prostration,
and shock. A rash that is characteristically petechial or, less
commonly, urticarial or morbilliform is often among the
earliest signs of generalized infection. The petechiae typi-
cally appear on the trunk and lower extremities but also can
be found on the palms, soles, and mucous membranes. They
are frequently palpable, with gun-metal-gray centers and
irregular borders.
Extensive hemorrhagic bullae and areas of necrosis
develop in patients with meningococcemia whose disease
is complicated by purpura fulminans. Obtundation,
hypotension, and death may ensue within hours despite
appropriate antimicrobial therapy. Absence of meningeal
signs is a feature of this acute fulminant form of meningo-
coccal disease. Children under age 2 have the highest mor-
tality rate, perhaps as a consequence of immaturity of the
protein C system.
Other complications of invasive meningococcal disease
are arthritis, myocarditis, pericarditis, cervicitis, and
Waterhouse-Friderichsen syndrome. More rare meningo-
coccal diseases include occult bacteremia and chronic
meningococcemia.
B. Laboratory Findings—Confirmation of the diagnosis
depends on demonstration of the organism. This
may be by culture, Gram stain, or immunologic tests.
Blood and cerebrospinal fluid cultures are indicated in
all patients suspected of having invasive disease. Naso-
pharyngeal and synovial cultures are positive in some cases.
Counterimmunoelectrophoresis or latex agglutination with
group-specific antisera of cerebrospinal fluid, urine, or tears
can facilitate rapid diagnosis. A Gram stain of material from
purpuric lesions may reveal the organism. Other laboratory
studies are otherwise nonspecific but should be performed
as indicated to assess and monitor the illness, including eval-
uation for DIC.
DERMATOLOGIC PROBLEMS IN THE INTENSIVE CARE UNIT
629
Differential Diagnosis
Meningococcal infection must be considered in patients
with the combination of fever and a petechial rash, espe-
cially in association with meningitis. Depending on the
clinical presentation, other infections, such as gram-negative
septicemia, Rocky Mountain spotted fever, echovirus and
coxsackievirus infections, and atypical measles, must be
excluded. Vasculitis and other causes of purpura also are
diagnostic possibilities.
Treatment
Intravenous penicillin G or ampicillin is the therapy of choice.
Ceftriaxone is an acceptable alternative (see Chapter 15).
Hemodynamic and other supportive measures must be pro-
vided as necessary to maintain organ system function.
Respiratory isolation is mandatory. Close contacts of patients
with meningococcal disease should be given rifampin pro-
phylaxis and monitored closely.
Ramos-e-Silva M, Pereira AL: Life-threatening eruptions due to
infectious agents. Clin Dermatol 2005;23:148–56. [PMID:
15802208]
Rocky Mountain Spotted Fever
ESSENTIALS OF DIAGNOSIS
Potential exposure to ticks in endemic area.
After incubation period of 1–14 days, sudden onset of
fever, headache, myalgia, and nausea or vomiting.
Appearance on days 2–4 of blanchable pinkish red mac-
ular rash over ankles, wrists, and forearms, spreading to
involve the soles, palms, extremities, trunk, and face
within hours; bilaterally symmetric petechiae of the
palms and soles are a major finding.
May be complicated by CNS, cardiac, pulmonary, renal,
or other organ involvement; DIC and shock leading to
death may occur.
Diagnosis can be confirmed by serologic tests, but these
are not reliable before the second week of illness.
General Considerations
Rocky Mountain spotted fever is an acute systemic illness
characterized by fever and a purpuric eruption. The disease
is transmitted to humans by the bite of a tick infected with
the causative organism Rickettsia rickettsii. Transmission
reflects the tick season in a particular geographic area, with
highest incidence in spring and summer. The disease is wide-
spread in the United States and Canada; most cases are from
the southeastern and Rocky Mountain states. All age groups
are affected, but most are between 5 and 9 years old.
Clinical Features
The incubation period is usually about 1 week, ranging from
1–14 days.
A. Symptoms and Signs—Sudden onset of fever, headache,
myalgia, and nausea or vomiting are initial features. On the sec-
ond to fourth days of illness, a blanchable pinkish red macular
rash appears over the ankles, wrists, and forearms, spreading to
involve the soles, palms, extremities, trunk, and face within
hours. Over the next 1–2 days, the eruption becomes papular
and nonblanchable (purpuric) and may evolve into gangrene
of the digits, nose, earlobes, scrotum, or vulva. Bilaterally sym-
metric petechiae of the palms and soles is a major finding. The
illness can persist up to 3 weeks and may be complicated by
CNS, cardiac, pulmonary, renal, or other organ involvement.
DIC and shock leading to death may occur.
B. Laboratory Findings—The diagnosis of Rocky Mountain
spotted fever can be established retrospectively by one of
many serologic techniques, including complement fixation,
latex agglutination, or microagglutination tests. However,
these tests are not reliably positive before the second week of
the illness.
Skin biopsy reveals a necrotizing vasculitis. A Giemsa-
stained smear of tissue sections occasionally may demon-
strate the organism. Immunofluorescent microscopic
examination of skin biopsy specimens may confirm the diag-
nosis as early as the fourth day of illness.
Differential Diagnosis
Rocky Mountain spotted fever must be differentiated from
other serious febrile illnesses such as viral and bacterial
meningitis, meningococcemia, measles, vasculitis, and
thrombotic thrombocytopenic purpura.
Treatment
Treatment should be initiated as soon as the diagnosis is sus-
pected. Doxycycline is the drug of choice for patients with
Rocky Mountain spotted fever. Gangrene of the earlobes,
digits, nose, etc. requires additional antibiotics if secondarily
infected.
