Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.


519.2 Summary of the Multicomponent Equations of Change
587
Table
19.2-1
Equations of Change for Multicomponent Mixtures in Terms of
the Combined Fluxes
Mass
of
a:
d
pw,
=
-(V
.
nu)
+
r,
(a
=
1,2,
...,A9
at
Momentum:
d
pv
=
-[V.+]
+
pg
dt
(A)"
(Eq. 19.1-6)
Energy:
d-'
Zp(u
+
:v2)
=
-(V.e)
+
(pv-g)
(CIb
(Eq. 11.1-6)
"
When all
N
equations of continuity are added, the equation of continuity for the fluid
mixture
is obtained. Here
v
is the mass average velocity defined in Eq.
17.7-1.
If species
a
is acted on by a force per unit volume given by
L,
then
pg
has to be
replaced by
X,p,g,
in
Eq.
(B),
and
(pv
.
g)
has to be replaced by
ZJn,
g,)
in
Eq.
(C).
These replacements are required, for example, if some of the species are ions with
different charges on them, acted on by an electric field. Problems of this sort are
discussed in Chapter
24.
evolved by experience and experiment and therefore are generally accepted by the scien-
tific community.'
The three "combined fluxes," which appear in Eqs.
(A)
to
(C)
of Table 19.2-1, can be
written as the
convective fluxes
plus the
molecular
(or
diffusive) fluxes.
These various fluxes
are displayed in Table 19.2-2, where the equation numbers corresponding to their first
appearance are given.
When the flux expressions of Table 19.2-2 are substituted into the conservation
equations of Table 19.2-1 and then converted to the
D/Dt
form by means
of
Eqs. 3.5-4
and
5,
we get the multicomponent equations of change in their usual forms. These are
tabulated in Table 19.2-3.
In addition to these conservation equations, one needs also to have the expressions
for the fluxes in terms of the gradients and the transport properties (the latter being func-
tions of temperature, density, and composition). Finally one nceds Aalso the thermal
equation of state,
p
=
p(p,
T,
x,),
and the caloric equation of state,
U
=
U(p,
T,
x,),
and in-
formation about the rates of any homogeneous chemical reactions occurring2
Actually the conservation laws for energy, momentum, and angular momentum follow from
Lagrange's equation of motion, together with the homogeneity of time, the homogeneity of space, and
the isotropy of space, respectively
(Noether's
theorem).
Thus there is something very fundamental about
these conservation laws, more than is apparent at first sight. For more on this, see
L.
Landau and
E.
M.
Lifshitz,
Mechanics,
Addison-Wesley, Reading, Mass.
(1960),
Chapter
2,
and Emrny Noether,
Nachr.
Kgl.
Ges.
Wiss.
Gottingen
(Math.-phys.
Kl.)
(19181,
pp.
235-257.
Amalie
Emmy
Noether
(1882-1935),
after
doing the doctorate at the University of Erlangen, was a protkgke of Hilbert in Gottingen until Hitler's
purge of
1933
forced her to move to the United States, where she became a professor of mathematics at
Bryn Mawr College; a crater on the moon is named after her.
One might wonder whether or not we need separate equations of motion and energy for species
a.
Such equations can be derived by continuum arguments, but the species momentum and energy fluxes
are not measurable quantities and molecular theory is required in order to clarify their meanings.
These separate species equations are not needed for solving transport problems. However, the
species equations of motion have been helpful for deriving kinetic expressions for the mass fluxes
in multicomponent systems [see
C.
F.
Curtiss and
R.
B. Bird,
Proc.
Nut.
Acad.
Sci.
USA,
93,7440-7445
(1996)
and
I.
Chem. Phys., 111,10362-10370 (199911.
