Bhadeshia H.K.D.H., Honeycombe R. Steels: Microstructure and Properties
Подождите немного. Документ загружается.


PREFACE TO THE SECOND EDITION
This new edition retains the basic framework of the original book; however, the
opportunityhas beentakento introduceseveral additionalchapters dealing with
areas which have emerged or increased in significance since the book was first
published in 1981. There is now a separate chapter on acicular ferrite which has
become a desirable structure in some steels. The control of microstructures dur-
ing welding is undoubtedly a crucial topic which now requires a chapter, while
the modelling of microstructures to achieve optimum properties has emerged
as an important approach justifying the inclusion of a further new chapter. The
opportunity has also been taken to include a completely revised chapter on
bainite transformations.
The overall aim of the book remains to introduce students to the principles
determining the microstructures of steels, and through these, the mechanical
properties and behaviour in service. Steels remain the most important group
of metallic alloys, possessing a very wide range of microstructures and mechan-
ical properties, which will ensure their continued extensive use far into the
foreseeable future.
RWKH
HKDHB
Cambridge
1995
x

PREFACE TO THE THIRD EDITION
Steel has the ability to adapt to changing requirements. This comes from the
myriads of ways in which its structure can be influenced by processing and
alloying. This is why it is the standard against which emerging materials are
compared. Added to this is the commercial success, with output at record levels
and a production efficiency which is uncanny. It is pleasing to see how, all over
the world, iron and its alloys contribute to improving the quality of life of so
many human beings. The technology is so good that most of these people rightly
take it for granted.
This new edition captures developments since 1995, e.g., the extremely fine-
grained alloys, steels with the ability to abnormally elongate and the properties
of minute particles of iron. Questions are posed as to the theoretical limit to the
finest crystals that can be manufactured on a large scale. In addition, there are
major revisions in the explanations of microstructure, strengthening, kinetics
and modelling.
The original aim of this book, to introduce students and technologists to the
principles determining the microstructure and properties of iron and its alloys,
has remained the guiding principle in the new edition.
HKDHB
RWKH
Cambridge
2006
xi
Supporting material accompanying this book
A full set of accompanying exercises and worked solutions for this book are
available for teaching purposes.
Please visit http://www.textbooks.elsevier.com and follow the registration
instructions to access this material, which is intended for use by lecturers
and tutors.
The compilation of questions has been designed to stimulate the student to
explore the subject within the context of the book.
Each question is accompanied by a complete answer, with the exception of
the proposed set of topics for essays. Most of the questions and answers have
been developed as a consequence of many years of teaching and have been
tested on a variety of undergraduates.

1
IRON AND ITS INTERSTITIAL
SOLID SOLUTIONS
1.1 INTRODUCTION
Steel is frequently the ‘gold-standard’ against which emerging structural mater-
ials are compared. What is often not realized is that this is a moving standard,
with notoriously regular and exciting discoveries being made in the context of
ironand itsalloys.This iswhy steel remainsthemost successfuland cost-effective
of all materials, with more than a billion tonnes being consumed annually in
improving the quality of life. This book attempts to explain why steels continue
to take this pre-eminent position, and examines in detail the phenomena whose
exploitation enables the desired properties to be achieved.
One reason for the overwhelming dominance of steels is the endless variety
ofmicrostructures and propertiesthat canbe generatedby solid-statetransform-
ation and processing. Therefore, in studying steels, it is useful to consider the
behaviour of pure iron first, then the iron–carbon alloys, and finally the many
complexities that arise when further solutes are added.
Pure iron is not an easy material to produce. It has nevertheless been made
with a total impurity content less than 60 parts per million (ppm), of which
10 ppm is accounted for by non-metallic impurities such as carbon, oxygen,
sulphur and phosphorus, with the remainder representing metallic impurities.
Iron of this purity can be extremely weak when reasonably sized samples are
tested: the resolved shear stress of a single crystal at room temperature can be as
low as 10 MN m
−2
, while the yield stress of a polycrystalline sample at the same
temperature can be well below 50 MN m
−2
. However,the shear strength of small
single crystals has been observed to exceed 19,000 MN m
−2
when the size of the
sample is reduced to about 2 µm. This is because the chances of finding crystal
defects such as dislocations become small as the size of the crystal is reduced.