Chapman AS et al: Diagnosis and management of tickborne
rickettsial diseases: Rocky Mountain spotted fever, ehrli-
chioses, and anaplasmosis—United States: A practical
guide for physicians and other health-care and public
health professionals. MMWR Recomm Rep 2006;55:1–27.
[PMID: 16572105]
Cunha BA. Clinical features of Rocky Mountain spotted fever.
Lancet Infect Dis 2008;8:143–4. [PMID: 18291332]
Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis
2007;7:724–32. [PMID: 17961858]
CHAPTER 28
630
Necrotizing Fasciitis
ESSENTIALS OF DIAGNOSIS
Typically occurs following surgery or penetrating
trauma; diabetes may be a predisposing condition.
Erythema, edema, and pain develop 1–2 days following
surgery or trauma with central areas of dusky gray-blue
discoloration, occasionally in association with serosan-
guineous blisters.
Involved areas become gangrenous within a few days;
culture frequently grows multiple aerobic and anaero-
bic bacteria.
Severe systemic toxicity is usually present.
General Considerations
Necrotizing fasciitis is a rare, life-threatening soft tissue
infection characterized by acute and widespread fascial
necrosis. It typically occurs following surgery or penetrating
trauma. Diabetes may be a predisposing condition. The
pathogenesis involves the introduction of organisms into the
subcutis with subsequent spread through fascial planes.
Many different virulent bacteria have been isolated in associ-
ation with necrotizing fasciitis, including β-hemolytic strep-
tococci, staphylococci, coliforms, enterococci, Pseudomonas,
and Bacteroides. Rhizopus and C. albicans have been cultured
from tissue. The process is often fatal unless diagnosed
quickly and treated aggressively.
Clinical Features
A. Symptoms and Signs—Erythema, edema, and pain
develop 1–2 days following introduction of the organism into
the subcutis. The infection spreads rapidly and deeply, result-
ing in local tissue ischemia. Clinically, there are central areas of
dusky gray-blue discoloration, occasionally in association with
serosanguineous blisters. Crepitus is usually absent in necro-
tizing fasciitis. Within a few days, these areas become gan-
grenous; liberation of toxins and organisms into the
bloodstream leads to severe systemic toxicity. The extremities
are the most commonly affected site, but the trunk, perineum,
and abdomen also may be affected. Fournier’s gangrene is
necrotizing fasciitis of the perineum, scrotum, or penis that
spreads rapidly to the anterior abdominal wall. Necrotizing
fasciitis may be confused with cellulitis, angioedema,
eosinophilic fasciitis, and clostridial myonecrosis.
B. Laboratory Findings—Incisional biopsy of both the
advancing edge and the involved tissue should be performed
early, looking for necrotic fascia and the causative organism.
Tissue cultures frequently grow multiple aerobic and anaer-
obic bacteria as well as fungi. Radiographs of soft tissue
rarely may reveal tissue gas.
Treatment
Radical surgical debridement, intravenous broad-spectrum
antibiotics, and general supportive care are the mainstays of
therapy. The major indication for operative treatment is fasciitis
spreading despite empirical antibiotics in an acutely ill patient.
Anaya DA, Dellinger EP: Necrotizing soft-tissue infection:
Diagnosis and management. Clin Infect Dis 2007;44:705–10.
[PMID: 17278065]
Gabillot-Carre M, Roujeau JC: Acute bacterial skin infections and
cellulitis. Curr Opin Infect Dis 2007;20:118–23. [PMID:
17496568]
Lopez FA, Lartchenko S: Skin and soft tissue infections. Infect Dis
Clin North Am 2006;20:759–72. [PMID: 17118289]
Mehta S et al: Morbidity and mortality of patients with invasive
group A streptococcal infections admitted to the ICU. Chest
2006;130:1679–86. [PMID: 17166982]
Miller LG et al: Necrotizing fasciitis caused by community-
associated methicillin-resistant Staphylococcus aureus in Los
Angeles. N Engl J Med 2005;352:1445–53. [PMID: 15814880]
Toxic Shock Syndrome (TSS)
ESSENTIALS OF DIAGNOSIS
Highest incidence in menstruating women, persons
with focal staphylococcal infection or colonization, and
women using a diaphragm or contraceptive sponge—
but may occur in others.
Rapid onset of fever, vomiting, watery diarrhea, sore
throat, and profound myalgias, with hypotension.
Diffuse, blanching erythema appears early, predomi-
nantly truncal, with accentuation in the axillary and
inguinal folds and spreading to the extremities; desqua-
mation of the involved skin and of the palms and soles
seen during the second or third week.
Acute renal failure, acute respiratory distress syndrome
(ARDS) , refractory shock, ventricular arrhythmias, and DIC
may occur.
General Considerations
Toxic shock syndrome is a multisystem illness characterized
by the acute onset of high fever associated with myalgias,
vomiting, diarrhea, headache, pharyngitis, and hypotension.
Mucocutaneous findings are prominent. Staphylococcal pyo-
genic toxin superantigens (TSST-1) and enterotoxins B and
C are involved in the pathogenesis. Streptococcal toxic shock
syndrome is caused mainly by toxin-producing group A
strains but also by strains of groups B, C, F, and G. In the
1980s, most cases occurred in menstruating women using
superabsorbent tampons. Currently, most cases are caused
by nonmenstrual S. aureus infection—postoperative,

DERMATOLOGIC PROBLEMS IN THE INTENSIVE CARE UNIT
631
influenza-associated, or recalcitrant erythematous desqua-
mating syndrome—or by colonization of contraceptive
diaphragms or sponges. Streptococcal toxic shock syn-
drome may or may not be associated with necrotizing fasci-
itis or myositis.