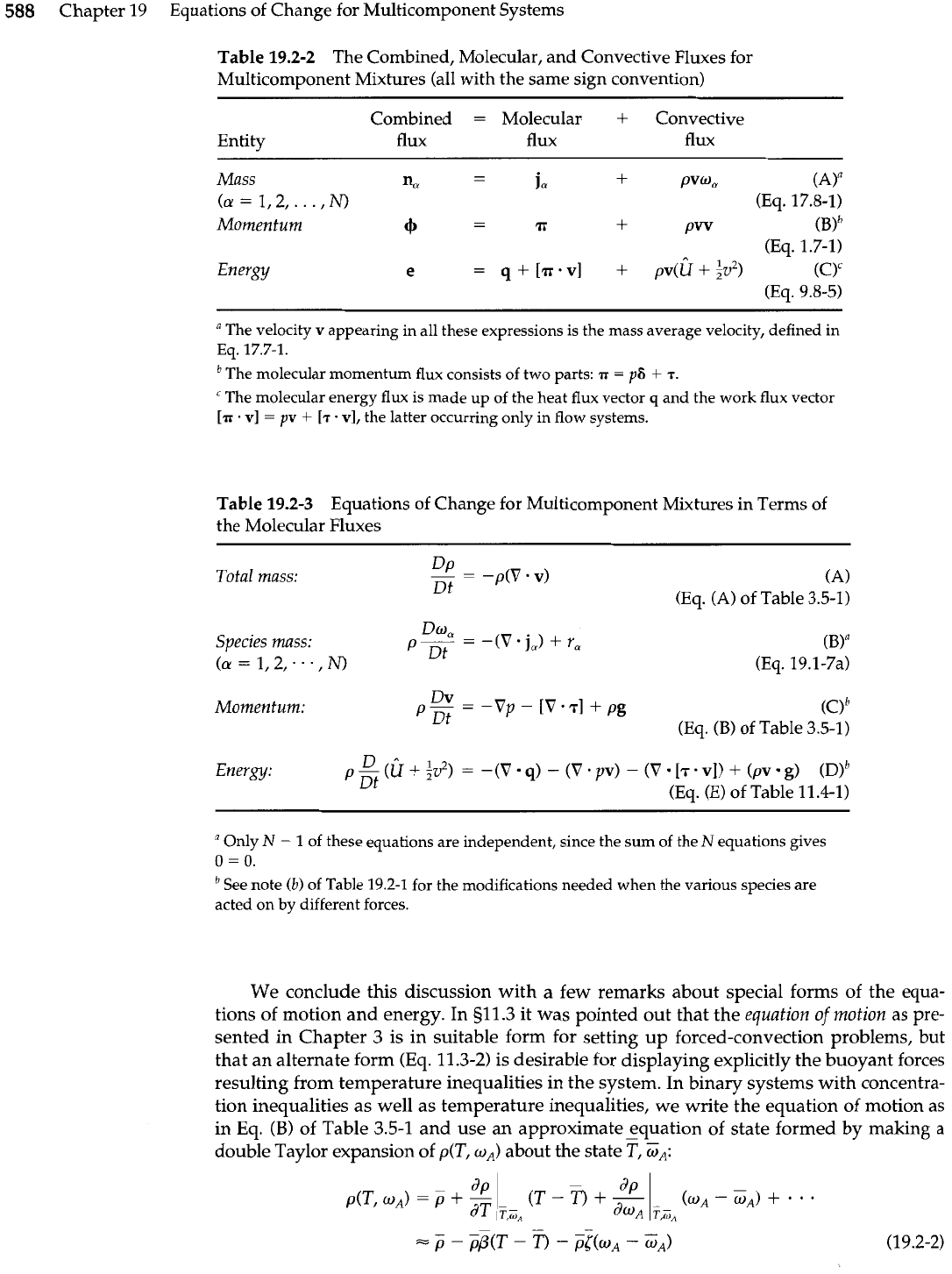
588
Chapter 19 Equations of Change for Multicomponent Systems
Table 19.2-2 The Combined, Molecular, and Convective Fluxes for
Multicomponent Mixtures (all with the same sign convention)
Combined
=
Molecular
+
Convective
Entity flux flux flux
Mass
-
-
na
lm
+
PV"a (AY
(a=
1,2,
...,A0
(Eq. 17.8-1)
Momentum
(P
m
+
P
W
mb
-
-
(Eq. 1.7-1)
Energy e
=
q+[msv1
+
pv(b+$v2) (CY
(Eq. 9.8-5)
"
The velocity
v
appearing in all these expressions is the mass average velocity, defined in
Eq.
17.7-1.
The molecular momentum flux consists of two parts:
TI
=
p6
+
T.
'
The molecular energy flux is made up of the heat flux vector
q
and the work flux vector
[P
.
V]
=
pv
+
[7
-
v],
the latter occurring only in flow systems.
Table 19.2-3
Equations of Change for Multicomponent Mixtures in Terms of
the Molecular Fluxes
Total mass:
--
Dp
-
-p(V
.
v)
Dt
(A)
(Eq. (A) of Table 3.5-1)
Species mass:
Dm'?
