Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

10 Interfacial Forces and Spectroscopic Study of Confined Fluids 535
0
0.5
Elapsed time t (s)
Normalized dissipative force
1000
1.5
1
250 500 750
0
0.5
1000
1.5
1
250 500 750
0
0
50
0
0.5
50
1.5
1
12.5 25 37.5
2
1
40302010
a)
b)
c)
d)
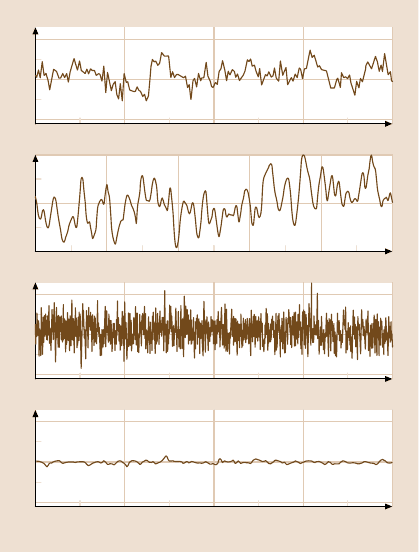
Fig. 10.10a–d. The prominence of fluctuations for water confined in a Janus interface is
illustrated. In panels (a)–(c), the viscous forces, normalized to the mean (at 1.3 Hz), are plot-
ted against time elapsed. In panel (a), the surfaces were first wetted with ethanol to remove
adsorbed gas, then flushed with degassed, deionized water. In panel (b), the ethanol rinse
was omitted. Panel (c) shows a long-time trace for data taken under the same conditions
as for panel (b). Panel (d) illustrates that water confined between symmetric hydrophilic–
hydrophilic surfaces (panel (d)) did not display noisy responses. Confined ethanol films like-
wise failed to display noisy responses (not shown). After [52] with permission
a quintessential instance of competing terms in the free energy, to satisfy which
there may be many metastable states that are equally bad (or almost equally bad)
compromises[91,92]. This suggests the physical picture of flickering capillary-type
waves, sketched hypotheticallyin Fig. 10.11. These proposed long-wavelengthcap-
illary fluctuationswould differ profoundlyfrom thoseat the free liquid–gasinterface
because they would be constricted by the nearby solid surface.
The power spectrum is the decomposition of the traces into their Fourier com-
ponents whose squared amplitudes are plotted, on log–log scales, against Fourier
frequency in Fig. 10.11. In the power spectrum one observes, provided the Fourier
frequency is sufficiently low, a high level of “white” frequency-independent noise.
But the amplitude began to decrease beyond a threshold Fourier frequency
(f ≈ 0.001Hz), about 10
3
times less than the drive frequency. Other experiments
536 Y. Elaine Zhu et al.
10
4
10
14
f (Hz)
S(f )
10
15
10
4
10
9
10
8
10
7
10
6
10
5
10
0
10
3
10
2
10
1
2
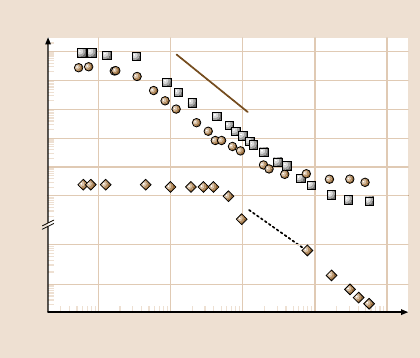
Fig. 10.11. The power spectrum for deionized water and the hydrophobized surface com-
prised of OTE deposited by the LB technique (squares); degassed deionized water and the hy-
drophobized surface comprised of an octadecanethiol monolayer (circles); and water contain-
ing 25 mM NaCl confined between symmetric hydrophilic–hydrophilic surfaces (diamonds).