Clinical Features
The Centers for Disease Control and Prevention (CDC) case
definition of toxic shock syndrome is based on six major
criteria—high fever, rash, desquamation, hypotension,
involvement of three or more organ systems (eg, GI, muscu-
lar, mucous membrane, renal, hepatic, hematologic, and
CNS)—and exclusion of other causes.
A. Symptoms and Signs—Patients usually present with
rapid onset of fever, vomiting, watery diarrhea, sore throat,
and profound myalgias. Significant hypotension develops
during the first 48–72 hours of illness. Multisystem organ
involvement probably results both from poor tissue perfu-
sion and from toxin-induced damage. Potentially devastating
complications include acute renal failure, ARDS with pul-
monary edema, refractory shock, ventricular arrhythmia,
and DIC. Some patients have relatively mild episodes.
The cutaneous and mucous membrane findings are
prominent but not diagnostic. A diffuse, blanching erythema
(scarlatiniform exanthem) appears early. The rash is pre-
dominantly truncal, with accentuation in the axillary and
inguinal folds and spreading to the extremities. Erythema
and edema of the palms and soles may develop. Generalized
nonpitting edema is also common. Intense hyperemia of the
conjunctival, oropharyngeal, and vaginal surfaces is a fre-
quent finding. Desquamation of the involved skin and of the
palms and soles is seen during the second or third week of ill-
ness. Toxic shock syndrome recurs in approximately 30% of
untreated patients. Mortality is higher (12%) in patients with
nonmenstrual causation.
B. Laboratory Findings—Laboratory studies are useful for
assessing and monitoring the severity and progression of the
illness. Patients often have leukocytosis with a left shift and
thrombocytopenia. If DIC is suspected, coagulation studies
should be obtained. Serum electrolytes, calcium, phospho-
rus, creatine kinase, renal function and liver function tests,
albumin, total serum protein, and amylase may be abnormal.
Urinalysis may show proteinuria and pyuria.
Chest x-ray, arterial blood gas determinations, and
echocardiography may provide useful information. Cultures
of blood, soft tissue sites of infection, and all mucosal surfaces
(including the trachea if intubation is performed) should be
obtained. Serologic tests should be ordered for Rocky
Mountain spotted fever, leptospirosis, or measles, as indicated
in individual patients, to exclude alternative diagnoses.
Differential Diagnosis
Toxic shock syndrome is a clinical diagnosis. Appropriate
laboratory tests help to distinguish it from several serious
and potentially life-threatening exanthematous diseases,
including streptococcal toxic shock–like disease, scarlet fever,
Kawasaki’s disease, Rocky Mountain spotted fever, SJS, drug
eruptions, bacterial sepsis, measles, and leptospirosis.
Treatment
Tampons or other contraceptive devices must be removed
immediately, followed by irrigation of the vagina. Any surgi-
cal packings also should be removed. Soft tissue abscesses,
empyema, and other sites of infection require surgical
drainage and irrigation.
An antistaphylococcal antibiotic should be administered
intravenously based on a presumptive diagnosis, although its
effect on the outcome of the acute episode is unclear.
Antibiotics do reduce the recurrence of menses-related toxic
shock syndrome. Treatment of group A streptococcal toxic
shock syndrome includes penicillin or ceftriaxone plus clin-
damycin or erythromycin.
Supportive care, including management of organ system
failure and treatment of hypotension, is the mainstay of ther-
apy. Systemic corticosteroids, if given within 3–4 days of dis-
ease, reduce its severity and shorten the duration of fever.
Andrews MM et al: Recurrent nonmenstrual toxic shock syn-
drome: Clinical manifestations, diagnosis, and treatment. Clin
Infect Dis 2001;32:1470–9. [PMID: 11317249]
Kain KC, Schulzer M, Chow AW: Clinical spectrum of nonmen-
strual toxic shock syndrome (TSS): Comparison with menstrual
TSS by multivariate discriminant analyses. Clin Infect Dis
1993;16:100–6. [PMID: 8448283]
Ramos-e-Silva M, Pereira AL: Life-threatening eruptions due to
infectious agents. Clin Dermatol 2005;23:148–56. [PMID:
15802208]
REFERENCES
James WD, Berger T, Elston D (eds): Andrew’s Diseases of the Skin:
Clinical Dermatology, 10th ed. Philadelphia: Saunders, 2006.
Habif TP: Clinical Dermatology: A Color Guide to Diagnosis and
Therapy, 4th ed. St. Louis: Mosby, 2004.
Lebwohl M et al: Treatment of Skin Disease: Comprehensive
Therapeutic Strategies, 2d ed. St. Louis: Mosby, 2006.
Provost TT, Flynn JA (ed): Cutaneous Medicine. New York: BC
Decker, 2002.
Wolff K et al (eds): Fitzpatrick’s Dermatology in General Medicine,
7th ed. New York: McGraw-Hill, 2008.

632
00
The critical care of patient with peripheral vascular disease
requires considerable diagnostic skill and clinical acumen.
Associated medical comorbidities—diabetes, renal insuffi-
ciency, coronary artery disease, and many others—necessitate
admission to an ICU for preoperative optimization and post-
operative observation. Acute arterial occlusion, pulmonary
embolism, and—with the advent of endovascular interven-
tions—pseudoaneurysm formation are among the more
common vascular-related complications encountered in the
otherwise routine care of medical and surgical patients.