PDt
=
-(V.j,)
+
r,
(a
=
1,2,...,N)
(BY
(Eq. 19.1-7a)
Momentum:
Dv
p-
Dt
=
-Vp
-
[V-T]
+
pg
(Ob
(Eq.
(B)
of Table 3.5-1)
Energy:
P
~t
D*
(U
+
iv
12
)
-
-
-(V
4)
-
(V
.
pv)
-
(8
17.
v])
+
(pv
8)
(D)~
(Eq.
(El of Table 11.4-1)
a
Only N
-
1
of these equations are independent, since the sum of the
N
equations gives
0
=
0.
See note
(b)
of Table
19.2-1
for the modifications needed when the various species are
acted on by different forces.
We conclude this discussion with a few remarks about special forms of the equa-
tions of motion and energy. In 511.3 it was pointed out that the equation of motion as pre-
sented in Chapter
3
is in suitable form for setting up forced-convection problems, but
that an alternate form
(Eq.
11.3-2) is desirable for displaying explicitly the buoyant forces
resulting from temperature inequalities in the system. In binary systems with concentra-
tion inequalities as well as temperature inequalities, we write the equation of motion as
in Eq.
(B)
of Table
3.5-1
and use an approximate equation of state formed by making
a
double Taylor expansion of p(T,
w,)
about the state
&:
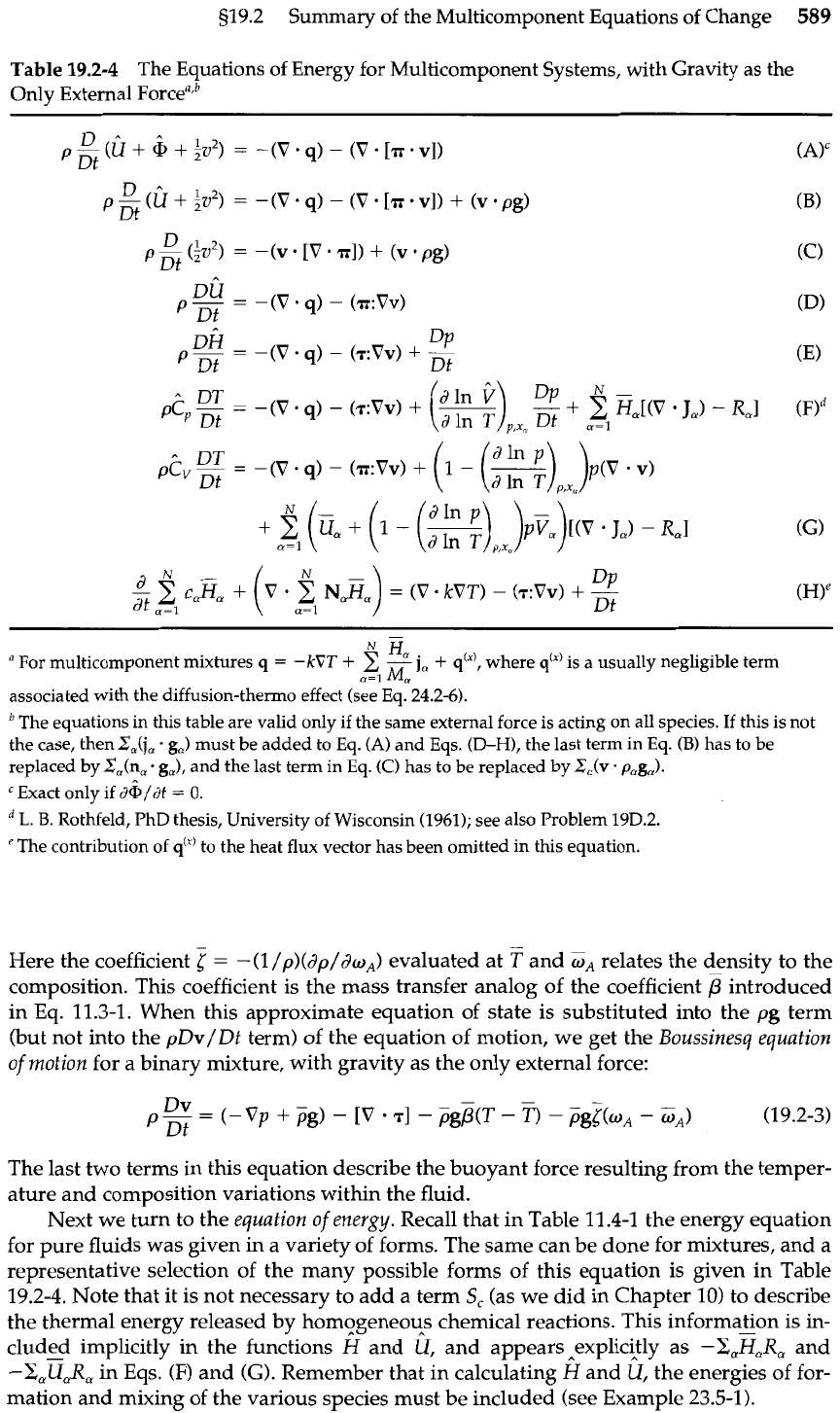
519.2
Summary of the Multicomponent Equations of Change
589
Table
19.2-4
The Equations of Energy for Multicomponent Systems, with Gravity as the
Only External Forcearb
H,
a
For multicomponent mixtures
q
=
-kVT
+
-
j,
+
q'"',
where
q'"
is a usually negligible term
w=l
M,
associated with the diffusion-thermo effect (see Eq. 24.2-6).