To calculate the power spectra the time elapsed was at least 10
5
s (the complete time series is
not shown). The power-law exponent is close to −2. After [52] with permission
(not shown) showed the same thresholdFourierfrequency when the drivefrequency
was raised from 1.3 to 80 Hz, and therefore it appears to be a characteristic fea-
ture of the system. It defines a characteristic time for rearrangement of some kind
of structure, ≈ 10
3
/2πs; we tentatively identify this with the lifetime of bubbles or
vapor (see below). The subsequent decay of the power spectrum as roughly 1/ f
2
suggests that these fluctuations reflect discrete entities, as smooth variations would
decay more rapidly. Noise again appeared to become “white” but with an amplitude
10
4
times smaller at a Fourier frequency of f > 0.1Hz but the physical origin of this
is not evident at this time.
The possible role of dissolved gas is clear in the context of our proposed physi-
cal explanation. Indeed, submicron-sized bubbles resulting from dissolved air have
been proposed to explain the anomalously long range of the hydrophobic attrac-
tion observed between extended surfaces [48, 51, 70–72]. To test this idea, we
performed control experiments using degassed water. The power spectrum, shown
in Fig. 10.12, was nearly the same. Althoughwe cannotexcludethat a small amount
of residualdissolved gas was responsible,this method of degassingis reportedto re-
movelong-range hydrophobicattraction [71], whereas the comparison in Fig. 10.12
shows the consequence in the present situation to be minor. We conclude that the
effects reported in this chapter do not appear to stem from the presence of dissolved
gas.
From recent theoretical analysis of the hydrophobic interaction the expectation
of dewetting emerges – it is predicted that an ultrathin gas gap, with a thickness on

10 Interfacial Forces and Spectroscopic Study of Confined Fluids 537
Hydrophilic
Hydrophobic
Fig. 10.12. Schematic il-
lustration of the capillary
waves of water meeting the
hydrophobic surface with
a flickering vapor phase in
between
the order of 1 nm, forms spontaneously when an extended hydrophobic surface is
immersed in water [50,61,67]. The resulting capillary-wave spectrum does not ap-
pear to have yet been considered theoretically, but for the related case of the free
water–vapor interface, measurements confirm that capillary waves with a broad
spectrum of wavelengths up to micrometers in size contribute to its width [93].
On physical grounds, the thin gas gap suggested by our measurements should also
be expected to possess soft modes with fluctuations whose wavelength ranges from
small to large. From this perspective,we then expectthat the experimentalgeometry
of a Janus-type water film, selected for experimentalconvenience,was incidental to
the main physical effect.
This has evident connections to understanding the long-standing question of the
structure of aqueous films near a hydrophobic surface and may have a bearing on
understanding the structure of water films near the patchy hydrophilic–hydrophobic
surfaces that are so ubiquitous in proteins.
10.4 Ultrafast Spectroscopic Study of Confined Fluids:
Combining Ultra-Fast Spectroscopy with Force Apparatus
The surface forces apparatus (SFA) modified to measure interfacial rheology has
been used widely in last few years to study the viscoelasticity of molecularly thin
fluid films [1–4, 94–98]. A recent application of this technique is described in
Sect. 10.3. Though the force-based techniques are powerful and sensitive, they are
indirect. The observation of structure-related transitions, e.g., oscillatory forces [1],
confinement-inducedsolidification [4,94,95], and stick–slip motion in SFA experi-
ments [17,96,97] have not been directly verified experimentally using an indepen-
dent technique.
The power of scattering, microscopyand spectroscopic techniquesin the studies
of confined fluids has been of speculativeinterest for a long time.However, thereare
only a few reported successes, primarily because of technicaldifficulties of combin-
ing these forms of techniques with SFA. Neutron and X-ray diffraction methods are
