Bird R.B., Stewart W.E., Lightfoot E.N. Transport Phenomena
Подождите немного. Документ загружается.

Problems
577
Surface
z
=
+
b
Fig.
18B.14.
Side view of a disk-shaped
catalyst particle.
Surface
z
=
-
b
18B.14.
Effectiveness factors for thin disks (Fig. 18B.14). Consider porous catalyst particles in the
shape of thin disks, such that the surface area of the edge of the disk is small in comparison
with that of the two circular faces. Apply the method of 518.7 to show that the steady-state
concentration profile is
where
z
and
b
are described in the figure. Show that the total mass transfer rate at the surfaces
z
=
+bis
1
WAI
=
21rR*c,,91~h tanh hb
(18B.14-2)
in which h
=
m.
Show that, if the disk
is
sliced parallel to the xy-plane into
n
slices, the
total mass transfer rate becomes
Obtain the expression for the effectiveness factor by taking the limit
Express this result in terms of the parameter
A
defined in 518.6.
18B.15.
Diffusion and heterogeneous reaction in a slender cylindrical tube with a closed end (Fig.
18B.15).
A
slender cylindrical pore of length
L,
cross-sectional area
Sf
and perimeter
P,
is in
contact at its open end with
a
large body of well-mixed fluid, consisting of species A and
B.
Species A, a minor constituent of this fluid, disappears into the pore, diffuses in the
z
direc-
tion and reacts on its walls. The rate of this reaction may be expressed as (n
.
n,)l,,,,,,
=
f(oA,);
that is, at the wall the mass flux normal to the surface is some function of the mass fraction,
WA~,
of A in the fluid adjacent to the solid surface. The mass fraction
w,
depends on
z,
the dis-
tance from the inlet. Because
A
is present in low concentration, the fluid temperature and density
may be considered constant, and the diffusion flux is adequately described by
jA
=
(a)
Side View
End
View
Fig.
18B.15.
(a)
Diffu-
sion and heterogeneous
reaction in a long, non-
circular cylinder.
(b)
Re-
gion of thickness
Az
over which the mass
balance is made.

578
Chapter 18
Concentration Distributions in Solids and in Laminar Flow
where the diffusivity may be regarded as a constant. Because the pore is long compared to its
lateral dimension, concentration gradients in the lateral directions may be neglected. Note the
similarity with the problem discussed in 510.7.
(a) Show by means of a shell balance that, at steady state,
(b)
Show that the steady-state mass average velocity
v,
is zero for this system.
(c)
Substitute the appropriate form of Fick's law into
Eq.
18.15-1, and integrate the resulting
differential equation for the special case that
f
(w,,)
=
k','wAo.
To obtain a boundary condition
at
z
=
L,
neglect the rate of reaction on the closed end of the cylinder; why is this a reasonable
approximation?
(dl Develop an expression for the total rate
WA
of disappearance of
A
in the cylinder.
(el Compare the results of parts (c) and (d) with those of s10.7 both from the standpoint
of
the mathematical development and the nature of the assumptions made.
188.16.
Effect of temperature and pressure on evaporation rate.
(a)
In
518.2 what is the effect of a change of temperature and pressure on the quantity x,,?
(b) If the pressure is doubled, how is the evaporation rate in
Eq.
18.2-14 affected?
(c)
How does the evaporation rate change when the system temperature is raised from
T
to
T'?
18B.17.
Reaction rates in large
and
small particles.
(a) Obtain the following limits for
Eq.
18.7-11:
Interpret these results physically.
(b) Obtain the corresponding asymptotes for the system discussed in Problem 18B.14. Com-
pare them with the results in (a).
18B.18.
Evaporation rate for small mole fraction of the volatile liquid. In
Eq.
18.2-15, expand
in a Taylor series appropriate for small mole fractions of
A.