The theoretical shear strength of a perfect crystal of iron is estimated to be about
21,000 MN m
−2
, equivalent to a tensile strength of about 11,000 MN m
−2
.
1

2 CHAPTER 1 IRON AND ITS INTERSTITIAL SOLID SOLUTIONS
For comparison purposes the breaking strength of a very small carbon
nanotube has been measured to be about 130,000 MN m
−2
; this number is so
astonishing that it has led to exaggerated statements about their potential in
structural applications. For example, the tubes are said to be a hundred times
stronger than steel; in fact, there is no carbon tube which can match the strength
of iron beyond a scale of 2 mm, because of the inevitable defects which arise as
the tubes are grown.
The lesson from this is that systems which rely on perfection in order to
achieve strength necessarily fail on scaling to engineering dimensions. Since
perfection is thermodynamically impossible to achieve in large samples, steels
must in practice be made stronger by other means which are insensitive to
size. The mechanisms by which the strength can be increased will be discussed –
suffice it to state here that it is possible to commercially buy steel with a strength
of 5500 MN m
−2
, with sufficient ductility to ensure safe application. Some of
the methods by which such impressive combinations of properties are achieved
without compromising safetywill be discussed,before thewide range ofcomplex
structures which determine the properties is dealt with.
1.2 THE ALLOTROPES OF PURE IRON
At least three allotropes of iron occur naturally in bulk form, body-centred
cubic (bcc, α, ferrite), face-centred cubic (fcc, γ, austenite) and hexagonal close-
packed (hcp, ǫ). The phase β in the alphabetical sequence α, β, γ, δ...is missing
because the magnetic transition in ferrite was at one time incorrectly thought
to be the β allotrope of iron. In fact, there are magnetic transitions in all of the
allotropes of iron. The phase diagram for pure iron is illustrated in Fig. 1.1. Each
point on any boundary between the phase fields represents an equilibrium state
in which two phases can coexist. The triple point where the three boundaries
intersect represents an equilibrium between all three phases which coexist. It
is seen that in pure iron, the hcp form is stable only at very large pressures,
consistent with its high density. The best comparison of the relative densities of
the phases is made at the triple point where the allotropes are in equilibrium
and where the sum of all the volume changes is zero:
V(bcc → hcp) =−0.34
V(hcp → ccp) =+0.13
V(ccp → bcc) =+0.21
cm
3
mol
−1
There may exist a fourth natural allotrope in the core of the earth, where the
pressure reaches some three million times that at the surface and where the tem-
perature is estimated to be about 6000
◦
C.The core of the earth is predominantly
iron, and consists of a solid inner core surrounded by a liquid outer core. Know-
ledge of the core is uncertain, but it has been suggested that the crystal structure
of the solid core may be double hcp, although calculations which assume pure
iron,indicate that theǫ-iron remainsthe moststable underinner-core conditions.
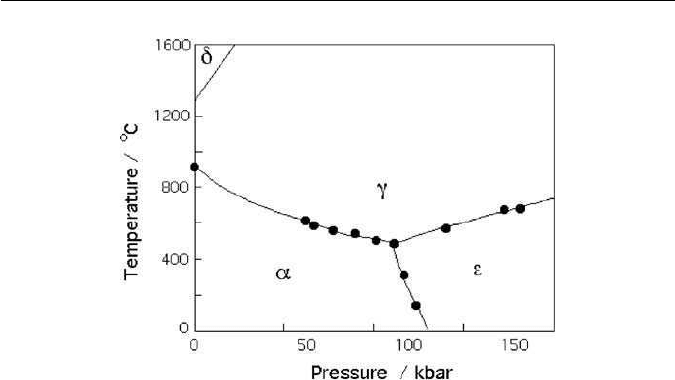
1.2 THE ALLOTROPES OF PURE IRON 3
Fig. 1.1 The phase diagram for pure iron (data from Bundy,1965).The triple point temperature
and pressure are 490
◦
C and 110 kbars, respectively. α, γ and ǫ refer to ferrite, austenite and
ǫ-iron, respectively. δ is simply the higher temperature designation of α.