This chapter addresses acute vascular emergencies in criti-
cally ill patients and discusses the management of complica-
tions following both elective and emergency vascular surgical
procedures.
VASCULAR EMERGENCIES IN THE ICU
Acute Arterial Insufficiency
ESSENTIALS OF DIAGNOSIS
The “six Ps”: pain, paralysis, paresthesia, pallor, pulse-
lessness, and poikilothermia.
Loss of light touch and position sense followed by
paralysis.
Absence of previously palpable distal pulses and slow
capillary refill.
Cool extremity with skin mottling, often with a detectable
line of demarcation.
Collapse of the superficial venous system and develop-
ment of venous thrombosis and rigid muscular com-
partments in prolonged ischemia.
General Considerations
The management of acute limb ischemia continues to chal-
lenge today’s critical care specialist. Patients often present in a
severely compromised state with unclear symptomatology and
may have multiple associated medical illnesses. Despite
improved therapeutic options in recent years, outcome
remains poor. In a review of 35 reported series, a mortality rate
of 26% and an amputation rate of 37% were documented.
Decreased arterial inflow in a previously normal limb
may be due to embolization from a remote origin (owing to
in situ thrombosis from preexisting occlusive disease) or may
occur in association with a low-flow state. Although throm-
bosis occurs more frequently than embolic occlusion in the
general population, progression to complete occlusion of an
atherosclerotic thrombus is an unusual cause of acute arterial
insufficiency in a critically ill patient admitted for other rea-
sons. Depending on the artery affected and the adequacy of
collateral circulation, clinical presentation may be along a
continuum from subtle to overt limb threat.
The most common site of origin of an embolus is the
heart, with atherosclerotic disease the predominant under-
lying factor. Other sources include aortic and peripheral
aneurysms, atherosclerotic debris from ulcerating plaques,
and less commonly, paradoxical embolus through a cardiac
anomaly, arteritis, or vascular trauma. Atrial fibrillation,
often seen in the postoperative setting, is currently associ-
ated with two-thirds to three-quarters of peripheral emboli.
Acute arterial insufficiency in the ICU is most commonly
caused by intrinsic obstruction produced by the emboliza-
tion of clot from distant sites. Atherosclerotic cardiac vascu-
lar disease accounts for 60–70% of all arterial emboli. Most
arise in patients with atrial fibrillation and stasis in the left
atrial appendage. Those who have sustained a recent myocar-
dial infarction also may develop mural thrombi, most com-
monly at the cardiac apex or in a trabeculation of the left
ventricle. No clear temporal relationship exists between the
time of the myocardial infarction and when embolization
29
Critical Care of Vascular
Disease & Emergencies
James T. Lee, MD
Frederic S. Bongard, MD
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.
CRITICAL CARE OF VASCULAR DISEASE & EMERGENCIES
633
occurs. When congestive heart failure or cardiomyopathy is
present, dyskinetic segments again result in areas of relative
stasis that lead to the formation of thrombi. After separation
from its site of origin, an embolus may be swept into the
innominate or left subclavian artery and travel distally in an
upper extremity until the arterial tree narrows sufficiently to
trap it. If it is carried into either of the internal carotid or ver-
tebral arteries, a bland (dry) stroke results. Distal emboli typ-
ically lodge where vessels taper or branch and consequently
are seen in the superior mesenteric artery, resulting in vis-
ceral ischemia, or in the iliac, femoral, or popliteal arteries.
Peripheral emboli travel to the lower extremities 10 times
more often than to the upper extremities. Commonly
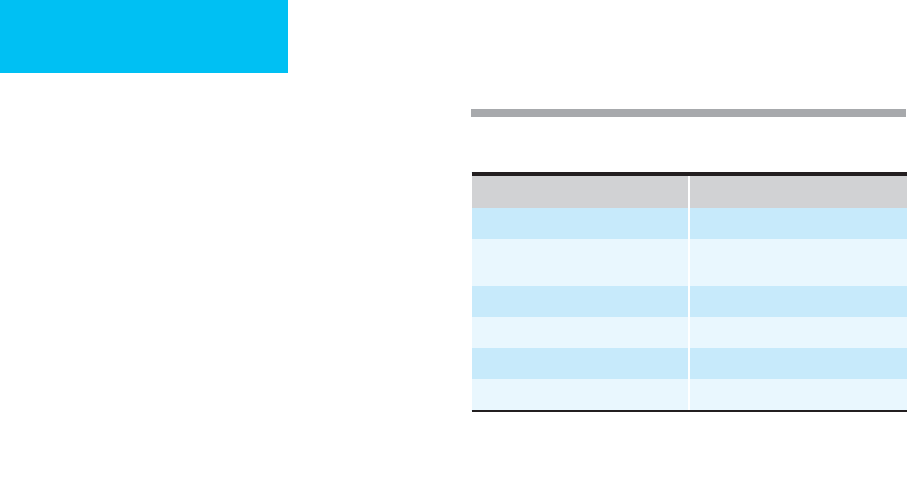
involved arterial segments are listed in Table 29-1.
Other intrinsic sources of arterial emboli are atheroscle-
rotic debris from aneurysms, fibrin plugs, or collections of
platelets. It is unusual for atherosclerotic emboli to present
de novo in a patient admitted to the ICU for another reason.
The exception to this is the blue toe syndrome, which results
from occlusion of digital vessels by atherosclerotic emboli.
