Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

394 Bharat Bhushan
Fig. 8.65 [42]. The results measured by these two methods are in good agreement.
Figure 8.65 shows that the presence of the mobile Z-15 lubricant film increases the
adhesive force compared to that of Si(100) by meniscus formation. Whereas, the
presence of solid-phase Z-DOL (BW) film reduces the adhesive force compared to
that of Si(100) because of the absence of mobile liquid. The schematic (bottom)
in Fig. 8.65 shows the relative size and sources of meniscus. It is well known that
the native oxide layer (SiO
2
) on top of a Si(100) wafer exhibits hydrophilic proper-
ties, and some water molecules can be adsorbed onto this surface. The condensed
water will form meniscus as the tip approaches the sample surface. The larger ad-
hesive force in Z-15 is not only caused by the Z-15 meniscus, the nonpolarized
Z-15 liquid does not have good wettability and strong bonding with Si(100). Con-
sequently, in the ambient environment, the condensed water molecules from the
environment will permeate through the liquid Z-15 lubricant film and compete with
the lubricant molecules present on the substrate. The interaction of the liquid lu-
bricant with the substrate is weakened, and a boundary layer of the liquid lubricant
forms puddles [39,40]. This dewetting allows water molecules to be adsorbed onto
the Si(100) surface as aggregates along with Z-15 molecules. And both of them can
form a meniscus while the tip approaches to the surface. Thus the dewetting of liq-
uid Z-15 film results in higher adhesive force and poorer lubrication performance.
In addition, as the Z-15 film is quite soft compared to the solid Si(100) surface, and
penetration of the tip in the film occurs while pushing the tip down. This leads to
the large area of the tip involved to form the meniscus at the tip–liquid (mixture of
Z-15 and water) interface. It should also be noted that Z-15 has a higher viscosity
than water, therefore Z-15 film provides higher resistance to motion and coefficient
of friction. In the case of Z-DOL (BW) film, both of the active groups of Z-DOL
molecules are mostly bonded on Si(100) substrate, thus the Z-DOL (BW) film has
low free surface energy and cannot be displaced readily by water molecules or read-
ily adsorb water molecules. Thus, the use of Z-DOL (BW) can reduce the adhesive
force.
To study the velocity effect on friction and adhesion, the variation of friction
force, adhesive force, and coefficient of friction of Si(100), Z-15 and Z-DOL(BW)
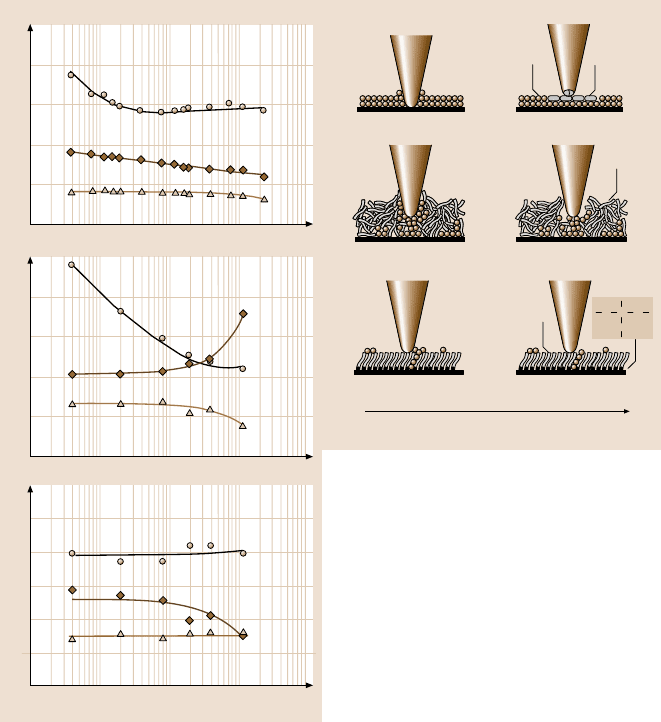
as a function of velocity are summarized in Fig. 8.66 [42]. It indicates that, for
silicon wafers, the friction force decreases logarithmically with increasing velocity.