538 Y. Elaine Zhu et al.
very powerful for direct determination of structures at the nanoscale. Recently de-
veloped X-ray surface forces apparatus permits simultaneous X-ray diffraction and
direct normal and lateral forces measurements of complex fluids under shear and
confinement [98]. Safinya et al. have investigated the structure of thin liquid crystal
films under confinement using this apparatus [99]. The deep penetrating power of
neutrons and the ability to substitute hydrogen with deuterium in many liquid sys-
tems can be exploited to measure the molecular density and orientation of confined
fluidsby using neutrondiffraction[100].The structureof end-graftedpolymerbrush
layers have been investigated in this manner. Successful utilization of this method
requires one to devise an apparatus that can keep single-crystal substrates of quartz
or sapphire with areas up to tens of square centimeters parallel at controlled and
well-defined separations [101]. So far, both neutron and X-ray confinement cells
are limited to confining gaps ranging from several hundred angstroms to millime-
ters and are not capable of studying ultrathin (≈ nm) liquid films. This intermediate
length scale is more suited to study complex fluids, e.g., long polymer chains, col-
loidal particles and biological cells, where self-organized structures of larger scale
come into play. For simple fluids, the difficulty arises because, as the film thick-
ness decreases, the total number of scatterers decreases and the signal-to-noise ratio
presents severe limitations. It is difficult to distinguish with sufficientconfidence the
structure of a molecularly thin fluid film from that of the thicker solids that envelop
it. Synchrotron X-ray sources, such as the advanced photon source at Argonne Na-
tional Laboratory have sufficient flux for experiments of extremely confined liquid
films possible. Recent X-ray reflectivity experiments have confirmed the expected
layering in the direction perpendicular to the confining surfaces [102],but questions
about in-plane order and responses to external fields remain conjectural.
The interaction of light with matter – for example Raman and infrared – has
impressive potential, but the problem is to distinguish the signal (the fluid mono-
layer) from all the noise (the sliding surfaces and the solids beneath them). The
microrheometerdeveloped by Dhinojwala et al. can readily be combinedwith spec-
troscopy (Fourier-transform infrared spectroscopy and dielectric spectroscopy) or
scattering (X-ray and neutron) techniques [103]. It uses two parallel optically flat
windows plates whose separation can be controlled from a few tens of nm to tens
of µm, but is more suited to thicker (0.1–10µm) films. It has been used for in situ
study of shear-induced molecular orientation of nematic liquid crystals by using
Fourier-transform infrared time-resolved spectroscopy (FTIR-TRS) synchronized
with the shear motion [104]. By replacing one of the plates with a prism, recently
it has been shown that this rheometer can be combined with the surface-sensitive
technique of infrared-visible sum-frequency generation (SFG) in the total internal
reflection (TIR) geometry [105]. This combination can be used to probe the orien-
tation, alignment and relaxation modes of organic molecules at the buried interface
in a condition of flow or shear. Some years back it was shown that SFG can be
combined with the surface forces apparatus to study nanometer-thick films of self-
assembled monolayer confined between atomically smooth mica surfaces [106], but
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 539
implementation of this approach to other experimental situations, such as confined
fluids still presents significant challenges.
Another problematic issue arises in interpreting experiments that measure me-
chanical properties, such as the SFA or AFM. The measurement generates a single
number, the force, but although the surface separations are molecular, the areas of
interaction are macroscopic. So this force is the result of the average response of
a large collection of molecules. Recent advances in optical spectroscopy and mi-
croscopy have made it possible not only to detect and image single molecules, but
to conduct spectroscopic measurements and monitor dynamic processes as well at
the level of single (or a handful of) molecules [107, 108]. These studies illustrate
their utility to dissect the distributions around the average for processes such as dif-
fusion or chemical reactions. In many of these experiments, a fluorescent molecule
is doped into the sample, which acts as a probe of its local environment[109]. Mon-
itoringmotions of the probeovertime and in the presenceof externalfields can offer
insightsinto changesin this localenvironmentwithin whichthe dye moleculeis em-
bedded.However,to integrateforce measurementsusing SFA withconcurrentmeas-
urements using fluorescence spectroscopy required significant modification of the
usual methods [110]. In the following we discuss the challenges of combining SFA
with single-molecule-sensitive spectroscopy techniques. This section is adapted
from discussions in several primary accounts published previously [109,110].