However, when the possibility of an expanding aneurysm or
symptomatic chronic aortic dissection was the reason for
admission, acute limb ischemia should arouse concern that
the atherosclerotic plaque or the grumous clot lining the
aneurysm has embolized.
Patients with atherosclerotic microemboli frequently
have transient focal ischemia associated with minor tissue
loss. The clinical distinction between arterial embolism and
arterial thrombosis is often difficult to make, although an
effort should be made to confirm the diagnosis because of
the differences in therapeutic approach and outcome
(Table 29–2). Fibrin plugs and platelet emboli occur most
commonly in patients with disseminated intravascular coag-
ulation (DIC) or in those anticoagulated with heparin who
develop antiheparin antibodies.
Extrinsic emboli are produced when foreign material
such as catheter tips, balloon fragments, and endovascular
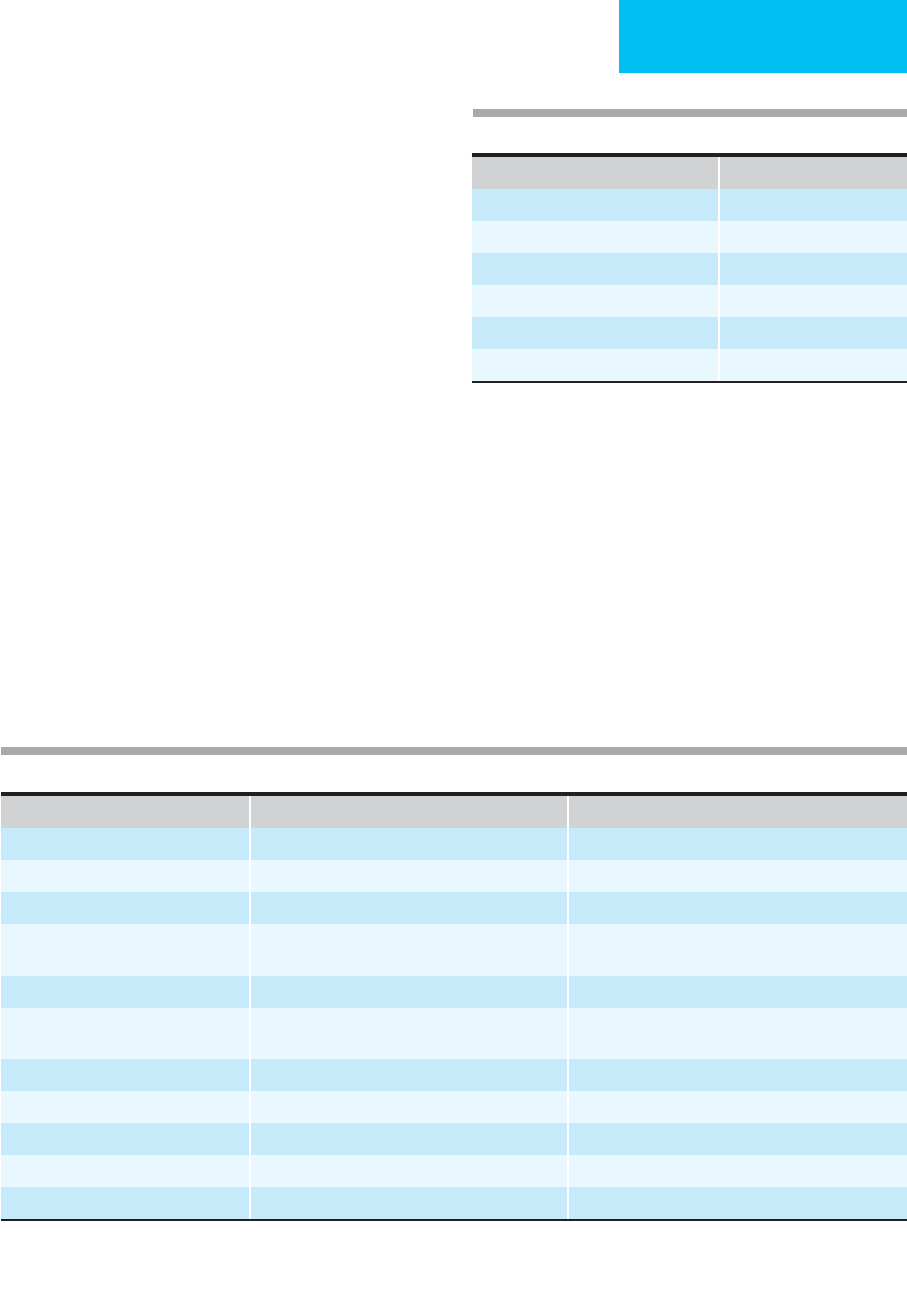
Characterisitics of Occlusion Embolus Thrombosis
Onset of symptons Rapid or immediate Slower or insidious
Prior symptoms: claudication Rare Frequent
Length of time to presentation Acute Chronic
Identifiable source Recent cardiac disease (eg, atrial fibrillation,
myocardial infarction)
None
Physical findings Normal contralateral extremity Bilateral peripheral vascular disease
Angiography Multiple sharp cutoffs, “reversed meniscus,” scant
collaterals
Diffuse peripheral vascular disease, irregular cutoff,
many collaterals
Goal of immediate therapy Eliminate embolus Correct disease
Long-term pharmacologic treatment Anticoagulation Platelet inhibition
Results of thromboembolectomy Good Poor
Amputation risk Lower Higher
Causes of mortality Cardiac disease Limb ischemia
Adapted from Young JR et al (eds): Peripheral Vascular Disease. St Louis: Mosby–Year Book, 1991; and from Rutherford RB (ed): Vascular
Surgery. Philadelphia: Saunders, 2000.
