Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

10 Interfacial Forces and Spectroscopic Study of Confined Fluids 525
much exceeds shear rate in those laboratory experiments that the direct connec-
tion to experiment is not evident. To quantify the influence of surface roughness,
Fig. 10.4 considers the limit up to which predictions based on the classical no-slip
boundary condition still described the data in Fig. 10.3. Since the no-slip boundary
condition still held, it was valid [7–9] to calculate the shear rate and shear stress by
known equations.
The data show that deviations from the no-slip prediction began at very low
levels of hydrodynamic stress – on the order of only 1–10Pa. Beyond this point, in
some sense the moving fluid was depinned from the surface.
Slip need not necessarily be predicated on having surfaces coated with self-
assembled monolayers to render them partially wetted, though this was the case in
most of the studies cited so far. The no-slip boundary condition switches to partial
slip when the fluid contains a small amount of adsorbing surfactant [30,32].
10.2.4 Slip Can Be Modulated by Dissolved Gas
When experimental observations deviate from expectations based on the stick
boundary condition, there are at least two alternative scenarios with microscopic
interpretations. One might argue that the fluid viscosity depends on distance from
the wall, but for Newtonian fluids this would not be realistic. Why then do experi-
mental data appear to undergo shear thinning with increasing values of the param-
eter υ
peak
/D, if it is unreasonable to suppose that the viscosity really diminished?
Inspection shows that the data for smooth surfaces at high flow rates are consistent
with a two-layer-fluidmodel in whicha layer < 1nm thick,but with viscosity 10–20
times less than the bulk fluid, adjoins each solid surface [9]. A possible mechanism
to explain its genesis was proposed by de Gennes [46], who conjectured that shear
may induce nucleation of vapor bubbles; once the nucleation barrier is exceeded
the bubbles grow to cover the surface, and flow of liquid is over this thin gas film
rather the solid surface itself. Indeed, it is likely that incomplete air removal from
the solid surfaces canprofoundlyinfluencefindings in thesesituationswhere surface
roughness is so low. This has been identified by recent research as a likely source
of the misnamed long-range hydrophobic attraction [47,48]; gases also appear to
influence the sedimentation rate of small particles in liquid [49].
Accordingly, similar experiments were performed, in which the surface forces
apparatus was used to measure hydrodynamic forces of Newtonian fluids that had
been purged with various gases. Dissolved gas strongly influences hydrodynamic
forces, in spite of the fact that gas solubility is low.
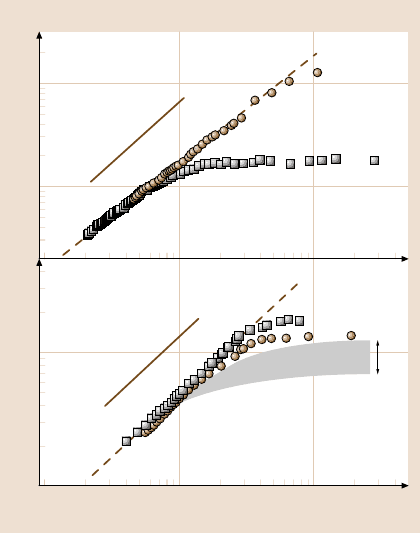
Figure 10.5 (top panel) illustrates experiments in which a simple nonpolar fluid
(tetradecane) was placed between a wetted mica surface on one side, and a partially
wetted methyl-terminated surface on the other, using methods described in detail
elsewhere [8,9]. The surface–surface spacing of 10–100nm substantially exceeded
the size of the fluid molecules. The spacings were vibrated with small amplitude
at these spacings where the fluid responded as a continuum, and the magnitude of
hydrodynamic force was measured as a function of the ratio υ
peak
/D suggested by
(10.2). The experiments showed that, whereas textbook behavior [10] was nearly
526 Y. Elaine Zhu et al.
1
Flow rate u
peak
/D (s
-1
)
F
H, peak
(mN)
10
0.1
101
10
Water
1
Ar
CO
2
100
Ar
CO
2
1
Tetradecane
Fig. 10.5. Illustration that the onset of slip depends on dissolved gas, when simple Newtonian
fluids flow past atomically smooth surfaces, either wetted or partially wetted. On log–log
scales, the hydrodynamic force F
H,peak
is plotted against reduced flow rate, ν
peak
/D,such
that a straight line of slope unity would indicate the no-slip condition assumed by (10.1). The
vibration frequency is 9 Hz. Top panel: tetradecane flowing between the asymmetric case
of a wetted mica surface on one side, a partially wetted surface of methyl-terminated self-
assembled monolayer on the other side, prepared as described elsewhere [33]. Filled symbols,
tetradecane saturated with carbon dioxide; open symbols, tetradecane saturated with argon.