For Z-15, the friction force decreases with increasing velocity up to 10µm/s, after
which it remains almost constant. The velocity has a very small effect on the friction
force of Z-DOL (BW); it reduced slightly only at very high velocity. Figure 8.66
also indicates that the adhesive force of Si(100) is increased when the velocity is
higher than 10µm/s. The adhesive force of Z-15 is reduced dramatically when the
velocity increases to 20µm/s, after which it is reduced slightly. The adhesive force
of Z-DOL (BW) also decreases at high velocity. In the tested velocity range, only
the coefficient of friction of Si(100) decreases with velocity, while the coefficients
of friction of Z-15 and Z-DOL (BW) almost remain constant. This implies that the
friction mechanisms of Z-15 and Z-DOL (BW) do not change with the variation of
velocity.
8 Nanotribology, Nanomechanics and Materials Characterization 395
25
20
15
10
5
0
125
100
75
50
25
0
0.15
0.10
0.05
0
1 1000100100.1
70 nN, 22°C, RH 45–55%
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Velocity (μm/s)
From friction force plot
Adhesive force (nN)
Si(100)
Z-DOL (BW)
Z-15
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Z-DOL (BW)
Si(100)
H
2
O
Increasing velocity
0.4
μm/s
240 μm/s
Si(OH)
4
Z-15
Z-15
Z-DOL
O
Si
O
O
Fig. 8.66. The influence of velocity on the friction force, adhesive force and coefficient of
friction of Si(100) and Z-15 and Z-DOL (BW) films at 70 nN, in ambient air. The schematic
(right) shows the change of surface composition (by tribochemical reaction) and formation
of meniscus while increasing the velocity [42]
The mechanisms of the effect of velocity on the adhesion and friction are ex-
plained based on the schematics shown in Fig. 8.66 (right) [42]. For Si(100), tri-
bochemical reactions play a major role. Although, at high velocity, the meniscus is
broken and does not have enough time to rebuild, the contact stresses and high ve-
locity lead to tribochemical reactions of the Si(100) wafer (which has native oxide
(SiO
2
)), and Si
3
N
4
tip with water molecules, forming Si(OH)
4
. The Si(OH)
4
is re-
movedand continuouslyreplenished during sliding. The Si(OH)
4
layer between the
tip and Si(100) surface is known to be of low shear strength and causes a decrease
396 Bharat Bhushan
in friction force and coefficient of friction [10,17]. The chemical bonds of Si−OH
between the tip and the Si(100) surface induce large adhesive force. For Z-15 film,
at high velocity the meniscus formed by condensed water and Z-15 molecules is
broken and does not have enough time to rebuild; therefore, the adhesive force and
consequently friction force is reduced. The friction mechanisms for the Z-15 film
still is shearing the same viscous liquid even at high velocity range, thus the coeffi-
cient of friction of Z-15 does not change with velocity. For Z-DOL (BW) film, the
surface can adsorb few water molecules in ambient condition, and at high velocity
these molecules are displaced, which is responsible for a slight decrease in friction
force and adhesive force. Koinkar and Bhushan [40,40] have suggested that, in the
case of samples with mobile films, such as condensed water and Z-15 films, align-
ment of liquid molecules (shearthinning)is responsiblefor the drop in friction force
with increasing scanning velocity. This could be another reason for the decrease in
friction force for Si(100) and Z-15 film with velocity in this study.