Table 29–2. Differentiation of emoblism from thrombosis.
Table 29–1. Sites of peripheral arterial emboli.
Segment Incidence (%)
Femoral 36
Aortoiliac 22
Popliteal-tibial 15
Upper extremity 14
Visceral 7
Other 6
Data complied from 1303 embolic events at the Massachusetts
General Hospital and Stanford University. Adapted from Rutherford RB
(editor): Vascular Surgery. Philadelphia: Saunders, 2000.
CHAPTER 29
634
occluding devices migrate to distant sites. Bullet emboli
should be remembered as a possible cause of acute arterial
insufficiency in a trauma victim in whom the missile was not
recovered or was unreachable at the time of surgery. Other
penetrating injuries, such as stab wounds, may partially dis-
rupt the vascular intima and begin the process of dissection
and thrombotic occlusion.
Once arterial flow is halted, three pathophysiologic events
occur, each worsening the overall ischemic insult. Initially,
propagation of thrombus can occlude potential collateral
vessel orifices and lend to the no-reflow phenomenon once
large vessel revascularization is established. Second, cellular
swelling owing to local hypoxia may cause red blood cell
trapping and effectively increase the ischemic period even
after adequate inflow is restored. The cause of cellular
swelling is debated but may consist of failure of the sodium
pump. This inability to reperfuse after ischemic intervals is
termed the no-reflow or low-reflow phenomenon. As fluid
leaves the interstitium and enters the cellular matrix, the
effective viscosity of the blood increases, raising the pressure
required to overcome the blood’s inertia (yield stress) and
causing significant narrowing and occlusion of the arterioles,
capillaries, and venules. The more protracted the ischemic
period, the greater is the fluid loss and the higher is the yield
stress. Muscle damage produced by ischemia and reperfusion
is more related to “reflow” than to the absolute period of
ischemia. Animal models have shown that graded return of
inflow over a period of time did result in improved postis-
chemic muscle function and less edema. The mediators of
capillary endothelial injury are highly active oxygen metabo-
lites such as superoxide (O
2
–
) and hydroxyl (-OH) radicals.
On reperfusion, the lactic acid, potassium, myoglobin, and
cardiodepressants such as thromboxane that have accumu-
lated in the ischemic limb are systemically released. The
resulting metabolic acidosis and biochemical insult can have
profound consequences in an already fragile patient.
Peripheral nerve fibers that mediate light touch and posi-
tion sense are much more vulnerable than skin and subcuta-
neous tissue. Thus deficits in these areas, although subtle,
present an illusion of surface viability while masking the
presence of complete functional loss.
Clinical Features
A. Symptoms and Signs—Acute ischemia is often mani-
fested by some or all of the six cardinal signs known as the
“six P’s.” Ischemic pain is profound, and most patients
require large doses of opioid analgesics before they obtain
relief. The diagnosis of acute arterial ischemia is usually
entertained because of the localized nature of the pain. The
level of the obstruction is typically in the artery lying one
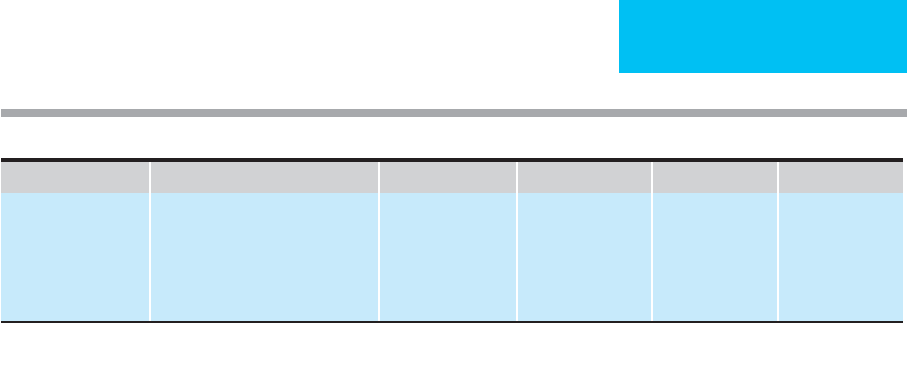
joint above the area of discomfort (Table 29–3). Emboli to
the axillary artery, which has excellent collateral flow, are
either asymptomatic, detected primarily by a pulse deficit,
or noted only with physical activity. Conversely, emboli to
the common femoral or popliteal arteries typically produce
profound ischemia, and symptoms appear rapidly. On exam-
ination, the extremity is pallid and cool. Unlike venous
thrombosis, arterial ischemia produces a white rather than a
violaceous limb. Occasionally, the sensorial perception of
numbness and paresthesias predominates and may mask the
primary component of pain. A late sign, paralysis is the result
of motor nerve ischemia followed by muscle necrosis. Distal
pulses are usually absent, although profound ischemia may
occur in the presence of a normal pulse when the embolus is
lodged distally in the small arteries of the hand or foot. In
some patients—especially those with chronic disease and
generalized edema or anasarca—pulses may be difficult or
impossible to detect. The extremity may be firm because of
muscle swelling. A compartment syndrome occurs when
muscle swelling limits venous outflow from within a fascial
compartment. An indurated and hard compartment is an
indication for release of the pressure by fasciotomy.