The equations
in
this table are valid only if the same external force is acting on all species. If this is not
the case, then
ZJj,
g,) must be added to Eq.
(A)
and Eqs.
(D-H),
the last term in Eq.
(B)
has to be
replaced byZ,(n,
. g,),
and the last term in Eq.
(C)
has to be replaced by
Z,(v.
page).
Txact only if
d&/dt
=
0.
L.
B.
Rothfeld, PhD thesis, University of Wisconsin (1961); see also Problem 19D.2.
'The contribution of
q"'
to the heat
flux
vector has been omitted in this equation.
Here the coefficient
5
=
-(l
/p)(dp/doA) evaluated at
T
and
relates the density to the
composition. This coefficient is the mass transfer analog of the coefficient
p
introduced
in Eq. 11.3-1. When this approximate equation of state is substituted into the pg term
(but not into the pDv/Dt term) of the equation of motion, we get the
Boussinesq equation
of motion
for a binary mixture, with gravity as the only external force:
The last two terms in this equation describe the buoyant force resulting from the temper-
ature and composition variations within the fluid.
Next we turn to the
equation of energy.
Recall that in Table 11.4-1 the energy equation
for pure fluids was given in a variety of forms. The same can be done for mixtures, and a
representative selection of the many possible forms of this equation is given in Table
19.2-4. Note that it is not necessary to add a term
S,
(as we did in Chapter 10) to describe
the thermal energy released by homogeneous chemical reactions. This information is in-
cluded implicitly in the functions
H
and
k
and appears explicitly as
-xaEa~,
and
-X,U,R,
in Eqs.
(F)
and
(G).
Remember that in calculating
H
and
fi/
the energies of for-
mation and mixing of the various species must be included (see Example 23.5-1).

590
Chapter 19 Equations of Change for Multicomponent Systems
519.3
SUMMARY OF THE MULTICOMPONENT
FLUXES
The equations of change have been given in terms of the fluxes of mass, momentum, and
energy. To solve these equations, we have to replace the fluxes by expressions involving
the transport properties and the gradients of concentration, velocity, and temperature.
Here we summarize the flux expressions for mixtures:
Mass:
jA
=
-p9ARV~A binary only
Momentum:
7
=
-,u[Vv
+
(vv)~]
+
($,u
-
K)(V
v)S
Energy:
Now we append a few words of explanation:
a.
The mass flux expression given here is for binary mixtures only. For multicom-
ponent gas mixtures at moderate pressures, we can use the Maxwell-Stefan
equations of Eq. 17.9-1. There are additional contributions to the mass flux cor-
responding to driving forces other than the concentration gradients: forced difu-
sion, which occurs when the various species are subjected to different external
forces; pressure diffusion, proportional to Vp; and thermal diffusion, proportional
to VT. These other diffusion mechanisms, the first two of which can be quite
important, are covered in Chapter
24.
b.
The momentum flux expression is the same for multicomponent mixtures as for
pure fluids. Once again we point out that the contribution containing the dilata-
tional viscosity
K
is seldom important. Of course, for polymers and other vis-
coelastic fluids, Eq. 19.3-2 has to be replaced by more complex models, as
explained in Chapter
8.
c.
The energy-flux expression given here for multicomponent fluids consists of two
terms: the first term is the heat transport by conduction which was given for pure
materials in Eq. 9.1-4, and the second term describes the heat transport by each
of
the diffusing species. The quantity is the partial molar enthalpy of species
a.