First rewrite the logarithm of the
quotient as the difference of the logarithms. Then expand ln(1
-
x,,) and ln(1
-
xA2)
in
Taylor
series about
XAl
=
1
and
XA2
=
1,
respectively. Verify that
Eq.
18.2-16 is correct.
18B.19.
Oxygen uptake by a bacterial aggregate. Under suitable circumstances the rate of oxygen
metabolism by bacterial cells is very nearly zero order with respect to oxygen concentration.
We examine such a case here and focus our attention on a spherical aggregate of cells, which
has
a
radius
R.
We wish to determine the total rate of oxygen uptake by the aggregate as
a
function of aggregate size, oxygen mass concentration po at the aggregate surface, the meta-
bolic activity of the cells, and the diffusional behavior of the oxygen. For simplicity we con-
sider the aggregate to be homogeneous. We then approximate the metabolic rate by an
effective volumetric reaction rate
rO2
=
-k!
and the diffusional behavior by Fick's law, with
an effective pseudobinary diffusivity
BO2,.
Because the solubility of oxygen is very low in this
system, both convective oxygen transport and transient effects may be neglected.12
l2
J.
A.
Mueller,
W.
C.
Boyle,
and
E.
N.
Lightfoot,
Biotechnol.
and
Bioengr.,
10,331-358 (1968).

Problems
579
(a)
Show by means of a shell mass balance that the quasi-steady-state oxygen concentration
profile is described by the differential equation
where
,y
=
po2/po,
5
=
v/R, and
N
=
(b)
There may be an oxygen-free core in the aggregate, if
N
is sufficiently large, such that
x
=
0
for
6
<
to.
Write sufficient boundary conditions to integrate
Eq.
18B.19-1 for this situation. To
do this, it must be recognized that both
x
and
dx/d&
are zero at
6
=
6,.
What is the physical
significance of this last statement?
(c)
Perform the integration of
Eq.
18B.19-1 and show how
6,
may be determined.
(d)
Sketch the total oxygen uptake rate and
6,
as functions of
N,
and discuss the possibility
that no oxygen-free core exists.
N
Answer:
(c)
,y
=
1
-
-
(1
-
p)
+
for
6
z
6,
2
0,
where
6,
is determined as a func-
tion of
N
from
6
18C.1.
Diffusion from a point source in a moving stream
(Fig. 18C.1).
A
stream of fluid
B
in lami-
nar motion has a uniform velocity v,.
At
some point in the stream (taken to be the origin of
coordinates) species
A
is injected at a small rate
WA
g-moles/s. This rate is assumed to be suf-
ficiently small that the mass average velocity will not deviate appreciably from
v,.
Species
A
is swept downstream (in the
z
direction), and at the same time it diffuses both axially and
radially.
(a)
Show that
a
steady-state mass balance on species
A
over the indicated ring-shaped ele-
ment leads to the following partial differential equation if
'?JAB
is assumed to be constant:
(b)
Show that
Eq.
18C.1-1 can also be written as
in which s2
=
v2
+
z2.
Uniform
stream
velocity
vo
Origin of coordinates placed at
point of iniection;
WA
moles
bf
A
are iijected per second
4
LAZ
Fig.
18C.1.
Diffusion of
A
from a point source into
a
stream of
B
that moves with
l+
a uniform velocity.

580
Chapter 18
Concentration Distributions in Solids and in Laminar Flow
(c)
Verify (lengthy!) that the solution
satisfies the differential equation above.
(d) Show further that the following boundary conditions are also satisfied by Eq. 18C.1-3:
B.C.
1:
(18C.1-4)
B.C.
3:
at
r
=
0,
~CA
-
0
--
dr
(18C.1-6)
Explain the physical meaning of each of these boundary conditions.
(e)
Show how data on cA(r,
z)
for given vo and %,, may be plotted, when the preceding solu-
tion applies, to give a straight line with slope v,/29JA, and intercept In
9,,.
18C.2.