Bottom panel: deionized water flowing between mica surfaces that are wetted by this fluid.
The hatched region of the graph shows the range of irreproducible results obtained when the
gas dissolved in the water was not controlled. Filled symbols, water saturated with carbon
dioxide; open symbols, water saturated with argon. After [7] with permission
followed when the tetradecane had been saturated with carbon dioxide gas, massive
deviations from this prediction were found when the tetradecane was saturated with
argon. This makes it seem likely that argon segregated to the solid walls, creating
a low-viscosity boundary layer, in this way greasing the flow of fluid past that sur-
face. Presumably, the amount of segregation is a material property of the fluid, the
chemical makeup of the surface, and the chemical identity of the dissolved gas. In
this example, the fact that argon possesses low solubility in tetradecane may have
made it more prone to segregate to the surfaces.
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 527
Indeed, when a solid wall is hydrophobic and immersed in water, the ideas of
Chandler andcoworkers[50] suggestthat thermodynamicsmay assist the formation
of a vaporphase near the wall. Recent forcemeasurementssupport this idea [51,52].
10.2.5 Slip Past Wetted Surfaces
The influence of dissolved gas (just discussed) casts doubt on a traditional assump-
tion of work in this field, which is that slip arises because fluid–fluid intermolecular
interactions are stronger than those between fluid and surface, i.e. that the surface
must be wetted only partially. Yet for severalyears, therehavebeen prominentcoun-
terexamples [28, 41]. Recent experiments show that dissolved gases can mediate
apparent slip even for solid surfaces that are fully wetted by the flowing fluid.
Figure 10.5 (bottom panel) summarizes experiments in which deionized water
was placed between wetted surfaces of mica and the surface–surface spacing of 10–
100 nm was vibrated with small amplitude in the manner described previously [7–9,
31–34]. Hydrodynamic force is plotted against the ratio, ν
peak
/D. It is obvious that
the prediction based on (10.2), a straight line on the log–log plot with a slope of
unity, was violated systematically when the hydrodynamic force reached a critical
level. An intriguing point is that initial findings were found to be irreproducible
(they varied within the range marked by the hatched lines in the graph) but became
reproducible when the water was first deliberately saturated with gas. One observes
that water saturated with argon appeared to slip at a slightly higher level of shear
stress than water saturated with carbon dioxide, and that in both cases the limiting
hydrodynamic force was larger than when the nature of the dissolved gas was not
controlled.
This rich and complex sensitivity to the detailed materials chemistry of the sys-
tem disappears, unfortunately, when surfaces are so rough that the stick boundary
condition is produced trivially by the influence of surface roughness [7,8,22–24].
Therefore for scientific and practical reasons alike, these issues of flow past nearly
smooth surfaces comprise fertile ground for future work.
10.2.6 The Purposeful Generation of Slip
Inspired by these ideas to design new engineering structures, one might strive to
“grease” the flow of liquids past solid surfaces by altering the boundary condition.
One strategy is to make the surfaces ultra-smooth [7–9]. Another (also mentioned
already) is to add processing aids that segregate to the surface [30,32,36]. A third
way is to purposefully use multicomponent fluids to generate concentration gradi-
ents and differential wetting to generate slip, as can occur even if there is no velocity
gradient in the fluid [38]. These methods could potentially be used in nanomotors
or nanopumps.