To study the effect of relative humidity on friction and adhesion, the variation
of friction force, adhesive force, and coefficient of friction of Si(100), Z-15, and
Z-DOL (BW) as a function of relative humidity is shown in Fig. 8.67 [42]. It shows
that, for Si(100) and Z-15 film, the friction force increases with relative humidity up
to 45%, and then shows a slight decrease with further increases in the relative hu-
midity. Z-DOL (BW) has a smaller frictionforce than Si(100) andZ-15 in the whole
testing range and its friction force shows an apparent relative ncrease when the rel-
ative humidity is higher than 45%. For Si(100), Z-15 and Z-DOL (BW), their adhe-
sive forces increase with relative humidity, and their coefficients of friction increase
with relative humidity up to 45%, after which they decrease with further increases
of relative humidity. It is also observed that the effect of humidity on Si(100) de-
pends on the history of the Si(100) sample. As the surface of Si(100) wafer readily
adsorb water in air, without any pretreatment the Si(100) used in our study almost
reaches to its saturate stage of adsorbedwater, and is responsible for a smaller effect
during increasing relative humidity. However,once the Si(100) wafer was thermally
treated by baking at 150
◦
C for 1hour, a bigger effect was observed.
The schematic (left) in Fig. 8.67 shows that Si(100), because of its high free
surface energy, can adsorb more water molecules while increasing relative humid-
ity [42]. As discussed earlier, for the Z-15 film in the humid environment, the con-
densed water from the humid environmentcompetes with the lubricant film present
on the sample surface, and the interaction of the liquid lubricant film with the sili-
con substrate is weakened and a boundary layer of the liquid lubricant forms pud-
dles. This dewetting allows water molecules to be adsorbed onto the Si(100) sub-
strate mixed with Z-15 molecules [39, 40]. Obviously, more water molecules can
be adsorbed onto the Z-15 surface with increasing relative humidity. The higher
number of adsorbed water molecules in the case of Si(100), along with the lubri-
cant molecules in the Z-15 film case, form a larger water meniscus, which leads to
an increase of friction force, adhesive force, and coefficient of friction of Si(100)
and Z-15 with humidity. However, at the very high humidity of 70%, large quan-
tities of adsorbed water can form a continues water layer that separates the tip
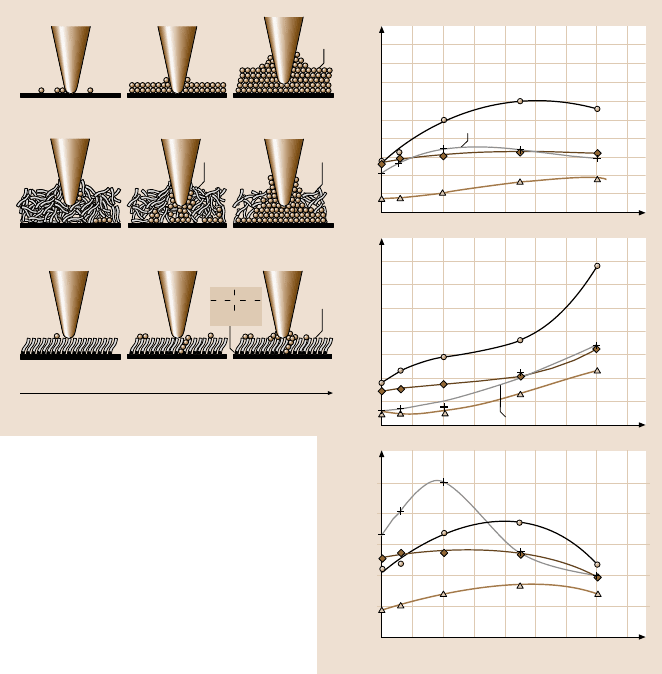
8 Nanotribology, Nanomechanics and Materials Characterization 397
25
20
15
10
5
0
200
175
150
125
100
75
50
25
0
0.15
0.10
0.05
0
20 8060400
70 nN, 2 μm/s, 22°C
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Relative humidity (%)
From friction force plot
Adhesive force (nN)
Si(100)
Z-DOL (BW)
Z-15
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Z-15
Z-DOL (BW)
O
Si
O
O
Si(100)
Increasing relative humidity
0%
Thermally treated Si(100)
Thermally treated Si(100)
Thermally treated Si(100)
Z-DOL
70 %
Z-15
Z-15
H
2
O
Fig. 8.67. The influence of relative humidity on the friction force, adhesive force, and coeffi-
cient of friction of Si(100) and Z-15 and Z-DOL (BW) films at 70 nN, 2 µm/s, and in 22
◦
C
air. The schematic (left) shows the change of meniscus while increasing the relative humid-
ity. In this figure, the thermally treated Si(100) represents the Si(100) wafer that was baked at
150
◦
C for 1hour in an oven (in order to remove the adsorbed water) just before it was placed
in the 0% RH chamber [42]
and sample surface and acts as a kind of lubricant, which causes a decrease in
the friction force and coefficient of friction. For the Z-DOL (BW) film, because
of its hydrophobic surface properties, water molecules can be adsorbed at humid-
ity higher than 45%, and this causes an increase in the adhesive force and friction
force.