Diffusion and reaction in a partially impregnated catalyst. Consider a catalytic sphere like
that in g18.7, except that the active ingredient of the catalyst is present only in the annular re-
gion between
r
=
KR
and r
=
R:
In region I (0
<
r
<
KR),
k"
-
,a
-
0
In region I1
(KR
<
r
<
R),
k;'a
=
constant
>
0
Such a situation may arise when the active ingredient is put on the particles after pelleting, as
is done for many commercial catalysts.
(a) Integrate Eq. 18.7-6 separately for the active and inactive regions. Then apply the appro-
priate boundary conditions to evaluate the integration constants, and solve for the concentra-
tion profile in each region. Give qualitative sketches to illustrate the forms of the profiles.
(b) Evaluate WAR, the total molar rate of conversion of
A
in a single particle.
18C.3.
Absorption rate
in
a falling
film.
The result in Eq. 18.5-18 may be obtained by an alternative
procedure.
(a) According to an overall mass balance on the film, the total moles of
A
transferred per unit
time across the gas-liquid interface must be the same as the total molar rate of flow of
A
across the plane
z
=
L.
The latter rate is calculated as follows:
Explain this procedure carefully.
(b)
Insert the solution for cA in Eq. 18.5-15 into the result of (a) to obtain:
In the second line, the new variable
u
=
X/~~%,,L/V~,~ has been introduced.
(c)
Change the order of integration in the double integral, to get
Explain by means of a carefully drawn sketch how the limits are chosen for the integrals The
integrals may now be done analytically to get Eq. 18.5-18.

Problems
581
18C.4.
Estimation of the required length of an isothermal reactor (Fig. 18.3-1). Let
a
be the area of
catalyst surface per unit volume of a packed-bed catalytic reactor and
S
be the cross-sectional
area of the reactor. Suppose that the rate of mass flow through the reactor is w (in lb,/hr, for
example).
(a) Show that a steady-state mass balance on substance
A
over a length
dl
of the reactor leads to
(b)
Use the result of (a) and Eq. 18.3-9, with the assumptions of constant
6
and
%,,,
to obtain
an expression for the reactor length
L
needed to convert an inlet stream of composition xA(0)
to an outlet stream of composition x,(L).
(Hint: Equation (P) of Table 17.8-1 may be useful.)
18C.5.
Steady-state evaporation. In a study of the evaporation of a mixture of methanol
(1)
and ace-
tone
(2)
through air (31, the concentration profiles of the three species in the tube were mea-
suredl%fter attainment of steady state. In this situation, species
3
is not moving, and species
1
and
2
are diffusing upward, with the molar fluxes N,, and Nz2, measured in the experi-
ments. The interfacial concentrations of these two species, x,, and
x~~~
were also measured. In
addition, the three binary diffusion coefficients were known. The interface was located at
z
=
0
and the upper end of the diffusion tube was at z
=
L.
(a) Show that the Maxwell-Stefan equation for species 3 can be solved to get
in which A
=
Vl13
+
"223,
with
vmpy
=
N,L/c~~, and
l
=
z/L.
(b)
Next verify that the equation for species
2
can be solved to get
"212
Cx30
x2
=
x2,eB[
+
---
(1
-
eB5)
+
-
(eA5
-
eB5)
B
A-B
where
B
=
vlI2
+
y,,
and
C
=
yl,
-
"223.
(c)
Compare the above equations with the published results.
(d)
How well do Eqs. 18C.5-1 and
2
fit the experimental data?
18D.1.
Effectiveness factors for long cylinders. Derive the expression for
77~
for long cylinders anal-
ogous to Eq. 18.7-16. Neglect the diffusion through the ends of the cylinders.
Zl(2N
Answer:
v,
=
---
No(2N'
where
I,,
and
I,
are "modified Bessel functions"
18D.2.
Gas absorption
in
a falling
film
with chemical reaction. Rework the problem discussed in
518.5 and described in Fig. 18.5-1, when gas A reacts with liquid
B
by a first-order irreversible
chemical reaction in the liquid phase, with rate constant
k;'.