Alternatively, one may seek to maximize contact with air, which is exceedingly
solvophobic. Readers will have noticed that water ubiquitously beads up on the
leaves of plants. Some plants can display a contact angle that approaches 180
◦
,even
528 Y. Elaine Zhu et al.
though water at a smooth surface of the same chemical makeup displays a much
lower contact angle. A beautiful recent series of experiments from the Kao Corpora-
tion in Japan providedinsight into why [53] – the surfaces are rough on many length
scales [54,55] and trap air beneath them. Readers will have noticed that, if one tilts
a leaf, a drop of water on it rolls smoothly, because it rides mainly on a cushion of
air, whose effect will be further discussed in the next section. It is the principle of
an ingenious method introduced recently to lower the viscous drag when fluids [56]
are caused to flow through pipes whose diameter is macroscopic. Of course, given
a long enough period of time it is likely that the trapped gas would dissolve into the
flowing fluid, but perhaps this effect could be enhanced by placing air nozzles along
the walls of the tube and replenishing the trapped gas with a stream of inlet air.
A final method by which flow of a Newtonian fluid past surfaces may be facil-
itated is to ciliate the surfaces by coating with chain molecules – polymers, pro-
teins, or sugars. Recent experiments using a surface forces apparatus (SFA) suggest
a similar (but less dramatic) rate-dependent slip in this case also [31]. This is pos-
sibly related to fluid flow in biological organs whose surfaces are also extensively
ciliated, such as blood vessels and the kidney [57].
10.2.7 Outlook
The textbook presentation of engineering fluid mechanics is often of a subject thor-
oughly understood, but recent experiments and simulations using smooth surfaces
show behavior that is richer and more complex than had been supposed. The cor-
rect boundary condition appears to depend on physical chemical properties of the
solid–liquid interface that are susceptible to rational control.
10.3 Hydrophobic Interaction and Water
at a Hydrophobicity Interface
The role of water in physical situations from biology to geology is almost univer-
sally thought to be important but the details are disputed [1,48,50–52,58–72]. For
example, as concerns proteins, the side-chains of roughly half the amino acids are
polar while the other half are hydrophobic; the non-mixing of the two is a major
mechanism steering the folding of proteins and other self-assembly processes. As
a second example, it is an everyday occurrence to observe beading-up of raindrops,
on raincoats or leaves of plants. Moreover, it is observed theoretically and experi-
mentally that, when the gap between two hydrophobic surfaces becomes critically
small, water is ejected spontaneously [50,51,70–72] whereas water films confined
between symmetric hydrophilic surfaces are stable [1]. Despite its importance, wa-
ter exhibits many anomalous behaviors when compared to other fluids. Particularly,
it presents some even more puzzling behaviors near hydrophobic surfaces. This
section is adapted from discussions in several primary accounts published previ-
ously [51,52].
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 529
In its liquid form, water consists of an ever-changing three-dimensional net-
work of hydrogen bonds. Hydrophobic surfaces cannot form hydrogen bonds, and
the hydrogen-bonding network must be disrupted. So what happens when water is
compelled to be close to a hydrophobic surface? Energetically, it is expected that
the system forms as many hydrogen bonds as possible, resulting in a preferential
ordering of the water. Entropically, it is expected that the system orients randomly
and thus samples the maximum number of states. Which of these two competing
interactions dominates? What effect does the competition have on the dynamic and
equilibrium properties of the system? The answers to these questions are still hotly
debated. To help resolve this debate, static and dynamic interaction of water con-
fined to a hydrophobic surface is studied by SFA.
10.3.1 Experimental
The atomically smooth clay surfaces used in this study, muscovite mica (hy-
drophilic) and muscovite mica blanketed with a methyl-terminated organic self-
assembled monolayer (hydrophobic), allowed the surface separation to be meas-
ured, by multiple beam interferometry, with a resolution of ±2−5 Å. Surfaces were
brought to the spacings described below using a surface forces apparatus modified
for dynamic oscillatory shear [44,73]. A droplet of water was placed between the
two surfaces oriented in a crossed cylinder geometry. Piezoelectric bimorphs were
used to produce and detect controlled shear motions. The deionized water was pre-
viously passed through a purification system, Barnstead Nanopure II (control ex-
periments with water containing dissolved salt were similar). In experiments using
degassed water, the water was either first boiled, then cooled in a sealed container,
or vacuumed for 5–10h in an oven at room temperature. The temperature of meas-
urements was 25
◦
C.