1.2.1 Thin films and isolated particles
There are two further allotropes which can be created in the form of thin films.
Face-centred tetragonal iron has been prepared by coherently depositing iron
as a thin film on a {100} plane of a substrate such as copper with which the iron
has a mismatch. The position of atoms in the first deposited layer in this case
replicates that of the substrate. A few monolayers can be forced into coherency
in the plane of the substrate with a corresponding distortion normal to the
substrate. This gives the deposit a face-centred tetragonal structure. Growing
iron on a misfitting {111} surface of a fcc substrate leads to trigonal iron.
Very thin films of iron retain their ferromagnetic character, but there are
special effects due to the small dimensions. The magnetic moment per atom
becomes very large: 3.1 Bohr magnetons compared with 2.2 for bulk α-iron.
This is due to the smaller coordination number for atoms in a thin film. The
second effect is that magnetic anisotropy greatly increases for thin films because
the spins tend to align normal to the surface. The Curie temperature is greatly
reduced, again because of the change in coordination. For a monolayer of iron
the temperature is just ≃280
◦
C.
Many classical studies of nucleation theory have been conducted on minute
(5–1000 nm) particles of iron where defects responsible for heterogeneous
nucleation can be avoided. Such particles have acquired new significance in
that they are exploited in the manufacture of carbon nanotubes. The particles
are deposited due to the decomposition of ferrocene in chemical mixtures which
also contain the ingredients necessary to grow the tubes.
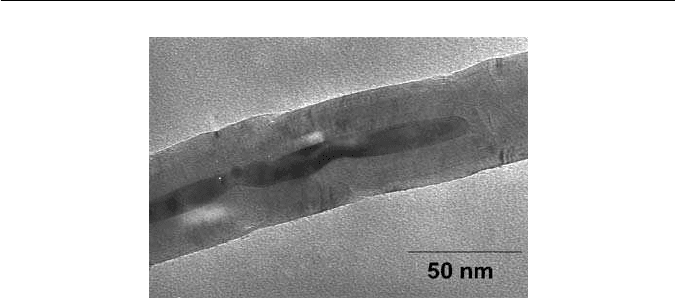
4 CHAPTER 1 IRON AND ITS INTERSTITIAL SOLID SOLUTIONS
Fig. 1.2 A multi-walled carbon nanotube containing a particle of iron (unpublished micrograph
courtesy of I. Kinloch).
It is expected that the coarser particles will have the bcc crystal structure
of ferrite, but it has to be appreciated that a 5 nm particle has about half its
atoms at the surface. Metal surfaces are prone to reconstruction into a variety
of two-dimensional structures which will complicate the interpretation of the
structure of the particle as a whole. The surface also plays another role, in that it
alters the total free energy of the particle leading to a depression of its melting
temperature. It has been estimated that a 5 nm diameter iron particle could
melt at a temperature as low as 500
◦
C. Figure 1.2 illustrates an iron particle
inside a carbon nanotube – its blobby character has been speculated to be due
to melting.
Small metal particles in the size range 1–5 nm are close to a metal/insulator
transition. When observed at the tips of carbon nanotubes using scanning elec-
tron microscopy, the iron particles have shown a tendency to charge, possibly
indicating a loss of metallic behaviour.
1.3 THE PHASE TRANSFORMATION: α- AND γ-IRON
The vast majority of steels rely on just two allotropes, α and γ. Iron is a peculiar
element in that at ambient pressure,bcc ferrite is stable from all temperatures up
to 910
◦
C (the A
3
point), when it transforms into the fcc austenite, only to revert
to ferrite at 1390
◦
C (theA
4
point). This high-temperature ferrite is traditionally
labelled δ, although it is no different in crystal structure from α.Theδ-ferrite
remains the stable phase until melting occurs at 1536
◦
C.