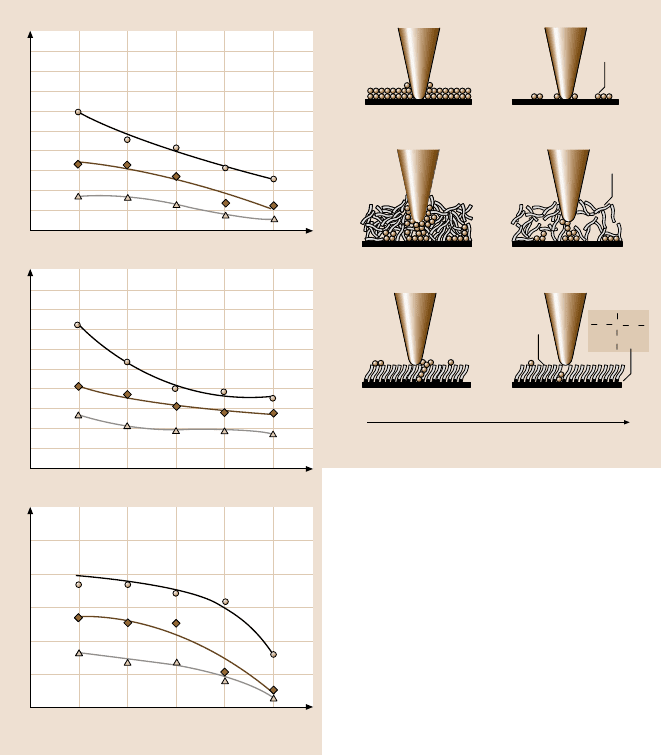
To study the effect of temperature on friction and adhesion,the variation of fric-
tion force, adhesive force, and coefficient of friction of Si(100), Z-15 and Z-DOL
(BW) as a function of temperature are summarized in Fig. 8.68 [42]. It shows that
increasing temperature causes a decrease of the friction force, adhesive force and
398 Bharat Bhushan
25
20
15
10
5
0
125
100
75
50
25
0
0.15
0.10
0.05
0
150100500
70 nN, 2 μm /s, RH 45–55%
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Temperature (°C)
From friction force plot
Adhesive force (nN)
Si(100)
Z-DOL (BW)
Z-15
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Si(100)
Increasing temperature
25 °C
125 °C
H
2
O
Z-15
Z-15
Z-DOL (BW)
Z-DOL
O
Si
O
O
Fig. 8.68. The influence of temperature on the friction force, adhesive force, and coefficient
of friction of Si(100) and Z-15 and Z-DOL (BW) films at 70 nN, at 2µm/s,andinRH40–
50% air. The schematic (right) shows that, at high temperature, desorption of water decreases
the adhesive forces. And the reduced viscosity of Z-15 leads to the decrease of coefficient of
friction. High temperature facilitates orientation of molecules in Z-DOL (BW) film, which
results in a lower coefficient of friction [42]
coefficient of friction of Si(100), Z-15 and Z-DOL (BW). The schematic (right) in
Fig. 8.68 indicates that, at high temperature, desorption of water leads to a decrease
of the friction force, adhesive forces and coefficient of friction for all of the samples.