Specifically, find the expression
for the total absorption rate analogous to that given in Eq. 18.5-18. Show that the result for ab-
sorption with reaction properly simplifies to that for absorption without reaction.
Answer:
wA
=
W~~ZJ,,~
JG
[(;
+
u)
era
+
$
in which u
=
k;'~/o,,,,.
k?
l3
H.
A.
Wilson,
Proc.
Camb.
Phil.
Soc.,
12,406423 (1904).
l4
R.
Carty and
T.
Schrodt,
Ind.
Eng.
Chem.,
14,276-278 (1975).

Chapter
19
Equations
of
Change
for
Multicomponent Systems
519.1 The equations of continuity for a multicomponent mixture
519.2 Summary of the multicomponent equations of change
s19.3
Summary of the multicomponent fluxes
519.4 Use of the equations of change for mixtures
919.5
Dimensional analysis of the equations of change for binary mixtures
In Chapter 18, problems in diffusion were formulated by making shell mass balances
on one or more of the diffusing species. In this chapter we start by making a mass bal-
ance over an arbitrary differential fluid element to establish the equation of continuity
for the various species in a multicomponent mixture. Then insertion of mass flux ex-
pressions gives the diffusion equations in a variety of forms. These diffusion equations
can be used to set up any of the problems in Chapter 18 and more complicated ones as
well.
Then we summarize all of the equations of change for mixtures: the equations of
continuity, the equation of motion, and the equation of energy. These include the equa-
tions of change that were given in Chapters 3 and 11. Next we summarize the flux ex-
pressions for mixtures. All these equations are given in general form, although for
problem solving we generally use simplified versions of them.
The remainder of the chapter is devoted to analytical solutions and dimensional
analyses of mass transfer systems.
519.1
THE
EQUATIONS OF CONTINUITY
FOR
A
MULTICOMPONENT MIXTURE
In this section we apply the law of conservation of mass to each species
a
in a mixture,
where
a
=
1,2,3,
. .
.
,
N.
The system we consider is a volume element
Ax
Ay
Az
fixed in
space, through which the fluid mixture is flowing (see Fig. 3.1-1). Within this mixture, re-
actions among the various chemical species may be occurring, and we use the symbol
r,
to indicate the rate at which species
a
is being produced, with dimensions of mass/vol-
ume time.
The various contributions to the mass balance are
rate of increase of mass of
(dp,/Jt)~x
~y
AZ
a
in the volume element
rate of addition of mass of
nffrlx
AY
a
across face at
x
519.1 The Equations of Continuity for a Multicomponent Mixture
583
rate of removal of mass of
n,,l,+~,
4
Az
a
across face at x
+
Ax
rate of production of mass of r,Ax Ay Az
a
by chemical reactions
The combined mass flux
n,,
includes both the molecular flux and the convective flux.
There are also addition and removal terms in the y and
z
directions. When the entire
mass balance is written down and divided by Ax Ay Az, one obtains, after letting the size
of the volume element decrease to zero,
This is the equation of continuity for species
a
in a multicomponent reacting mixture. It de-
scribes the change in mass concentration of species
a
with time at a fixed point in space
by the diffusion and convection of
a,
as well as by chemical reactions that produce or
consume a. The quantities n,,, n,,, n,, are the Cartesian components of the mass flux vec-
tor
n,
=
p,v, given in Eq.
(D)
of Table 17.8-1.
Equation 19.1-5 may be rewritten in vector notation as
Alternatively we can use Eq.
(S)
of Table 17.8-1 to write
I
rate of net rate of net rate of
rate of
increase addition addition
production
of mass of mass of of mass of
of mass of
of
A
per
A
per
unit
A
per
unit
A
per unit
unit volume
by
volume
by
volume
by
volume convection
diffusion reaction
Addition of all
N
equations in either Eq. 19.1-6 or 7 gives
which is the equation of continuity for the mixture. This equation is identical to the equation
of continuity for a pure fluid given in
Eq.