10.4.1 Challenges
Oneofthemajordifficulties is to detect and collect fluorescence efficiently and
to separate it from background noise. Background originates from many sources:
Raman and Rayleigh scattering, fluorescence owing to impurities in the solvent,
and from the substrates, which includes the lens, glue and mica (the glue attaches
a cleaved mica sheet onto the supporting cylindrical lens in SFA experiments).Typ-
ical background counts can far exceed those from a dilute concentration of fluo-
rophore molecules doped inside the sample of interest.
Another type of challenge comes from the limited photochemical lifetime of
a fluorophore.A common fluorophore photobleachesirreversibly after emitting a fi-
nite number of photons (10
5
–10
6
). This problem becomes severe in ultrathin films,
where the dynamics can become slower and a dye molecule resides for a long time
within the laser focus.
A third difficulty is the necessity to perform spectroscopy at the same time as
multiple-beam interferometry (MBI) to determine the film thickness. Traditionally
a silver coating of thickness ≈ 63 nm is used at the back side of mica for the purpose
of MBI, but the high reflectivity of silver from the infrared to UV regime excludes
this possibility here.
Thefinal challengeis to incorporatethe SFA and the neededoptics.As the signal
must be as large as possible, the maximum possible amount of fluorescence from
the fluorophore of interest should be detected for a successful experiment. A high
numerical aperture (N.A.) objective is desirable but such objectives have a very
540 Y. Elaine Zhu et al.
small working distance (≈ 1−2mm). This requires significant modification of the
traditional SFA in order to make it possible to focus the laser beam on the sample.
We recently succeeded in implementing the technique of fluorescence correla-
tion spectroscopy (FCS), which can measure the translational diffusion with surface
forces measurement and friction studies within the SFA [111]. The scientific objec-
tive of building this integrated platform was to answer questions such as: how is
the rate of molecular probe diffusion, within a confined fluid, related to the stress
relaxation time? Is there significant collective molecular motion or dynamical het-
erogeneity? What happens to the molecules during the stick–slip motion? The prin-
ciple of the FCS technique and the experimental set-up of the combined platform
are described below.
10.4.2 Principles of FCS Measurement
Fluorescence correlation spectroscopy (FCS) is an experimental method to extract
information on dynamical processes from the fluctuation of fluorescence inten-
sity [112]. The technique has enjoyed widespread application recently in the field of
chemical biology because of its ability to access to a multitude of parameters with
biologicalrelevance[113,114].The fluctuationof fluorescence,whendye molecules
are dilute, can in principle result from diffusion, aggregation, or chemical reaction.
Compared to other techniques for studying diffusion problems, such as quasi-elastic
light scattering (QELS), fluorescence recovery after photobleaching (FRAP), and
laser-induced transient grating spectroscopy, FCS presents the unique capability of
measuring extremely dilute systems with high spatial resolution (downto the optical
diffraction limit). On the average there can be as few as 1–5 dye molecules within
the ≈ 1 fl volume element of the focused laser beam. However, these dye molecules
move in and out due to Brownian motion, causing intensity fluctuations which can
be observed as low-frequency noise on the mean fluorescence signal (Fig. 10.13).
By inspecting the autocorrelation function of this fluctuation,
G(τ) ≡δI(t)δI(t+ τ)/δI(t)
2
, (10.4)
(here I denotes fluorescence intensity and t is the time variable), and by choosing
a suitable model to analyze it, the rate of dynamic process is obtained [112]. If the
primary reason for fluctuation is translational diffusion, and assuming that the flu-
orescence characteristics of the diffusing molecules do not change while traversing
the laser volume, one can use Fick’s second law to calculate the translational diffu-
sion coefficient (D) from the autocorrelation function by using the relation [115],
G(τ) = G(0)/
1+ 8Dτ/ω
2
0
. (10.5)
This result follows from the convolution of the concentration correlation with the
spatial profile of the laser focus, which has been assumed to be a two-dimensional
Gaussian of width ω
0
. The magnitude of the autocorrelation function at time zero,
G(0), is related to the averagenumber of fluorophores(N) in the observationvolume

10 Interfacial Forces and Spectroscopic Study of Confined Fluids 541
by the relation [116]
G(0) = 1/(2
√
2N) . (10.6)
Molecular mobilities can be measured over a wide range of characteristic time con-
stants from ≈10
−3
to 10
3
ms by using this technique.