For Z-15 film, the reduction of viscosity at high temperature also makes a contri-
bution to the decrease of friction force and coefficient of friction. In the case of the
8 Nanotribology, Nanomechanics and Materials Characterization 399
Z-DOL (BW) film, molecules are more easily oriented at high temperatures, which
may be partly responsible for the low friction force and coefficient of friction.
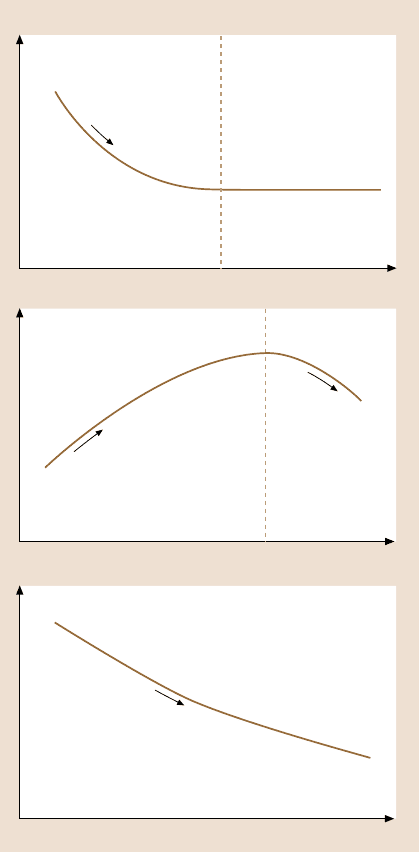
As a brief summary, the influence of velocity, relative humidity, and temperature
on the frictionforce of mobile Z-15 film is presented in Fig. 8.69 [42].The changing
trends are also addressed in this figure.
To study the durability of lubricant films at nanoscale, the friction of Si(100),
Z-15, and Z-DOL (BW) as a function of the number of scanning cycles are shown
Friction force (nN)
Log velocity
Lack of meniscus
reformation decreases
friction with
increasing velocity
Molecularly thick Z-15 film
Reaches to
equilibrium
Friction force (nN)
Relative humidity
Meniscus formation
increases friction with
increasing RH
Thick H
2
O
film serves
as a lubricant
Friction force (nN)
Temperature
Desorption of water and
decrease of viscosity decrease
friction with increase of
temperature
Fig. 8.69. Schematic shows
the change of friction force of
molecularly thick Z-15 films
with log velocity, relative
humidity, and temperature.
The changing trends are also
addressed in this figure [42]
400 Bharat Bhushan
0 50 75 10025 125
0.15
0.10
0.05
0
25
20
15
10
5
0
70 nN, 0.4 μm/s, 22°C, RH 45–55%
Friction force (nN)
Si(100)
Z-DOL (BW)
Z-15
Number of cycles
Coefficient of friction
Si(100)
Z-DOL (BW)
Z-15
Increasing scan number
Z-15
Z-15
H
2
O
Fig. 8.70. Friction force and coefficient of friction versus number of sliding cycles for Si(100)
and Z-15 and Z-DOL (BW) films at 70 nN, 0.8 µm/s, and in ambient air. The schematic
(bottom) shows that some liquid Z-15 molecules can be attached onto the tip. The molecular
interaction between the attached molecules onto the tip with the Z-15 molecules in the film
results in an increase of the friction force with multiple scanning [42]
8 Nanotribology, Nanomechanics and Materials Characterization 401
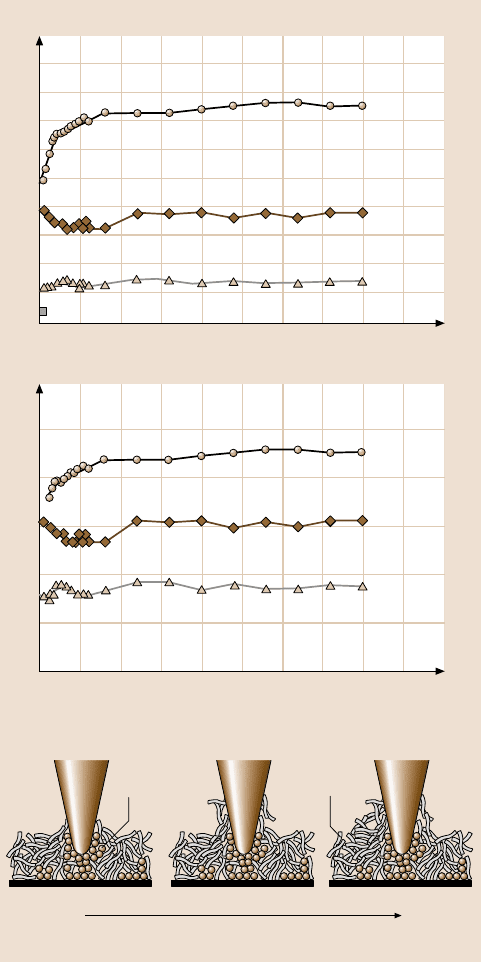
in Fig. 8.70 [42]. As observed earlier, the friction force and coefficient of friction
of Z-15 are higher than that of Si(100), with the lowest values for Z-DOL(BW).