Although indicative of arterial insufficiency, these symp-
toms are in essence nonspecific. They serve to alert the clini-
cian to the presence of ischemia but do not lend themselves
to grading or quantification. It is of paramount importance
to assess the degree of ischemia, which is stratified into three
categories based on the physical findings: viable, threatened,
and irreversible. Symptoms depend on the location of the
embolus and the adequacy of the collateral circulation. The
Joint Council of the Society for Vascular Surgery and the
North American Chapter of the International Society for
Cardiovascular Surgery has developed a consensus that
divides severity of limb ischemia into three categories
(Table 29–4). Category I (viable) limbs usually present as an
acute on chronic process. Patients in this category have abun-
dant collaterals and develop an acute femoral artery throm-
bosis overlying a chronic stenosis. The prognosis of category
II patients is dictated by the time interval between diagnosis
and revascularization. Prompt versus immediate intervention
Site of Occlusion Line of Demarcation
Infrarenal aorta Mid abdomen
Aortic bifurcation and common
iliac arteries
Groin/pelvis
External iliac arteries Proximal thigh
Common femoral artery Lower third of thigh
Superficial femoral artery Upper third of calf
Popliteal artery Lower third of calf
Adapted from Way LW (editor): Current Surgical Diagnosis and
Treatment, 10th ed. Originally published by Appleton & Lange.
Copyright © 1994 by The McGraw-Hill Companies, Inc.
Table 29–3. Demarcation of physical findings in relation
to site of arterial occlusion.
CRITICAL CARE OF VASCULAR DISEASE & EMERGENCIES
635
is dictated by the severity of presentation. Amputation is the
only recourse in category III patients.
B. Noninvasive Diagnostic Studies
1. Doppler examination—A Doppler flow probe is useful
in patients who are edematous or in whom a pulse may
not be detectable for other reasons. Evidence of flow by
Doppler examination indicates only that obstruction is not
complete—it does not mean that flow is adequate. One should
guard against grading “Doppler pulses” because they are
influenced by several factors, including the angle of
insonation, the gain of the system, and the flow in the artery.
The more superficial the vessel under consideration, the
higher is the frequency of the probe that should be used.
2. Ankle-brachial index (ABI)—Although used primarily
in patients with chronic arterial disease, the ABI may be use-
ful in patients who complain of subtle changes in their
extremities. The ABI is also useful in postoperative vascular
patients for monitoring graft patency. The index is calculated
by placing a blood pressure cuff at the high calf position, just
below the knee, where it will occlude the tibial arteries. A
Doppler probe is placed over either the posterior tibial or the
dorsalis pedis artery, and the cuff is then deflated. The pres-
sure at which flow resumes is documented to obtain an
opening pressure. The brachial artery pressure is measured in
a similar manner, taking the arm with the higher systolic
pressure. The ankle-brachial index then is calculated (by
dividing ankle Doppler pressure by brachial Doppler pres-
sure). An index greater than 1.0 is normal, and an index less
than 0.4 signifies a threat to the limb. Of greatest value are
changes in the index from previous values or a discrepancy
between the two extremities.
3. Duplex scanning—In equivocal cases or when angiogra-
phy is not available, noninvasive color-flow duplex ultra-
sonography has been a major advance in the diagnosis and
treatment of vascular diseases. It has several components. A
two-dimensional real-time image is projected in the B mode,
which can locate vessels in soft tissues, measure vessel
diameters, and reveal irregularities within the lumen. This
may be helpful in locating the position of the embolus.
Pulsed-wave Doppler technology determines the velocity of
blood flow at a specified location that is superimposed on the
B-mode image. Turbulent flow is seen with a mosaic pattern,
whereas a color-flow void signifies occlusion. Although
duplex scanning can be performed conveniently at the bed-
side, it has the disadvantage of being highly operator-
dependent. Duplex scanning can provide essentially the same
information as arteriography with respect to localization of
arterial segments with either stenosis or total occlusion.
4. Air plethysmography—Although it is seldom used, air
plethysmography remains a valuable noninvasive diagnostic
technique. Several types are available, but all measure the
same physiologic parameter: change in volume. A blood
pressure cuff is placed around the affected extremity and
inflated to 65 mm Hg. A pressure waveform tracing is then
recorded, and occlusive disease is graded based on pulse con-
tour. An advantage of this application is that the recording
obtained is not affected by vessel wall stiffness. In conjunc-
tion with segmental pressure measurements, an accurate
assessment in patients with peripheral occlusive disease can
be made. However, in the setting of acute limb ischemia,
other more specific modalities are necessary.
C. Angiography—Radiographic studies are best obtained in
consultation with a vascular surgeon to avoid unnecessary
delays. Arteriography remains the standard and is extremely
useful in the planning of operative procedures and is recom-
mended in all but the most straightforward cases. Only when
the location of the occlusion is apparent (eg, femoral embolus)
and coupled with an acutely ischemic limb is preoperative
angiography required. However, when symptoms are atypi-
cal in a threatened limb, arteriography is helpful in deter-
mining the surgical strategy or in the institution of
catheter-directed thrombolytic therapy. The major disadvan-
tage of radiologic studies in this setting is the time required
to obtain them. Delayed can lead to irreversible soft tissue
ischemic injury.
Category Description Sensory Loss Muscle Weakness Arterial Doppler Venous Doppler
I. Viable
II. Threatened
Marginal
Immediate
III. Irreversible
No immediate threat
Salvage with prompt treatment
Salvage with immediate treatment
Permanent tissue loss
None
Minimal
Rest pain
Anesthetic
None
None
Mild to moderate
Paralysis
+
–
–
–
+
+
+
–
Adapted from Rutherford RB et al: Recommended standards for reports dealing with lower extremity ischemia: Revised version.
J Vasc Surg 1997;26:517.