In order to determine firmly that findings did not depend on details of surface
preparation,three methods were used to render one surface hydrophobic.In order of
increasing complexity, these were: (a) atomically smooth mica coated with a self-
assembled monolayer of condensed octadecyltriethoxysilane (OTE), using meth-
ods described previously [44]; (b) mica coated using Langmuir–Blodgett methods
with a monolayer of condensed octadecyltriethoxysilane; and (c) a thin film of sil-
ver sputtered onto atomically smooth mica, then coated with a self-assembled thiol
monolayer. In method (a), the monolayer quality was improved by distilling the
OTE before self-assembly. In method (b), OTE was spread onto aqueous HCl (pH
= 2.5), 0.5 h was allowed for hydrolysis, the film was slowly compressed to the sur-
face pressure π = 20mN/m (3–4 h), and the close-packed film was transferred onto
mica by the Langmuir–Blodgetttechniqueat a creep-up speed of2 mm/min. Finally
the transferred films were vacuum baked at 120
◦
C for 2h. In method (c), 650Å of
silver were sputtered at 120V (1 Å/s) onto mica that was held at room temperature,
and then octadecanethiol was deposited from 0.5 mM ethanol solution. In this case,
AFM (atomic force microscopy; Nanoscope II) showed the rms (root mean square)
roughness to be 0.5nm. All three methods led to the same conclusions, summarized
below.
530 Y. Elaine Zhu et al.
10.3.2 Hydrophobic Interaction
A puzzling aspect of the hydrophobic attraction is that its intensity and range ap-
pear to be qualitatively different as concerns extended surfaces of large area, and
small molecules of modest size [50,60,67,74]. One difference is fundamental: the
hydrogen-bond network of water is believed for theoretical reasons to be less dis-
rupted near a single alkane molecule than near an extended surface [50,60,67,74].
A second difference is phenomenological: direct measurement shows attractive
forces between extended surfaces starting at separations too large to be reason-
ably explained by disruption of the hydrogen-bondnetwork. This conclusion comes
from 20years of research using the surface forces apparatus (SFA) and, more re-
cently, atomic force microscopy (AFM). The onset of attraction, ≈10nm in the first
experiments [69,75–78], soon increased by nearly an order of magnitude [79–81]
and has been reported, in the most recent work, to begin at separations as large
as 500nm [71]. This has engendered much speculation because it is unreasonably
large compared to the size of the water molecule (≈ 0.25 nm). The range of interac-
tion decreases if the system (water and the hydrophobic surfaces) are carefully de-
gassed [48,82–86]. Water in usual laboratoryexperimentsis not degassed, however,
so it is relevantto understandthe origin of long-rangeattraction in that environment.
A recent review summarizes the experimental and theoretical situation [81].
In the course of experiments intended to probe the predicted slip of water over
hydrophobicsurfaces [9,45] (seethe previoussection), weakeningof the long-range
hydrophobic force to the point of vanishing was observed when the solid surfaces
experienced low-level vibrations around a mean static separation.
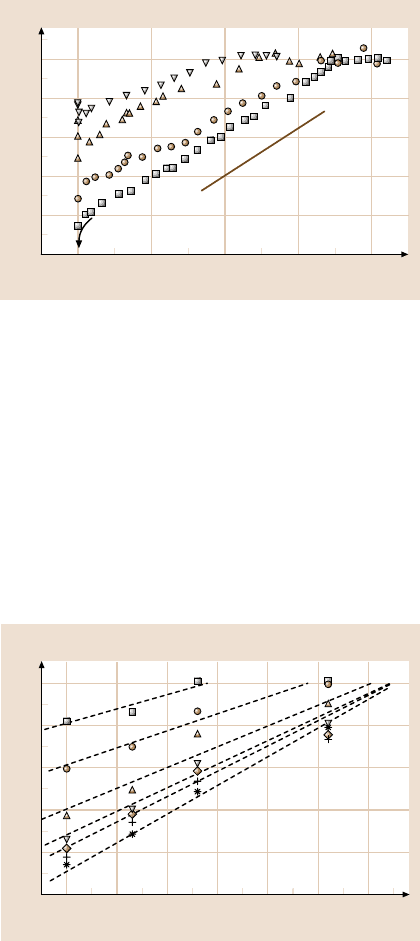
The attraction recorded during the approach of OTE surfaces with a droplet of
deionized water in between is plotted in Fig. 10.6 as a function of surface–surface
separation (D). D = 0 here refers to a monolayer–monolayer contact in air. In wa-
ter, the surfaces jumped into adhesive contact at 0 ±2Å. This jump in was very
slow to develop, however. The pull-off force to separate the surfaces from contact
at rest (113mN/m in Fig. 10.6) implies, from the Johnson–Kendall–Roberts (JKR)
theory [76], the surface energy of about 12mJ/m
2
(and up to about 30% less than
this when oscillations were applied). The onset of attraction at 650nm for the hy-
drophobic surfaces at rest is somewhat larger than in any past study of which we are
aware. However, we emphasize that the level of pull-off force was consistent with
the prior findings of other groups using other systems [1,60–72].