Fluctuation analysis is best performed if the system under observation is re-
stricted to very small ensembles and if the background is efficiently suppressed.
These can be accomplished by a combination of very low sample concentrations (≈
nanomolar) with extremely small measurements volumes (≈femtoliter). The excita-
tion of the fluorophores can be performed either with two photons using a pulsed
laser or with one photon using continuous-wave lasers [115]. In one-photon FCS,
spatial resolution is obtained with a confocal set-up, in which a small pinhole in-
serted into the image plane can reject the out-of-focusfluorescence. For two-photon
excitation on the other hand, simultaneous (within ≈ 10
−15
s) absorption of two
lower-energyphotonsof approximatelytwicethe wavelengthis requiredfor a transi-
tion to the excited state. Mode-locked lasers providing short pulses (≈10
−13
s) with
a high repetition rate (10
8
Hz) can provide sufficient photon flux densities for two-
photon processes. As the excitation probability is proportional to the mean square
of the intensity, it results in inherent depth discrimination. Additionally, two-photon
excitationimprovesthe signal-to-backgroundratioconsiderably.As the mostpromi-
nent scattering came from the incident light, which is well separated in wavelength
from the induced fluorescence, this makes it easy to separate the fluorescence emis-
sion from the excitation light and the scattered light. However, the photobleaching
rates with two-photon excitation are significantly enhanced with respect to one-
photon excitation at comparable photon-emission yields [117].
10.4.3 Experimental Set-up
A schematic diagram of the method of combing fluorescence correlation spec-
troscopy with the surface forces apparatus is shown in Fig. 10.12. The FCS por-
tion of the set-up consists of three major parts: light source, microscope and data
acquisition [110]. A femtosecond Ti:sapphire laser, which typically generates laser
pulses with full width at half maximum (FWHM) of 100 fs at a repetition rate of
80MHz can be used for the two-photon excitation of the fluorophores. An inverted
microscope serves as the operational platform for the whole experiment. The exci-
tation light is focused onto the sample with an objective lens of high N.A. and the
emitted light is collected through the same objective and is detected by a photomul-
tiplier tube (PMT) or avalanche photodiode (APD). The photon counting output is
recorded by an integrated FCS data-acquisition board and data analysis can be per-
formed with commercial or home-written software. By introducing the laser beam
through the objective lens, a small excitation volume (≈1fl) is generated within the
sample.The lateral dimension of the excitation spot is about ≈0.5 µm, which can be
determined by a calibration experiment using widely used dyes, such as fluorescein,
whose diffusion coefficient in water is known to be ≈ 300µm
2
/s. The excitation
542 Y. Elaine Zhu et al.
power at the sample needs to be less than 1 mW to avoid photobleaching and heat-
ing effects of the sample.
The modified surface forces apparatus sat directly on the microscope stage.
The traditional crossed-cylindrical geometry produced a circular contact of parallel
plates when the crossed cylinders were squeezed together such that they flattened
at the apex. Using an inchworm motor, separation of the surfaces can be controlled
from nanometers to millimeters. To determine the separation between the surfaces,
the traditional silver sheets for interferometric measurements of surface spacing in
the SFA need to be replaced by multilayer dielectric coatings [118]. These multi-
layers can be produced by successive evaporation of layers (typically 13 or 15) of
TiO
x
and Al
2
O
3
by electron-beam evaporation. The optical thickness of each layer
was approximately λ/4(λ ≈ 650nm), as determined by the optical monitor within
the coating chamber. The thickness of each coating determines the windows of re-
flectivity and translucency. This approach can produce high reflectivity in the region
600–700nm, as well as translucent windows in the region ≈ 800nm (to allow flu-
orescence excitation) and 400–550nm (to detect fluorescence). The reflectivity as
a function of wavelength is shown in Fig. 10.13 for the bare mica surface and for
surfaces with different numbers of multilayers.