Table 29–4. Clinical categories of acute limb ischemia.

CHAPTER 29
636
Differential Diagnosis
Acute arterial insufficiency owing to an embolus may be
mimicked by low-flow states produced by congestive heart
failure and hypovolemic shock. In the latter conditions, how-
ever, global ischemia is present, and the localizing symptoms
associated with an embolus are lacking. Acute stroke or tran-
sient ischemic attacks may produce muscle weakness but are
seldom associated with pain. Aneurysmal disease or aortic
dissection not only may be the source of emboli but also may
result in rupture. If a dissection extends distally, it may
become thrombotic, producing acute ischemia of the organs
that receive blood from its false channel. Diabetic neuropa-
thy and neuritis may produce hypesthesias in the extremities
but seldom are a diagnostic dilemma.
Treatment
Prompt restoration of inflow is the most important manage-
ment priority. In general, the extent of tissue necrosis and the
resulting disability are directly proportional to the duration
of ischemia. Tolerance of ischemia varies widely among dif-
ferent tissues, extremities, and individuals. Thus a safe upper
limit for arterial compromise cannot be established,
although most authorities cite 4–6 hours as the usual time
limit beyond which irreversible injury of muscles and nerves
may have occurred, even though the overlying skin still may
be viable. For this reason, once a threat to limb survival has
been recognized, prompt treatment is paramount.
A. Anticoagulation—Systemic anticoagulation with heparin
is used unless life-threatening contraindications such as active
GI or cerebral bleeding is present. Heparin prevents the distal
propagation of thrombus, protects the distal vascular bed,
and preserves the extremity’s outflow. The usual dose of
heparin is 100 units/kg given as a bolus, followed by 10–20
units/kg per hour. Before heparin is started, one should
record a baseline partial thromboplastin time (aPTT), pro-
thrombin time (PT), and platelet count. Heparin is cleared
when bound to receptors on endothelial cells and macrophages,
where it is depolymerized. Consequently, its half-life depends
on the initial bolus. The half-life increases from approximately
30 minutes following an intravenous bolus of 25 units/kg to
60 minutes with a bolus of 100 units/kg and to 150 minutes
with a bolus of 400 units/kg. Based on the standard dosage,
most authors recommend titrating a continuous heparin drip
to lengthen the aPTT to twice baseline. Heparin should be
started before any diagnostic maneuvers and may be contin-
ued through to the time of surgery. Titration of the heparin
dosage should not substitute for or delay appropriate surgical
management. The use of heparin should be followed by oral
anticoagulation to prevent recurrent embolism in patients
undergoing thromboembolectomy.
Some surgeons recommend nonoperative management
for acute arterial ischemia, in which case heparin in “high”
doses (20,000 units as an IV bolus followed by 4000 units/h)
is used as the sole form of treatment. Extreme caution must
be exercised in recommending such therapy, however, and
only patients without signs of limb threat should be treated
with anticoagulation alone. This therapy is best reserved for
upper extremity lesions in which collateral flow is good—or
in lower extremity cases in patients whose neural function is
not diminished or in whom it improves quickly after institu-
tion of therapy.
B. Rheologic Agents—The increase in blood viscosity asso-
ciated with acute ischemia has led some vascular surgeons to
recommend the use of either mannitol or low-molecular-
weight dextran (dextran 40; MW 40,000) to reduce cellular
swelling. An additional benefit of these agents is that they
produce an osmotic diuresis and may help to prevent renal
failure owing to myoglobin released from ischemic and
necrotic muscle. Mannitol is started with an intravenous
bolus dose of 25–50 g. Care must be exercised in patients
with congestive heart failure because the increased intravas-
cular volume may worsen cardiac symptoms.
C. Platelet-Active Agents—Aspirin, the agent prescribed
most commonly for this purpose, has been thoroughly eval-
uated and found to prevent vascular death by approxi-
mately 15% and nonfatal vascular events by about 30% in a
meta-analysis of over 50 secondary prevention trials in var-
ious groups of patients. The role of aspirin in acute limb
ischemia is more restricted to postoperative adjunctive
cardiac prophylaxis.
Integrin glycoprotein IIb/IIIa receptor antagonists (eg,
abciximab) inhibit the final common pathway of platelet
aggregation. Their development objective was to prevent
restenosis in patients undergoing percutaneous coronary
intervention. Three large randomized trials involving
approximately 27,000 patients resulted in a higher mortality
and excessive bleeding complications when compared with
aspirin. The role of this class of medications is evolving.
Thienopyridines such as clopidogrel inhibit ADP-induced
platelet aggregation with no direct effects on arachidonic acid
metabolism. Use of this agent in the acute setting has not been
studied; however, in a comparison trial with aspirin involving
a subset of 6400 patients, virtually all the benefit associated
with clopidogrel was observed in the group with symptomatic
peripheral vascular disease. As a group, these patients had
fewer myocardial infarctions and fewer vascular-related
deaths than did the aspirin-treated group. The main disad-
vantage is the permanent platelet defect encountered, which
can be replaced only with platelet turnover.
D. Thrombin Inhibitors—Direct thrombin inhibitors (eg,
lepirudin, desirudin, bivalirudin, and argatroban) have been
used successfully to treat arterial and venous thrombotic com-
plications of heparin-induced thrombocytopenia. Despite
producing a more predictable anticoagulant response than
heparin, direct thrombin inhibitors have yet to find a place in
the treatment of acute arterial thrombosis. Potential disadvan-
tages include the irreversible nature of this complex, because
no antidote is available if bleeding occurs, and its narrow