3.1-4.
In
obtaining Eq. 19.1-8 we had to use Eq.
(J)
of Table 17.8-1 and also the fact that the law of conservation of total mass gives Car,
=
0.
Finally we note that Eq. 19.1-8 becomes
for a fluid mixture of constant mass density p.
In
the preceding discussion we used mass units. However, a corresponding deriva-
tion
is
also possible in molar units. The equation of continuity for species
a
in molar
quantities is
'
J.
Crank,
The Mathematics of Diffusion,
2nd edition, Oxford University Press
(1975).
584
Chapter
19
Equations of Change for Multicomponent Systems
where
R,
is the molar rate of production of
a!
per unit volume. This equation can be
rewritten by use of Eq.
(V)
of Table 17.8-1 to give
rate of net rate of rate of
rate of
increase addition
addition production
in moles
in
moles of of moles of of moles of
of
A
per
A
per unit
A
per unit
A
per unit
unit volume
by
volume
by
volume
by
volume convection diffusion
reaction
--
-
-
When all
N
equations in Eq. 19.1-10 or
11
are added we get
for the equation of continuity for the mixture. To get this we used Eq.
(M)
of Table 17.8-1.
We also note that the chemical reaction term does not drop out because the number of
moles is not necessarily conserved in a chemical reaction. Finally we note that
for a fluid mixture of constant
molar density
c.
We have thus seen that the equation of continuity for species
a
may be written in
two forms,
Eq.
19.1-7 and Eq. 19.1-11. Using the continuity relations in Eqs. 19.1-8 and
19.1-12 the reader may verify that the equation of continuity for species
a!
can be put into
two additional, equivalent forms:
These two equations express exactly the same physical content, but they are written in
two different sets of notation-the first in mass quantities and the second in molar quan-
tities. To use these equations we have to insert the appropriate expressions for the fluxes
and the chemical reaction terms. In this chapter we give only the results for
binary sys-
tems
with constant
p%,,,
with constant
or with zero velocity.
Binary Systems with Constant
p9lAB
For this assumption, Eq. 19.1-14 becomes, after inserting Fick's law from Eq.
(A)
of Table
17.8-2,
with a corresponding equation for species
B.
This equation is appropriate for describing
the diffusion in
dilute liquid solutions
at constant temperature and pressure. The left side
can be written as
pDoA/Dt.
Equation 9.1-16 without the
r,
term is of the same form
as
Eq. 11.2-8 or
9.
This similarity is quite important, since it is the basis for the analogies
that are frequently drawn between heat and mass transport in flowing fluids with con-
stant physical properties.
s19.1 The Equations of Continuity for a Multicomponent Mixture
585
Binary Systems with Constant
&,,
For this assumption, Eq. 19.1-15 becomes, after inserting Fick's law from Eq.
(B)
of Table
17.8-2,
with a corresponding equation for species
B.
This equation is useful for low-density gases
at constant temperature and pressure. The left side can not be written as cDx,/Dt be-
cause of the appearance of
v"
rather than
v.
Binary Systems with Zero Velocity
If there are no chemical reactions occurring, then the chemical production terms are all
zero. If, in addition
v
is zero and
p
constant in Eq. 19.1-16, or
v"
is zero and c constant in
Eq. 19.1-17, then we get
which is called Fick's second law of diffusion, or sometimes simply the diffusion equation.
This equation is usually used for diffusion in solids or stationary liquids (that is,
v
=
0 in
Eq. 19.1-16) and for equimolar counter-diffusion in gases (that is,
v"
=
0 in Eq. 19.1-17). By
equimolar counter-diffusion we mean that the net molar flux with respect to stationary
coordinates is zero; in other words, that for every mole of
A
that moves, say, in the posi-
tive
z
direction, there is a mole of
B
that moves in the negative
z
direction.