These observations clearly imply some kind of rate-dependent process. As
shown in Fig. 10.7, the force F diminished with increasing velocity and its mag-
nitude at a given D appeared in every instance to extrapolate smoothly to zero. The
possible role of hydrodynamic forces was considered but discarded as a possible
explanation. Similar results (not shown) were also obtained when the surfaces were
vibratedparallel to oneanotherrather thanin the normaldirection.Some precedence
is found in a recent AFM study that reported weakened hydrophobic adhesion force
with increasing approach rate [85].
These observations remove some of the discrepancy between the range of hy-
drophobic forces between extended surfaces of macroscopic size [1,60–72] and the
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 531
25
Distance D (nm)
F/R (mN/m)
0
20
15
10
5
0
200 400 600 800
~K
sp
/R
Fig. 10.6. Force–distance profiles of deionized water between hydrophobic surfaces (OTE
monolayers on mica). Force F, normalized by the mean radius of curvature (R ≈ 2cm)of
the crossed cylinders, is plotted against surface separation. Forces were measured during
approach from static deflection of the force-measuring spring, while simultaneously applying
small-amplitude harmonic oscillations in the normal direction with peak velocity v
peak
=
d ×2π f where d denotes displacement amplitude and f denotes frequency. This velocity
was zero (solid squares), 7.6nm/s(d = 1.6nm, f = 0.76 Hz; circles), 26 nm/s(d = 3.2nm,
f = 1.3Hz;up triangles), 52 nm/s(d = 3.2nm, f = 2.6Hz;down triangles). The pull-off
adhesion forces (“jump out”), measured at rest and with oscillation, are indicated by the
respective semi-filled symbols. The approach data follow the straight line with slope K
sp
/R
(drawn separately as a guide to the eye), indicating that they represented a spring instability
(“jump in”) such that the gradient of the attractive force exceeded the spring constant (K
sp
),
930 N/m. After [51] with permission
25
v
peak
(nm/s)
F/R (mN/m)
25
0
20
15
10
5
0123456
Fig. 10.7. The attractive
force (F/R)atsevendiffer-
ent surface separations (D)
is plotted against peak ve-
locity. The film thickness
was D = 720 nm (squares),
540 nm (circles), 228 nm (up
triangles), 116 nm (down tri-
angles), 63 nm (diamonds),
17 nm (crosses), 5 nm (stars).
After [51] with permission
532 Y. Elaine Zhu et al.
range that is expected theoretically [50,60,67,74]. A tentative explanation is based
on the frequent suggestion that the long-range hydrophobic attraction between ex-
tended surfaces stems from the action of microscopic or submicron-sized bubbles
that arise either from spontaneous cavitation or the presence of adventitious air
droplets that form bridges between the opposed surfaces [48, 81,83–86]. The ex-
periments reported here show that this effect required time to develop. Hydrophobic
attraction at long range was softened to the point of vanishing when the solid sur-
faces were not stationary.