There is actually one further contribution to the energy flux, related to a concen-
tration driving force-usually quite small-and this diffusion-thermo effect will be
discussed in Chapter 24. The thermal conductivity of
a
mixture-the
k
in
Eq.
19.3-3-is defined as the proportionality constant between the heat flux and the
temperature gradient in the absence of any mass fluxes.
We conclude this discussion with a few comments about the combined energy flux
e.
By substituting
Eq.
19.3-3 into Eq.
(C)
of Table 19.2-2, we get after some minor rearranging:
In some situations, notably in films and low-velocity boundary layers, the contributions
ipv2v
and
[T
.
V]
are negligible. Then the dashed-underlined terms may be discarded.
This leads to
919.3 Summary of the Multicomponent Fluxes
591
Then use of Eqs.
(G)
and
(H)
of Table 17.8-1 leads finally to
Finally, for ideal gas mixtures, this expression can be further simplified by replacing the
partial molar enthalpies by the molar enthalpies
&.
Equation
19.3-6
provides a stan-
dard starting point for solving one-dimensional problems in simultaneous heat and
mass transfer.'
The partial molar enthalpy E,, which appears in Eqs. 19.3-3 and 19.3-6, is defined for a multi-
component mixture as
The
Partial Molar
,
~
Enthalp
y
in which n, is the number of moles of species
a
in the mixture, and the subscript np indicates
that the derivative is to be taken holding the number or moles of each species other than
a
constant. The enthalpy H(n,, n,, n,,
. .
.)
is an "extensive property," since, if the number of
moles of each component is multiplied by k, the enthalpy itself will be multiplied by
k:
H(kn,, kn,, kn,,
.
a)
=
kH(n,, n,, n,,
.
.)
(19.3-8)
Mathematicians refer to this kind of function as being "homogeneous of degree 1." For such
functions Euler's theorem2 can be used to conclude that
(a)
Prove that, for a binary mixture, the partial molar enthalpies at a given mole fraction can
be determined by plotting the enthalpy per mole as a function of mole fraction, and then de-
termining the intercepts of the tangent drawn at the mole fraction in question (see Fig. 19.3-1).
This shows one way to get the partial molar enthalpy from data on the enthalpy of the
mixture.
(b)
How else could one get the partial molar enthalpy?
Fig.
19.3-1.
The "method of inter-
cepts" for determining partial molar
quantities in a binary mixture.
'
T.
K.
Sherwood,
R.
L.
Pigford, and
C.
R.
Wilke,
Mass Transfer,
McGraw-Hill, New York (1975),
Chapter 7.
Thomas Kilgore Sherwood
(1903-1976) was a professor at
MIT
for nearly 40 years, and then
taught at the University of California in Berkeley. Because of his many contributions to the field of mass
transfer, the Sherwood number (Sh) was named after him.
M.
D.
Greenberg,
Foundations
of
Applied
Mathematics,
Prentice-Hall, Englewood Cliffs,
N.J.
(1978),
p. 128;
R.
J.
Silbey and
R.
A.
Alberty,
Physical Chemistry,
3rd edition, Wiley, New York (2001), §§1.10,4.9,
and
6.10.

592
Chapter 19 Equations of Change for Multicomponent Systems
SOLUTION
(a)
Throughout this example, for brevity we omit the subscripts
p,
T
indicating that these
quantities are held constant. First we write expressions for the intercepts as follows:
in which
H
=
H/(nA
+
n,)
=
H/n. To verify the correctness of Eq. 19.3-10, we rewrite the
ex-
pression in terms of
H:
NOW
the expression
HA
=
(dH/dnA),B implies that
H
is a function of nA and nB, whereas
(dH/dxA), implies that His a function of
x,
and n. The relation between these kinds of deriva-
tives is given by the chain rule of partial differentiation. To apply this rule we need the rela-
tion between the independent variables, which, in this problem, are
Therefore we may write
Substitution of this into
Eq.
19.3-12 and use of Euler's theorem
(H
=
nAEA
+
n,~,)
then gives
an identity. This proves the validity of Eq. 19.3-10, and the correctness of Eq. 19.3-11 can be
proved similarly.
(b)
One can also get
HA
by using the definition in Eq. 19.3-7 and measuring the slope of the
curve of
H
versus n~, holding n, constant. One can also get
HA
by measuring the enthalpy of
mixing and using
Often the enthalpy of mixing is neglected and the enthalpies of the pure substances are given
as
=
cPA(~
-
T")
and a similar expression for
i?,.