During cycling, the friction force and coefficient of friction of Si(100) show a slight
decrease during the initial few cycles and then remain constant. This is related to
the removal of the top adsorbed layer. In the case of the Z-15 film, the friction force
and coefficient of friction show an increase during the initial few cycles and then ap-
proach higher,stable values. This is believedto be caused by the attachment of Z-15
molecules to the tip. The molecular interaction between these attached molecules on
the tip and molecules on the film surface is responsible for an increase in the fric-
tion. However, after several scans, this molecular interaction reaches an equilibrium
and the friction force and coefficient of friction then remain constant. In the case
of the Z-DOL (BW) film, the friction force and coefficient of friction start low and
remain low during the entire test for 100cycles. This suggests that Z-DOL (BW)
molecules do not become attached or displaced as readily as Z-15.
8.6.2 Self-Assembled Monolayers
For lubrication of MEMS/NEMS, another effective approach involves the deposi-
tion of organized and dense molecular layers of long-chain molecules. Two com-
mon methods to produce monolayers and thin films are the Langmuir–Blodgett
(LB) deposition and self-assembled monolayers (SAMs) by chemical grafting of
molecules. LB films are physically bonded to the substrate by weak van der Waals
attraction, while SAMs are chemically bonded via covalent bonds to the substrate.
Because of the choice of chain length and terminal linking group that SAMs of-
fer, they hold great promise for boundary lubrication of MEMS/NEMS. A num-
ber of studies have been conducted to study tribological properties of various
SAMs [38,41,43,129–135].
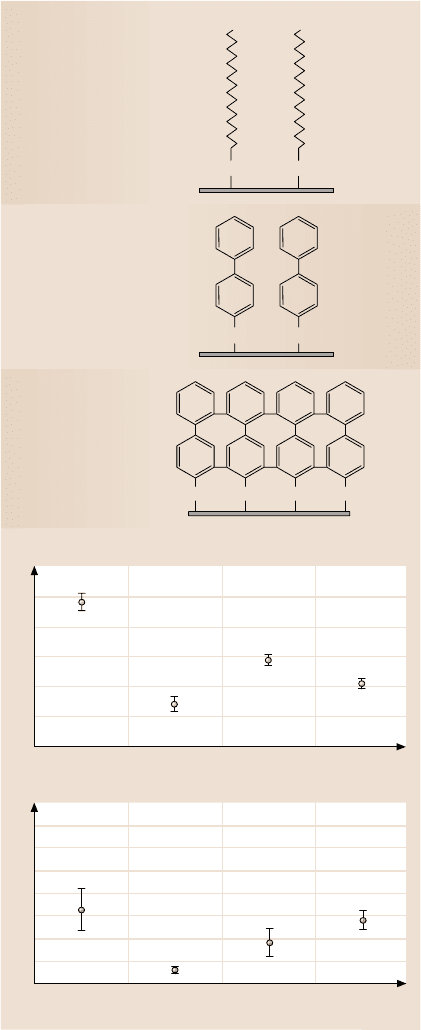
Bhushanand Liu [41]studied the effect of film complianceon adhesion and fric-
tion.Theyused hexadecanethiol(HDT), 1,1,biphenyl-4-thiol(BPT),and crosslinked
BPT (BPTC) solventdepositedon Au(111)substrate,Fig. 8.71a.The averagevalues
and standard duration of the adhesive force and coefficient of friction are presented
in Fig. 8.71b. Based on the data, the adhesive force and coefficient of frictions of
SAMs are less than those of corresponding substrates. Among various films, HDT
exhibits the lowest values. Based on stiffness measurements of various SAMs, HDT
was the most compliant,followed by BPT and BPTC. Based on friction and stiffness
measurements, SAMs with high-compliance long carbon chains exhibit low fric-
tion; chain compliance is desirable for low friction. Friction mechanism of SAMs
is explained by a so-called molecular spring model (Fig. 8.72). According to this
model, the chemically adsorbed self-assembled molecules on a substrate are just
like assembled molecular springs anchored to the substrate. An asperity sliding on
the surface of SAMs is like a tip sliding on the top of molecular springs or a brush.