10.3.3 Hydrophobicity at a Janus Interface
Due to the strong propensity to repel water completely out of two hydrophobicsur-
faces, it is then interesting to consider the antisymmetric situation, a hydrophilic
surface on one side (A) and a hydrophobic surface (B) on the other. The surface A
prevents water from being expelled, to successfully retain a stabilized aqueous thin
film at intimately close contact. The surface B introduces the hydrophobic effect.
This Janus situation is shown in the cartoon of Fig. 10.8.
Similar to the force between two OTE surfaces, long-range attraction was also
observed at the Janus interface, as shown in the inset of Fig. 10.8. The opposed
surfaces ultimately sprang into contact from D ≈ 5nm and upon pulling the sur-
faces apart, an attractive minimum was observed at D = 5.4nm. The surfaces could
be squeezed to a lower thickness, D ≈ 2.0nm. Knowing that the linear dimension
of a water molecule is ≈ 0.25nm [1], the thickness of the resulting aqueous films
amounted to the order of 5–20 water molecules, although (see below) it is not clear
that molecules were distributed evenly across this space. Below we discuss shear
results.
In the shear measurements, the sinusoidal shear deformations were gentle – the
significance of the resulting linear response being that the act of measurement did
not perturb their equilibrium structure (linear responses were verified from the ab-
sence of harmonics in the time dependence of shear motion). Using techniques
that are well known in rheology, from the phase lag and amplitude during oscil-
latory excitation the responses to shear excitation were decomposed into one in-
phase component (the elastic force, f
) and one out-of-phase component (the vis-
cous force, f
) [87]. Figure 10.8 (main portion) illustrates responses at a single
frequencyand variable thickness. The shear forces stiffened by more than one order
of magnitude as the films were squeezed. It is noteworthy that, when molecularly
thin aqueous films are confined between clay surfaces that are symmetrically hy-
drophilic, deviations from the response of bulk water appear only at smaller separa-
tions [88]; evidently the physical origin is different here. Moreover, at each separa-
tion the elastic and viscous forces were nearly identical. The equality of elastic and
viscous forces proved to be general, not an accident of the shear frequency chosen.
Again this contrasts with recent studies of molecularly thin water films between sur-
faces that are symmetrically hydrophilic [88]. The magnitudes of the shear moduli
in Fig.10.8are “soft”– somethinglikethoseof agaror jelly. Theywere considerably
softer than for molecularly thin aqueous films between symmetrically hydrophilic
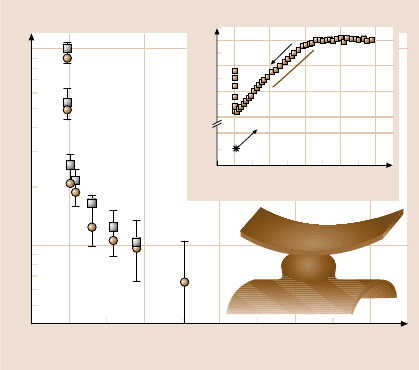
10 Interfacial Forces and Spectroscopic Study of Confined Fluids 533
D (nm)
f', f'' (mN)
1
10
2
3 4 5 6
0 200 400 600 800
D (nm)
Inward
K
sp
/R
Jump out
30
28
0
15
10
5
F/R (mN/m)
Hydrophilic
Hydrophobic
Fig. 10.8. Deionized water confined between a hydrophilic surface on one side and a hy-
drophobic surface on the other (cartoon). The cartoon is not to scale because the gap thick-
ness is nanometers at closest approach and the droplet size (≈2 mm on a side) vastly exceeds
the contact zone (≈ 10 µm on a side). The main figure shows the time-averaged viscous (cir-
cles) and elastic (squares) shear forces measured at 1.3Hzand0.3-nm deflection, plotted
semilogarithmically against surface separation for deionized water confined between OTE
deposited onto mica using the Langmuir–Blodgett (LB) technique (shear impulses were ap-
plied to this hydrophobic surface). The inset shows the static force–distance relations. Force,
normalized by the mean radius of curvature (R ≈ 2 cm) of the crossed cylinders, is plotted
against the thickness of the water film (D = 0 refers to contact in air). The pull-off adhesion
at D ≈ 5.4 nm is indicated by a star.Thestraight line with slope K
sp
/R indicates the on-
set of a spring instability where the gradient of attractive force exceeds the spring constant
(K
sp
), 930 N/m. Following this jump into contact, films of stable thickness resulted, whose
thickness could be varied in the range D = 1−4 nm with application of compressive force.