This is a standard assumption for gas
mixtures at low to moderate pressures.
Other methods for evaluating partial molar quantities may be found in current textbooks
on thermodynamics.
519.4
USE
OF
THE EQUATIONS
OF
CHANGE FOR MIXTURES
The equations of change in
519.2
can be used to solve all the problems of Chapter
18,
and
more difficult ones as well. Unless the problems are idealized or simplified, mixture
transport phenomena are quite complicated and usually numerical techniques are re-
quired. Here we solve a few introductory problems by way of illustration.
(a)
Develop expressions for the mole fraction profile x,(y) and the temperature profile
T(y)
for the system pictured in Fig. 19.4-1, given the mole fractions and temperatures at both film
Simulfaneous
Heat
and
boundaries
(y
=
0 and
y
=
6).
Here a hot condensable vapor,
A,
is diffusing at steady state
Mass Transporf'
through a stagnant film of noncondensable gas,
B,
to a cold surface at
y
=
0,
where
A
con-
denses. Assume ideal gas behavior and uniform pressure. Furthermore assume the physical
A.
P.
Colburn
and
T.
B.
Drew,
Trans.
Am.
Inst.
Chem.
Engrs.,
38,197-212 (1937).
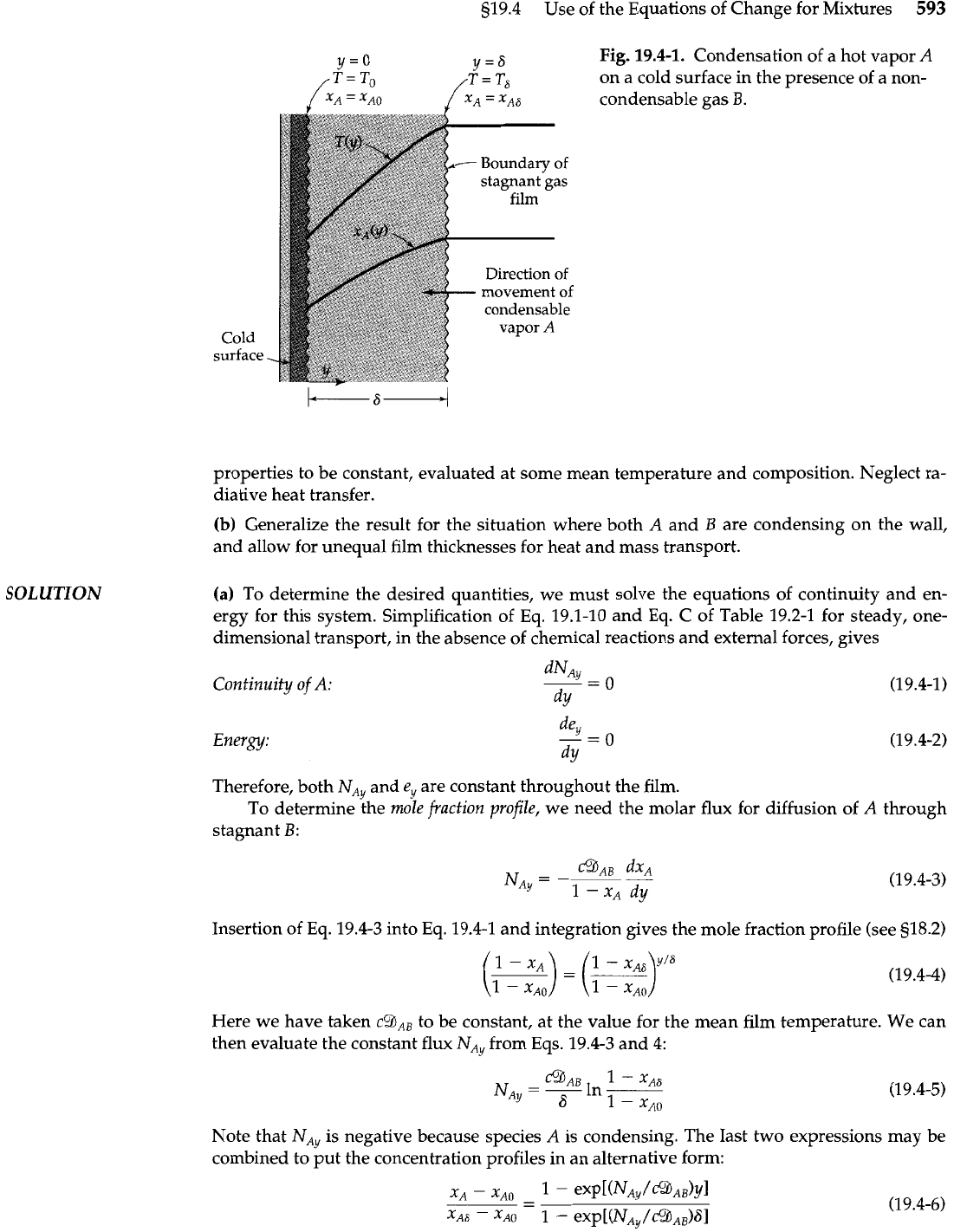
919.4 Use of the Equations of Change for Mixtures
593
SOLUTION
Fig.