Note that Eq. 19.1-18 has the same form as the heat conduction equation in Eq. 11.2-10.
This similarity is the basis for analogies between many heat conduction and diffusion
problems in solids. Keep in mind that many hundreds of problems described by Fick's
second law have been solved. Solutions are tabulated in the monographs of Crank1 and
of Carslaw and Jaeger.'
In Tables B-10 and 11 we give Eq. 19.1-14 (multicomponent equation of continuity in
terms of
j,)
and Eq. 19.1-16 (binary diffusion equation for constant
p
and '?JAB) in the
three standard coordinate systems. Other forms of the equation of continuity can be pat-
terned after these.
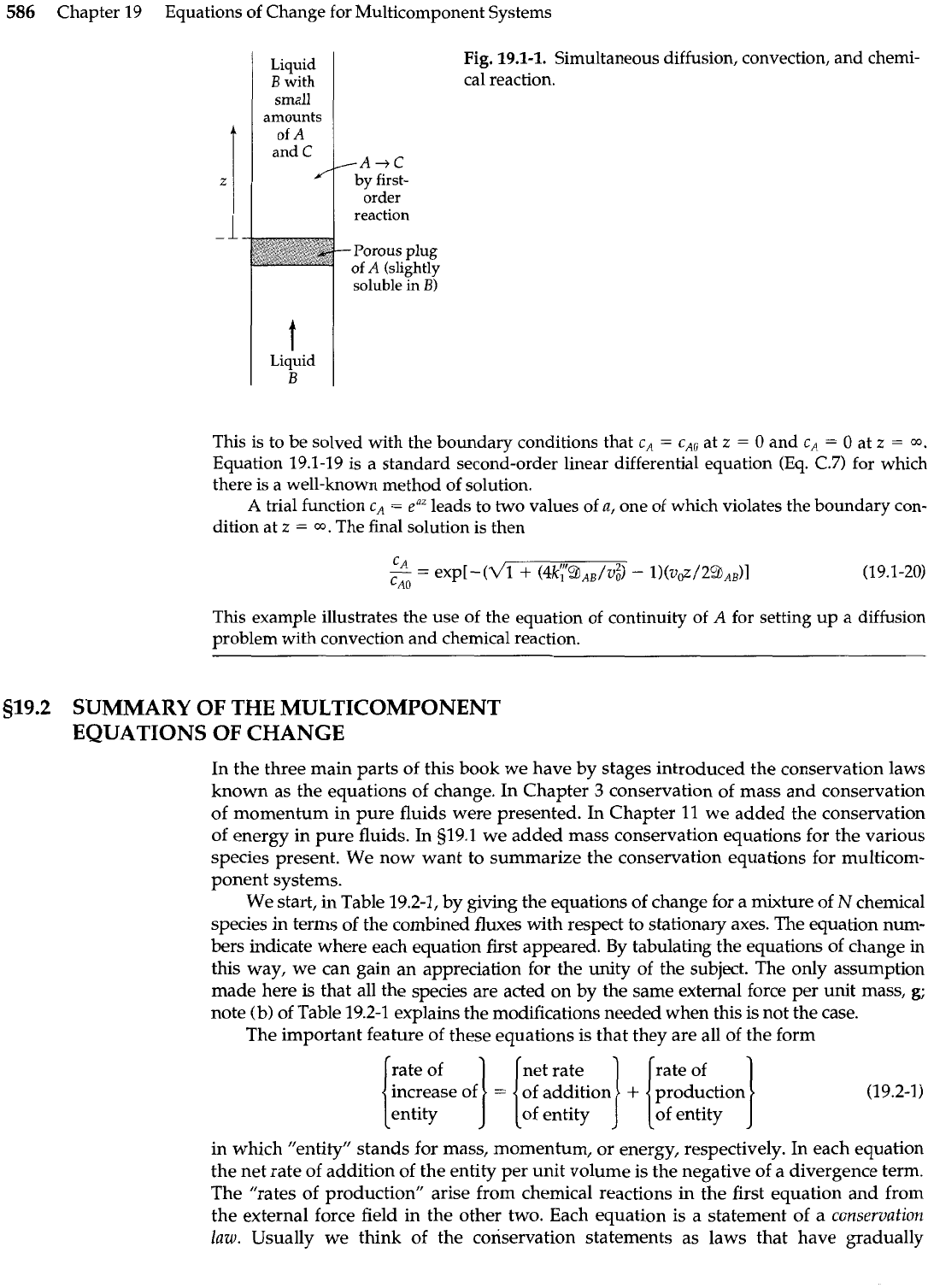
In Fig. 19.1-1 we show a system in which a liquid,
B,
moves slowly upward through a slightly
soluble porous plug of
A.