The same set-up (Fig. 10.14) with some modification can be used to probe
molecular rotational diffusion. In the ground state fluorophores are all randomly
oriented. When excited by polarized light, only those fluorophores that have their
dipole moments oriented along the electric vector of the incident light are prefer-
10
5
0
ô (s)
g(ô)
0.75
0.25
0.5
10
1
10
4
10
3
10
2
2
8
1200200 600
Time (ms)
d)
Count
c)
b)a)
6
4
10
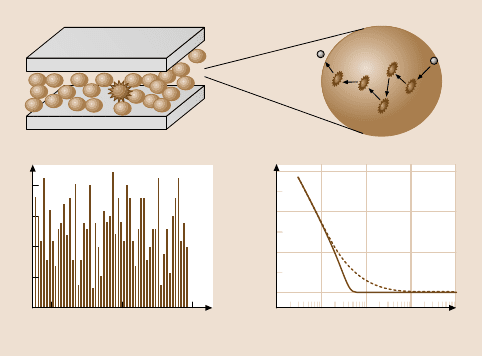
Fig. 10.13a–d. Schematic illustration of the utility of fluorescence correlation spectroscopy
in a confined geometry: (a) A fluorescent molecule is doped within an ultrathin film of fluids
(e.g., simple alkanes, polymers, colloidal particles) confined between two solid surfaces. Pho-
ton emission counts can fluctuate with time (c) resulting from the diffusion of fluorophores
through the laser focus (b). From the autocorrelation function of this fluctuation G(τ), the
rate of dynamic process can be obtained (d). Calculated G(τ) for pure Brownian diffusion
(dashed curve) and flow superimposed with diffusion (solid curve)areshown
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 543
SFA with
shear assembly
Inverted
microscope
Femtosecond
laser
C
B
A
D
E
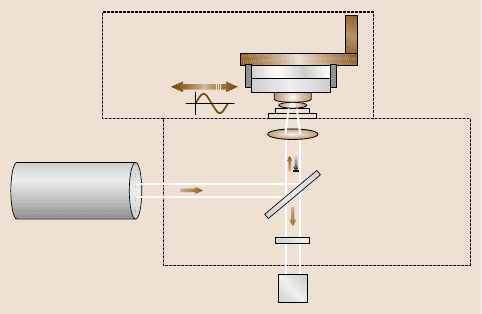
Fig. 10.14. Schematic diagram of the assembly used to perform fluorescence correlation
spectroscopy within a surface forces apparatus equipped for shear experiments. A miniatur-
ized surface forces apparatus sits on a microscope stage. A femtosecond pulsed laser excites
fluorescent dye molecules within a molecularly thin liquid film contained between two op-
posed surfaces of muscovite mica. Colored filter (A) to remove the residual excitation light
(λ = 800 nm) from the fluorescence light (400–550 nm) which is collected by the single-
photon counting module (D). Dichroic mirror (B) and the objective lens (C) used to focus
and collect the light. An inchworm motor (E) controls the separation of the surfaces from
nanometers up to several micrometers
entially excited. So the excited-state population is not randomly oriented, instead
there is a larger number of excited molecules having their transition moments ori-
ented along the polarization direction of the incident light. This anisotropy of orien-
tation can be determined by measuring the intensity of light polarized parallel to the
incident light and perpendicular to the incident light. This preferential anisotropy
at time zero decays due to the rotational diffusion of the dye molecule, following
an exponential law with a characteristic rotation time (τ). As the typical rotational
timeranges from picosecondsto nanoseconds,the dynamicson much smaller length
scales, that of only one or two nm, can be investigated by this method. Translational
diffusion experimentsby FCS, on the other hand, (as discussed in the following sec-
tions) involves a large distance – the probe molecules travel hundreds of nm into
and out of the laser spot – to produce a signal. Therefore, it is more sensitive to the
global environmentof the molecules.