19.4-1.
Condensation of a hot vapor
A
on a cold surface in the presence of a non-
condensable gas
B.
Cold
surface
.
properties to be constant, evaluated at some mean temperature and composition. Neglect ra-
diative heat transfer.
(b) Generalize the result for the situation where both
A
and
B
are condensing on the wall,
and allow for unequal film thicknesses for heat and mass transport.
(a)
To determine the desired quantities, we must solve the equations of continuity and en-
ergy for this system. Simplification of Eq. 19.1-10 and Eq. C of Table 19.2-1 for steady, one-
dimensional transport, in the absence of chemical reactions and external forces, gives
Continuity of
A:
Energy:
Therefore, both
NAY
and e, are constant throughout the film.
To determine the mole fraction profile, we need the molar flux for diffusion of
A
through
stagnant
B:
Insertion of Eq. 19.4-3 into Eq. 19.4-1 and integration gives the mole fraction profile (see 918.2)
Here we have taken
to be constant, at the value for the mean film temperature. We can
then evaluate the constant flux
NAY
from Eqs. 19.4-3 and 4:
Note that
NAY
is negative because species
A
is condensing. The last two expressions may be
combined to put the concentration profiles in an alternative form:

594
Chapter 19 Equations of Change for Multicomponent Systems
To get the temperature profile, we use the energy flux from Eq. 19.3-6 for an ideal gas along
with Eq. 9.8-8:
Here we have chosen To as the reference temperature for the enthalpy. Insertion of this
ex-
pression for e, into Eq. 19.4-2 and integration between the limits T
=
To at
y
=
0, and
T
=
T,
at
y
=
6
gives
It can be seen that the temperature profile is not linear for this system except in the limit as
NA,&/k
+
0. Note the similarity between Eqs. 19.4-6 and 8.
The conduction energy flux at the wall is greater here than in the absence of mass trans-
fer. Thus, using a superscript zero to indicate the conditions in the absence of mass transfer,
we may write
We see then that the rate of heat transfer is directly affected by simultaneous mass transfer,
whereas the mass flux is not directly affected by simultaneous heat transfer. In applications at
temperatures below the normal boiling point of species
A,
the quantity
~,,&/k
is small, and
the right side of Eq. 19.4-9 is very nearly unity (see Problem 19A.1). The interaction between
heat and mass transfer is further discussed in Chapter
22.
(b)
If both
A
and
B
are condensing at the wall, then Eqs. 19.4-1 and
2,
when integrated, lead
to NAY
=
NAO and e,
=
e,, where the subscript
"0"
quantities are evaluated at
y
=
0.
We
also in-
tegrate the analog of Eq. 19.4-1 for
B
to get N,,
=
NBo and obtain
In the second of these equations, we replace
HA
by ?,*(T
-
To) and by
$.
(T
-
To), and
since the reference temperature is To, we may replace e, by
q,,
the conductive heat flux at
the
wall. In the first equation, we subtract xAO(NAO
+
NBO) from both sides to make the equation
similar in form to the temperature equation just obtained. Thus
Integration with respect to
y
and application of the boundary conditions at
y
=
0 gives

s19.4
Use of the Equations of Change for Mixtures
595
These are the concentration and temperature profiles in terms of the mass and heat fluxes.
Applications of the boundary conditions at the outer edges of the films-that is, at
y
=
6,
and
y
=
ST,
respectively-give
These equations relate the fluxes to the film thicknesses and the transport properties. When
Eq.
19.4-14
is divided by Eq.
19.4-16
and Eq.
19.4-15
is divided by Eq.
19.4-17,
we get the con-
centration profiles in terms of the transport coefficients (analogously to Eqs.
19.4-6
and
8).