Figure 1.3 shows the phase changes in a plot of the mean volume per
atom of iron as a function of temperature. It should be noted that the γ-to
α-transformation is accompanied by an atomic volume change of approximately
1%, which can lead to the generation of internal stresses during transformation.
The detailed geometry of unit cells of α- and γ-iron crystals is particularly
relevant to, e.g., the solubility in the two phases of non-metallic elements such
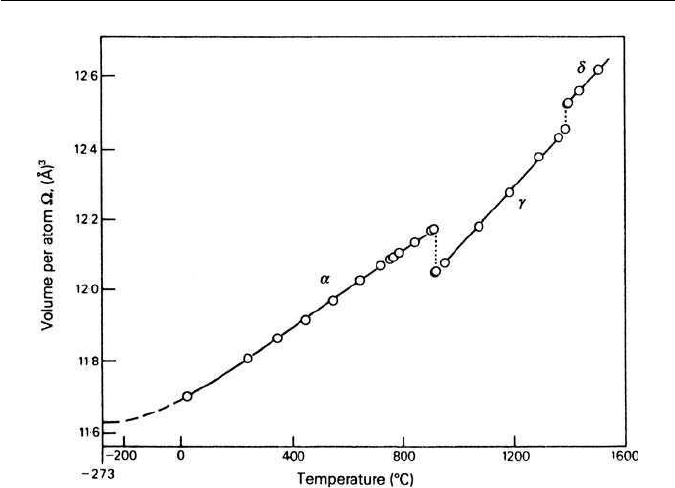
1.3 THE PHASE TRANSFORMATION: α- AND γ-IRON 5
Fig. 1.3 Temperature dependence of the mean volume per atom in iron crystals
(Hume-Rothery,The Structure of Alloys of Iron, Pergamon Press, Oxford, UK, 1966).
as carbon and nitrogen, the diffusivity of alloying elements at elevated tem-
peratures and the general behaviour on plastic deformation. The bcc structure
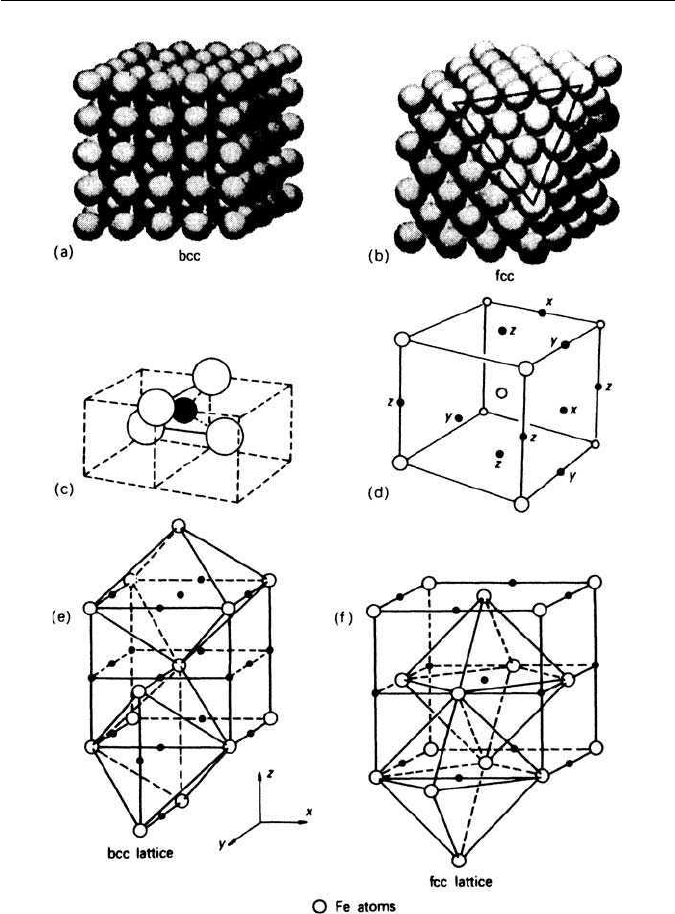
of α-iron is more loosely packed than that of fcc γ-iron (Figs 1.4a, b). The
largest cavities in the bcc structure are the tetrahedral holes existing between
two edge and two central atoms in the structure, which together form a tetra-
hedron (Fig. 1.4c). The second largest are the octahedral holes which occupy
the centres of the faces and the 001 edges of the body-centred cube (Fig.