10.5 Contrasting Friction with Diffusion
in Molecularly Thin Films
In FCSexperiments,the magnitudeof the fluctuationautocorrelationfunction scales
inversely with the number of fluorescent molecules in the observation volume
(10.6). Large fluorophore concentrations more than micromolar are not efficient in
544 Y. Elaine Zhu et al.
FCS, because G(0) eventually becomes too small for fluctuation analysis. Typical
dye concentration for confined fluid experiments is kept at 50nM. A key point for
these experiments is to find systems in which adsorption of the fluorophore would
not complicate the situation. In other words, the fluids themselves, not the fluo-
rophores, should be attracted preferentially to the confining solid surfaces [111].
This point can be verified by scanning the laser focus vertically from within the
mica, through the surface, into the bulk fluids, and observing that there is no jump
in fluorescence counts as the surface is crossed. Finally, one needs to be sensitive
to the concern that, when using fluorophores to probe local micro-environments,
micro-environments might be perturbed by their presence. Therefore, it is essen-
tial to perform normal and shear force experiments with and without the presence
of dye molecules to verify that these are not affected. This section is adapted from
discussions in several primary accounts published previously [111].
In SFA experiments, a drop of the fluid for study is placed between the two
mica sheets, oriented as crossed cylinders so that in projection the geometry os
a sphere against a flat (Fig. 10.16). In the study of surface forces, it is well known
that, as surfaces separated by fluid are brought together, fluid drains smoothly until
a thickness of 5–10 molecular dimensions, at which point the fluid supports stress
owing to packing of molecules at the surface [1]. When rounded surfaces of this
kind are pressed together, separated by fluid, the curved surfaces flatten at the apex
to form parallel plates. The resulting inhomogeneous pressure distribution over the
contact region is well known in the field of tribology. It is approximately Hertzian;
zero at the edges of the contact zone and, at the center, 3/2 the mean value [119].
The Hertzian model is generally a good approximation in the absence of strongly
attractive forces.
Figure 10.15 shows results for two different fluid systems: (a) propane diol con-
taining ≈ 50nM rhodamine 123, and (b) octamethylcyclotetrasiloxane (OMCTS),
containing ≈ 50nM coumarin. Propane diol is a low-viscosity fluid (≈ 0.4Poise)
with a glass-transition temperature far below room temperature (T
g
= −105
◦
C).
The OMCTS molecule is ring-shaped; it is the cyclic tetramer of dimethylsilox-
ane. It has a viscosity much like water (≈ 0.002Poise) and possesses the intriguing
featurethat it crystallizes at 1atm near room temperature(T
m
= 17
◦
C), thusenhanc-
ing the possibility that a confinement-induced elevation of the melting temperature
might be detected. There is a long tradition of considering it to constitute a model
system when studying friction and surface-induced structure of nonpolar confined
fluids because numerous computer simulations designed to model lubrication have
concerned particles of spherical shape [2,95,120]. As the typical size of the con-
tact area is ≈ 50 µm and the size of the laser spot is ≈ 0.5µm, it is possible to scan
the laser focus laterally, as sketched in Fig. 10.14. From time series of fluctuations
of the fluorescence intensity, the intensity–intensity autocorrelation function can be
calculated and is plotted against logarithmic time lag. From Fig. 10.15 it is obvious
that the characteristic diffusion time increased with increasing proximity to the cen-
ter of the contact. Their physical meaning is to describe the time to diffuse through
the spot of calibrated diameter, ≈ 0.5 µm, at which the interrogatory laser beam was