Equations
19.4-16
and
17
will be encountered again in
522.8.
A catalytic tubular reactor is shown in Fig.
19.4-2.
A
dilute solution of solute
A
in a solvent
S
is in fully developed, laminar flow in the region
z
<
0.
When it encounters the catalytic wall
in the region
0
5
z
5
L,
solute
A
is instantaneously and irreversibly rearranged to an isomer
in
a
Tddar Reactor
B.
Write the diffusion equation appropriate for this problem, and find the solution for short
distances into the reactor. Assume that the flow is isothermal and neglect the presence of
B.
SOLUTION
For the conditions stated above, the flowing liquid will always be very nearly pure solvent
S.
The product
@BAS
can be considered constant, and the diffusion of
A
in
S
can be described by
the steady-state version of Eq.
19.1-14
(ignoring the presence of a small amount of the reaction
product
B).
The relevant equations of change for the system are then
Continuity
of
A:
Motion:
We make the usual assumption that axial diffusion can be neglected with respect to axial con-
vection, and therefore delete the dashed-underlined term (compare with Eqs.
10.8-11
and
12).
Equation
19.4-19
can be solved to give the parabolic velocity profile v,(r)
=
v,,,,,[l
-
(r/M21.
When this result is substituted into Eq.
19.4-18,
we get
From
z
=
0 to
z
=
L
the
~il~t~ solution
of
inner surface of the tube is
A
B
in
coated
with
a catalyst
/
/'
Fully-developed
A
+
B
irreversibly
laminar flow before and instantaneously
z
=
0 is reached on catalyst surface
Fig.
19.4-2.
Boundary conditions for a tubular reactor.
596
Chapter 19 Equations of Change for Multicomponent Systems
This is to be solved with the boundary conditions
B.C. 1:
B.C. 2:
B.C. 3:
at
z
=
0,
c,
=
c,,
at
r
=
R,
c,
=
0
at
r
=
0,
c,
=
finite
For short distances
z
into the reactor, the concentration
c,
differs from
c,,
only near the wall,
where the velocity profile is practically linear. Hence we can introduce the variable
y
=
R
-
r,
neglect curvature terms, and replace
B.C.
3 by a fictitious boundary condition at
y
=
w
(see
Example 12.2-2 for a detailed discussion of this method of treating the entrance region of the
tube).
The reformulated problem statement is then
with the boundary conditions
B.C. 1:
B.C. 2:
B.C. 3:
This problem can be solved by the method of combination of independent variables by seeking
a solution of the form
c,/cA0
=
f($,
where
r]
=
(y/R)(2~~,,,,~~/9~~~z)"~.
One thus obtains the
ordinary differential equation
f"
+
3r12f'
=
0, which can be integrated to give (see
Eq.
C.l-9)
This problem is mathematically analogous to the Graetz problem of Problem 12D.4,
O
of
that
problem being analogous to 1
-
(c,/c,,)
here.
Experiments of the type described here have proved useful for obtaining mass transfer
data at high Schmidt numbers.'
A
particularly attractive reaction is the reduction of ferri-
cyanide ions on metallic surfaces according to the reaction
in which ferricyanide and ferrocyanide take the place of
A
and
B
in the above development.
This electrochemical reaction is quite rapid under properly chosen conditions. Furthermore,
since it involves only electron transfer, the physical properties of the solution are almost en-
tirely unaffected. The forced diffusion effects neglected here may be suppressed by the addi-
tion of an indifferent electrolyte in ex~ess.~,~
Figure 19.4-3 shows schematically how oxygen and carbon monoxide combine at a catalytic
surface (palladium) to make carbon dioxide, according to the technologically important
Catalytic Oxidation
reaction5
of Carbon Monoxide
0,
+
2C0
+
2C02
(1 9.4-30)
D.
W. Hubbard and
E.
N.
Lightfoot,
Ind. Eng. Chem. Fundam.,
5,370-379 (1966).
1.
S.
Newman,
Electrochical Systems,
2nd edition, Prentice-Hall, Englewood Cliffs, N.J. (1991), §1.10.
J.
R.
Selman and
C.
W.
Tobias,
Advances in Chemical Engineering,
10,
Academic Press, New York,
N.Y. (1978),
pp.
212-318.
%.
C.
Gates,
Catalytic Chemistry,
Wiley, New York (1992),
pp.
356-362; C. N. Satterfield,
Heterogeneous Catalysis in Industrial Practice,
McGraw-Hill, New York, 2nd edition (1991), Chapter 8.