1.4d). The surrounding iron atoms are at the corners of a flattened octahedron
(Fig. 1.4e). It is interesting that the fcc structure, although more closely packed,
has larger holes than the bcc structure. These holes are at the centres of the
cube edges, and are surrounded by six atoms in the form of an octagon, so they
are referred to as octahedral holes (Fig. 1.4f). There are also smaller tetrahe-
dral interstices. The largest sizes of spheres which will enter these interstices are
given in Table 1.1.
The α ⇋ γ transformation in pure iron occurs very rapidly, so it is not gen-
erally possible to retain the high-temperature fcc form at room temperature.
Rapid quenching can substantially alter the morphology of the resulting α-iron,
but it still retains its bcc structure. It follows that any detailed study of austenite
in pure iron must be done at elevated temperatures, e.g. using X-ray or neutron
diffraction. The transformation of the austenite on cooling can also be followed
6 CHAPTER 1 IRON AND ITS INTERSTITIAL SOLID SOLUTIONS
Fig. 1.4 (a) bcc structure, (b) fcc structure (Moffat, Pearsall and Wulff,The Structure and Prop-
erties of Materials: Vol. 1, Structure, John Wiley, USA, 1964), (c) tetrahedral interstices in bcc
structure, (d) octahedral interstices in bcc structure (Hume-Rothery,The Structure of Alloys of
Iron, Pergamon Press, Oxford, UK, 1966), (e) and (f) octahedral interstices in bcc and fcc iron
(Cohen,Transactions of the Metallurgical Society of AIME 224, 638, 1962).
1.3 THE PHASE TRANSFORMATION: α- AND γ-IRON 7
Table 1.1 Size of largest spheres fitting interstices in bcc and
fcc iron
Radius Radius in iron (Å)
bcc Tetrahedral 0.29r 0.37
Octahedral 0.15r 0.19
fcc Tetrahedral 0.23r 0.28
Octahedral 0.41r 0.51
r =atomic radius of iron.
using diffraction based on the intense X-rays generated in a synchrotron, or
using precision dilatometry. The latter technique relies on the volume change
accompanying the transformation from austenite to ferrite.
There are circumstances where it is necessary to study pure austenite at
temperatures well below ambient. Pure iron can be retained in its austenitic
state to very low temperatures by coherent precipitation in copper. Copper has
an fcc crystal structure and hence prevents the coherent particles of austenitic
iron from transforming during cooling.This technique has been used to establish
the antiferromagnetic nature of the austenite with a Néel temperature of about
−190
◦
C (the austenite is ferromagnetic at high temperatures, with a Curie point
of some 1525
◦
C).
1.3.1 Mechanisms of transformation
One of the reasons why there is a great variety of microstructures in steels is
because the same allotropic transition can occur with a variety of ways in which
the atoms can move to achieve the change in crystal structure. The transform-
ation can occur either by breaking all the bonds and rearranging the atoms into
an alternative pattern (reconstructive transformation), or by homogeneously
deforming the original pattern into a new crystal structure (displacive or shear
transformation) (Fig. 1.5).
In the displacive mechanism the change in crystal structure also alters the
macroscopic shape of the sample when the latter is not constrained. The shape
deformation during constrained transformation is accommodated by a com-
bination of elastic and plastic strains in the surrounding matrix. The product
phase grows in the form of thin plates to minimize the strains. The atoms are
displaced into their new positions in a coordinated motion. Displacive transfor-
mations can, therefore, occur at temperatures where diffusion is inconceivable
within the time scale of the experiment. Some solutes may be forced into the
product phase, a phenomenon known as solute trapping. Both the trapping of
atoms and the strains make displacive transformations less favourable from a
thermodynamic point of view.
It is the diffusion of atoms that leads to the new crystal structure during
a reconstructive transformation. The flow of matter is sufficient to avoid any