The molecular spring assembly has compliant features and can experience orienta-
tion and compression under load. The orientation of the molecular springs or brush
under normal load reduces the shearing force at the interface, which in turn reduces
the friction force. The orientation is determined by the spring constant of a single
402 Bharat Bhushan
a)
b)
Adhesive force (nN)
60
40
20
0
Au
HDT BPT BPTC
Coefficient of friction
0.08
0.06
0.04
0.02
0
Materials
Au
HDT BPT BPTC
Hexadecane thiol
(HDT)
1,1'–biphenyl–4–thiol
(BPT)
Cross-linked 1,1'–
biphenyl–4–thiol
(BPTC)
Au(111)
SSSS
Biphenyl
–(C
6
H
6
)
2
–
Au(111)
S
S
CH
3
S
Alkyl
–(CH
2
)
n
–
Au(111)
CH
3
S
Fig. 8.71. (a) Schematics
of the structures of hex-
adecane thiol and biphenyl
thiol SAMs on Au(111) sub-
strates, and (b) adhesive force
and coefficient of friction of
Au(111) substrate and vari-
ous SAMs

8 Nanotribology, Nanomechanics and Materials Characterization 403
α
2
α
1
Substrate
Fig. 8.72. Molecular spring
model of SAMs. In this fig-
ure, α
1
<α
2
, which is caused
by the further orientation un-
der the normal load applied
by an asperity tip [41]
molecule as well as the interaction between the neighboring molecules, which can
be reflected by packing density or packing energy. It should be noted that the orien-
tation can lead to conformational defects along the molecular chains, which lead to
energy dissipation.
The SAMs with high-compliance long carbon chains also exhibit the best wear
resistance [41,130]. In wear experiments, the wear depth as a function of normal
loadcurves showa critical normalload. A representativecurveis shownin Fig. 8.73.
Below the critical normal load,SAMs undergoorientation;at the critical load SAMs
wear away from the substrate due to weak interface bond strengths, while above the
critical normal load severe wear takes place on the substrate.
Bhushan et al. [43], Kasai et al. [131], and Tambe and Bhushan [134] stud-
ied perfluorodecyltricholorosilane(PFTS), n-octyldimethyl (dimethylamino) silane
(ODMS) (n = 7), and n-octadecylmethyl(dimethylamino)silane (ODDMS) (n = 17)
vapor deposited on Si substrate, and octylphosphonate(OP) and octadecylphospho-
nate (ODP) on Al substrate, Fig. 8.74a. Figure 8.74b presents the contact angle,
adhesive force, friction force, and coefficient of friction of two substrates and with
Decrease of surface height (nm)
Normal load (μN)
0
7
7
5
3
1
–1
123456
Critical load
Fig. 8.73. Illustration of
the wear mechanism of
SAMs with increasing normal
load [130]