Then
A
slowly disappears by a first-order reaction after it has dis-
solved. Find the steady-state concentration profile c,(~), where
z
is the coordinate upward
and
Chemical Reaction3
from the plug. Assume that the velocity profile is approximately flat across the tube. Assume
further that
CAO
is the solubility of unreacted
A
in B. Neglect temperature effects associated
with the heat of reaction.
SOLUTION
Equation 19.1-16 is appropriate for dilute liquid solutions. Dividing this equation by the mol-
ecular weight
MA
and specializing for the one-dimensional steady-state problem at hand, we
get for constant
p:
H.
S.
Carslaw and
J.
C. Jaeger,
Conducfion ofHeaf in Solids,
2nd edition, Oxford University Press (1959).
W.
Jost,
Diffusion,
Academic Press, New York (1952),
pp.
58-59.
586
Chapter 19 Equations of Change for Multicomponent Systems
Liquid
Fig.
19.1-1.
Simultaneous diffusion, convection, and chemi-
B
with
cal reaction.
small
amounts
and
C
A+C
order
reaction
Porous plug
of
A
(slightly
soluble
in
B)
t
Liquid
B
This is to be solved with the boundary conditions that
cA
=
c,,
at
z
=
0
and
c~
=
0
at
z
=
m.
Equation 19.1-19 is a standard second-order linear differential equation (Eq. C.7) for which
there is a well-known method of solution.
A
trial function
CA
=
eaz
leads to two values of
a,
one of which violates the boundary con-
dition at
z
=
a.
The final solution is then
This example illustrates the use of the equation of continuity of
A
for setting up a diffusion
problem with convection and chemical reaction.
519.2
SUMMARY OF
THE
MULTICOMPONENT
EQUATIONS
OF
CHANGE
In the three main parts of this book we have by stages introduced the conservation laws
known as the equations of change. In Chapter
3
conservation of mass and conservation
of momentum in pure fluids were presented. In Chapter 11 we added the conservation
of energy in pure fluids. In 519.1 we added mass conservation equations for the various
species present. We now want to summarize the conservation equations for multicom-
ponent systems.
We start,
in
Table 19.2-1, by giving the equations of change for a mixture of
N
chemical
species in terms of the combined fluxes with respect to stationary axes. The equation num-
bers indicate where each equation first appeared. By tabulating the equations of change in
this way, we can gain an appreciation for the unity of the subject. The only assumption
made here
is
that all the species are acted on by the same external force per unit mass,
g;
note (b) of Table 19.2-1 explains the modifications needed when this is not the case.
The important feature of these equations is that they are all of the form
rate of
net rate
rate of
increase of
=
of addition
+
production (19.2-1)
{entity
]
[ofentity
]
jOf
entity
]
in which "entity" stands for mass, momentum, or energy, respectively. In each equation
the net rate of addition of the entity per unit volume is the negative of a divergence term.
The "rates of production" arise from chemical reactions in the first equation and from
the external force field in the other two. Each equation is a statement of a
conservation
law.
Usually we think of the conservation statements as laws that have gradually