After [52] with permission
surfaces. This again emphasizes the different physical origin of shear forces in the
present Janus situation.
Figure 10.9 illustrates the unusual result that the shear forces scaled in mag-
nitude with the same power law, the square root of excitation frequency. This be-
havior, which is intermediate between solid and liquid, is often associated in other
systems with dynamical heterogeneity [89,90]. By known arguments it indicates
a broad distribution of relaxation times rather than any single dominant one [87].
The slope of 1/2 is required mathematically by the Kramers–Kronig relations if
G
(ω) = G
(ω) [87]; its observation lends credibility to the measurements. Fig-
ure 10.9 (main panel) illustrates this scaling for an experiment in which data were
averaged over a long time. The inset shows that the same was observed using other
methods to prepare a hydrophobic surface. In all of these instances, ω
1/2
scaling
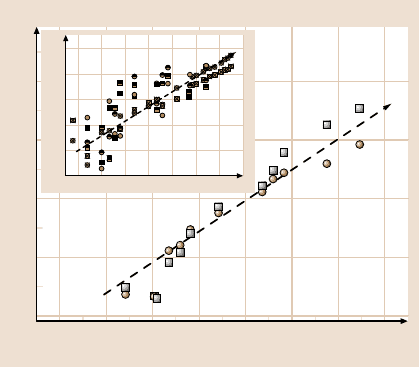
534 Y. Elaine Zhu et al.
3.5
0
6
4
4.5
5
5.5
3.50.5 1 1.5 2 2.5 3
4
log w (rad/s)
log G', G'' (Pa)
6
4.5
5
5.5
0.5 0 0.5 1 1.5 2 2.5 3
Fig. 10.9. The frequency dependence of the momentum transfer between the moving sur-
face (hydrophobic) and the aqueous film with an adjoining hydrophilic surface is plotted on
log–log scales. Time-averaged quantities are plotted. On the right-hand ordinate scale are the
viscous, g
(circles), and elastic, g
(squares) spring constants. The equivalent loss moduli,
G
eff
(circles), and elastic moduli, G
eff
(squares), are on the left-hand ordinate. All measure-
ments were made just after the jump into contact shown in Fig. 10.6, i.e. at nearly the same
compressive pressure ≈3 MPa. The main panel, representing LB-deposited OTE, shows ω
1/2
scaling after long-time averaging, 0.5–1 h per datum. The inset shows comparisons with us-
ing other methods to produce a hydrophobic surface. In those experiments the thickness was
generally D = 1.5−1.6 nm but occasionally as large as 2.5 nm when dealing with octade-
canethiol monolayers. Symbols in the inset show data averaged over only 5–10 min with
hydrophobic surfaces prepared with (a) a self-assembled monolayer of OTE (half-filled sym-
bols), (b) Langmuir–Blodgett deposition of OTE (crossed symbols), or (c) deposition of oc-
tadecanethiol on Ag (open symbols). As in the main figure, circles denote viscous forces,
squares denote elastic forces. Scatter in this data reflects shorter averaging times than in the
main part of this figure (Fig. 10.8). After [52] with permission
was observed regardless of the method of renderingthe surface hydrophobic.But to
observe clean ω
1/2
scaling required extensive time averaging – see later.
In repeated measurements at the same frequency, we observed giant fluctuations
(±30−40%) arounda definite mean, as illustrated in Fig. 10.10, although water con-
fined between symmetric hydrophilic–hydrophilic surfaces (bottom panel) did not
display this. It is extraordinary that fluctuations did not average out over the large
contact area (≈ 10µm on a side) that far exceeded any molecular size. The struc-
tural implication is that the confined water film comprised some kind of fluctuating
complex – seeking momentarily to dewet the hydrophobic side by a thermal fluctu-
ation in one direction, but unable to because of the nearby hydrophilicside; seeking
next to dewet the hydrophobic side by a thwarted fluctuation in another direction;
and so on. Apparently, nearby hydrophobic and hydrophilic surfaces may produce
